Abstract
The effective control of the release of endogenous phosphorus is an urgent problem in the management of urban malodorous rivers. This research explored the fraction and regeneration of phosphorus of urban malodorous river in the context of sulfate reduction. It was found that sulfate reduction could promote sediment phosphorus release. The contents of total phosphorus (TP) and soluble reactive phosphorus (SRP) in the overlying water presented a decreasing trend after the initial increase during the operation of 120 days. The phosphorus release was positively related to the input of sulfate, and the maximum values of TP and SRP (14.01 mg/L and 12.27 mg/L, respectively) in the overlying water were observed when 8 mM Na2SO4 was added. Moreover, the addition of sulfate could significantly affect the distribution of phosphorus fraction in the sediment and promote the transformation of moderately active phosphorus (NaOH-P, D. HCI-P) to more active phosphorus Resin-P), which resulted in more release of phosphorus to the overlying water. In addition, it was observed that sulfate input could increase the relative abundance of phosphate solubilizing bacteria (PSB) and sulfate-reducing bacteria (SRB) from 0.69 to 1.1% and 4.92 to 9.03%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is the key limiting factor for water eutrophication (Loganathan et al. 2014; Pant and Reddy 2001; Tang et al. 2014). Furthermore, the concentration of sulfur (S), an important biogenic element of the hydrosphere geochemical cycle, is often higher by 2–3 orders of magnitude than that of phosphorus in the aquatic system (Baldwin and Mitchell 2012), and its role in the geochemical cycle is receiving increasing attention. Sulfate reduction is the main route of organic matter mineralization in sediments, and it is the beginning of the sulfur cycle. How it affects the endogenous P release is a scientific question worthy of discussion.
Nowadays, only a part of the research on phosphorus regeneration in water have focused mainly on the oceans (Yang et al. 2016), estuaries (Myrbo et al. 2017; Pollman et al. 2017; Stagg et al. 2017), wetlands (Coleman Wasik et al. 2015; Johnson et al. 2016), and lake sediments (Zhang et al. 2017; Wang and Benoit 2017; Ren et al. 2018). Some studies have investigated the spatiotemporal distribution of sulfur and phosphorus in water environments (Wang et al. 2018) and the effect of exogenous sulfate on phosphate migration and transformation (Yuan et al. 2012; Fan et al. 2014). These previous studies confirmed the effects of sulfate on P release in sediments, since sulfate reduction significantly changed the physical and chemical properties of the sediments and thus affected the environmental behavior of phosphate. Different P fractions in the sediment have different effects on water eutrophication (Huang et al. 2003; Liu et al. 2019; Zhu et al. 2017). Nevertheless, none of these studies involved the effects of sulfate on phosphorus fractions. And few studies have focused on the effect of sulfate on phosphorus regeneration at the sediment–water interface.
Moreover, malodorous urban rivers have been common in many cities especially in developing countries (Lu et al. 2010; He et al. 2013). Due to human activities, the sulfate content in the urban black malodorous river water changes significantly. (Coleman Wasik et al. 2015). Therefore, it is of scientific importance to ascertain how sulfate reduction affects the endogenous P release in urban malodorous rivers. However, little information is available regarding the effect of sulfate reduction on the fraction and regeneration of phosphorus in an urban malodorous river.
The objectives of this study are to (1) investigate the distribution characteristics of phosphorus and sulfur fractions in the sediments of an urban malodorous river, (2) analyze the effects of sulfate reduction on the distribution and regeneration of phosphorus, and (3) identify the possible coupling mechanism of sulfate reduction and phosphorus release.
Materials and methods
Sample characteristics
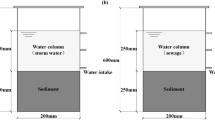
All of the water and sediments in the study are sampled from the river Taopu located in the town of Taopu in Shanghai. Sediments were collected using a bottom sampler and stored at 4 °C. The basic physicochemical properties of overlying water and sediment are measured and shown in Table 1.
Experimental setup
For all the experiments, each reactor was filled with 1 L of sediments and 3 L of the overlying water siphoned over the sediments. The outer flank of each column reactor was covered in order to prevent light exposure. The whole experiment included six runs, i.e., run 0 (control), run 1 (10 mM Na2MoO4 as a sulfate-reduction inhibitor), run 2 (2 mM Na2SO4), run 3 (4 mM Na2SO4), run 4 (6 mM Na2SO4), and run 5 (8 mM Na2SO4).
Different P fractions
Different P fractions were analyzed by the modified Hedley fractionation method by Tiessen (Tiessen and Moir 1993; He et al. 2008). The sediment-derived phosphorus was classified into six fractions: Resin-P, NaHCO3-P, NaHCO3-P, NaOH-P, dilute-HCl-extractable P (D. HCl-P), concentrated HCl-extractable P (C. HCl-P), and Residual-P. Resin-P denotes freely exchangeable inorganic phosphorus, while NaHCO3-P stands for organic and inorganic phosphorus loosely adsorbed on the surface of the sediment. Further, NaOH-P denotes inorganic phosphorus that was tightly bound to Fe–Al compounds and organic phosphorus bound to humic acid (Wang et al. 2006). In addition, D. HCl-P and C. HCl-P refer to inorganic phosphorus synthesized with calcium and occluded phosphorus, respectively, whereas Residual-P is unusable phosphorus or other occluded phosphorus in the residue after all of the extraction steps.
Microbial community analyses
After genomic DNA extraction according to the instructions of the DNA extraction kit corresponding to each type of sample, the integrity and purity of the DNA were detected by 1% agarose gel electrophoresis, and the concentration and purity of the DNA were detected by using NanoDrop One. For the polymerase chain reaction (PCR) amplification and product electrophoresis detection, genomic DNA was used as the template. Furthermore, primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTW TCTAA T-3′) with barcodes and Premix Taq (TaKaRa) were used for PCR amplification according to the specifically selected sequencing segment.
Subsequently, the NEBNext® Ultra™ DNA Library Prep Kit for Illumina® was employed for database construction. After its completion, the high-throughput sequencing platform HiSeq and MiSeq were used to perform on-line sequencing. The sequences analyses were done directly on I-Sanger Cloud platform.
Analytical and statistical methods
All of the chemical analyses were performed in accordance with standard methods (APHA 1998). The TP, soluble reactive phosphorus (SRP), sulfate, and acidic volatile sulfide (AVS) concentrations were measured colorimetrically. Furthermore, the pH, DO, and temperature (T) were measured using a relevant portable electrode.
Accession numbers
The sequence data reported in this study was deposited in the NCBI Sequence Read Archive database under accession number SRR11516698-SRR11516699.
Results and discussions
Variations in sulfate and acid volatile sulfide contents in different treatment groups
Figure 1 shows the profiles of sulfate concentration in each group. The sulfate content decreased gradually for all the experimental groups, which was mainly due to the sulfate reduction in the anoxic environment at the sediment–water interface. When sulfate enters the water body, it is used by soluble reactive phosphorus (SRB) as an electron acceptor (Berner and Westrich 1985; Dornblaser et al. 1994). Sulfates react in the system as follows (Herlihy and Mills 1985; van Houten et al. 1994):
During sulfate reduction, some organic phosphorus is converted to HPO4− and released. The sulfate content in the presence of 10 mM Na2MoO4 remained basically unchanged, indicating that the presence of Na2MoO4 had an inhibitory effect on sulfate reduction. As a sulfate-reduction inhibitor, excess Na2MoO4 can greatly inhibit the growth and anaerobic respiration of sulfate-reducing bacteria (Nair et al. 2015), thereby decreasing the consumption of sulfates and resulting in the sulfate concentration being higher than that in the control group.
In an anaerobic reduction environment, the organic matter in the sediments can be utilized by sulfate-reducing bacteria with SO42− receiving electrons to be reduced. Both the oxidation of sulfur-containing organic matter and the reduction of sulfate can produce S2− (Thode-Andersen and Jorgensen 1989). Fe(II) and Fe(III) and S2− in sediments react to form various iron sulfide minerals (Oehm et al. 1997).
The results showed that the AVS content in the sulfate-addition groups increased significantly in the following order: 8 mM > 6 mM > 4 mM > 2 mM (Fig. 2). This was because of the generated S2− from sulfate reduction to combine with Fe2+ from active iron reduction to form FeS2, which is the main form of AVS. The increase in sulfate content will promote the formation of reduction sulfur include the production of AVS in sediments.
Variations in TP and SRP contents in overlying water
Figure 3 demonstrates that the concentrations of SRP and TP in the overlying water increased first and then decreased in the first 30 days. After 30 days, the concentrations of SRP and TP showed an obvious increasing trend, and the increase was positively related to the sulfate level. The SRP and TP contents in the overlying water in the 8 mM sulfate group were significantly higher than the initial value and reached the maximum values of 14.01 mg/L and 12.27 mg/L, respectively, in 120 days (Fig. 3f). The P release followed the order: 2 mM < 4 mM < 6 mM < 8 mM. The addition of sulfate affects the P release in the sediment, which causes the migration of P from the sediment to the water body and gradually increases the P concentration in the overlying water.
Comparison of total phosphorus (TP) and soluble reactive phosphorus (SRP) contents in the overlying water between different treatment groups (a (control), b (10 mM Na2MoO4), c (2 mM Na2SO4), d (4 mM Na2SO4), e (6 mM Na2SO4), and f (8 mM Na2SO4)) during 140 days. Red dots denote SRP; black squares denote TP
In the later stage of the experiment, SRP and TP concentrations in the overlying water showed a decreasing trend (Fig. 3). With the lower sulfate concentration in the later stage, the sulfate reduction rate decreased, and the released phosphate was absorbed into the sediment. Before being permanently deposited, phosphorus generally undergoes multiple release-deposition-re-release-redeposition processes, and only a small part of phosphorus is finally deposited in sediments (Hupher et al. 1995).
SRP and TP concentrations in the overlying water were highest in the 10 mM Na2MoO4 group (Fig. 3b). Its concentration of SO42− was higher than that in the other groups from 70 to 140 days. However, the high sulfate concentration did not produce a high concentration of released P, indicating sulfate reduction was important for inducing the endogenous phosphorus.
Transformation of P fractions in sediments after sulfate input
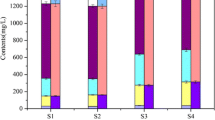
The P fractions are critical for the bioavailability of phosphorus. As such, the changes of P fractions in sediments after different levels of sulfate input were investigated (Fig. 4). Generally, sediment phosphorus is present in six different fractions and the moderate active phosphorus (NaOH-P and D. HCl-P) were dominant forms (46.97–76.25%) for all groups. An interesting observation was that the percentage of these two dominant P fractions decreased with the addition of sulfate. Additionally, the degree of decrease was positively related to the content of sulfate.
NaOH-P consists of inorganic phosphorus closely bound to iron and aluminum compounds as well as organic phosphorus bound to humic acid (Norton et al. 2008; Jan et al. 2013). Since aluminum and aluminum hydroxide are relatively stable under aerobic and anaerobic conditions (Jansen et al. 2003; Steinman et al. 2004), the change of Al-bound phosphorus content in the sediment is very small (Zhang and Huang 2007). Hence, the active inorganic phosphorus of sediments can be basically represented by the iron-bound phosphorus and sulfate reduction promoted the transformation and release of iron-bound phosphorus. The reason for the sulfate-driven transformation and release of iron-bound phosphorus (FeOOH-PO4 compound) is mainly attributed to the competitive advantage of reductant sulfur for iron ions with phosphate. Sulfate reduction can produce the reductant sulfur (S2−) to react with FePO4 to replace PO43−.
(Eq. 2) (Rutterberg and Berner 1993).
D. HCl-P is an inorganic phosphorus bound to Ca. Compared with the control group, the percentage of D. HCI in the sediment in runs 2–5 also decreased (Fig. 4). Phosphate-solubilizing bacteria (PSB) are bacteria associated with P release. Through the series of desorption, decomposition, and mineralization, phosphorus from insoluble inorganic phosphorus and phosphorous organic compounds is converted into soluble orthophosphates and released into water, producing organic acids—such as lactic acid, citric acid, succinic acid, acetic acid, and formic acid—and inorganic acids—such as nitric acid and sulfuric acid—and releasing positrons from Ca3(PO4)2 (Adnan et al. 2019).
Different from the decrease in NaOH-P and D. HCl-P, the increase in the percentage of active phosphorus (Resin-P) was observed with the input of sulfate. These observations showed the transformation of NaOH-P and D. HCl-P to Resin-P. Resin-P is free exchangeable inorganic phosphate that can enter the overlying water following the concentrate gradient and is the direct source of the phosphorus release. Compared with moderately active phosphorus, Resin-P is relatively more active. The transformation from NaOH-P and D. HCl-P to Resin-P, from less active to more active, and from the potential source to the direct source of phosphorus release increased the contents of TP and SPR in the overlying water. Moreover, our previous work showed that Resin-P is more favorable for algal growth, compared with NaOH-P and D. HCl-P (Zhu et al. 2017). Additionally, the contents of total phosphorus in the sediments decreased with the addition of sulfate. The decreases for 2 mM-, 4 mM-, 6 mM-, and 8 mM sulfate-addition group were separately 495 ± 16.4, 609 ± 10.8, 717 ± 15.3, and 846 ± 15.0 mg/kg, which further confirms the sulfate-driven release of endogenous P. Therefore, it is concluded that the sulfate input increases the risk of the eutrophication of water body.
Analysis of diversity and structure of bacterial flora
The alpha diversity reflects the species richness and diversity of microbial flora in samples. It was found that no obvious differences between the control and 8 mM sulfate group, as evidenced by the values of the richness of operational taxonomic units (OTUs) and Chao1 index. However, according to the Simpson index, the evenness of bacterial flora for 8 mM sulfate group (0.125) was higher than that of the control group (0.0937).
Figure 5 illustrates the bacterial flora structure and relative abundance at the genus level in the control and 8 mM Na2SO4 groups. The relative abundance of norank_o_MSBL5 (OTU_2) in the control and 8 mM sulfate-addition group was 36.61% and 34.8%, respectively. It belongs to the category of Dehalococcoidia, which includes microorganisms that exhibit strictly anaerobic organohalide respiration. Furthermore, the organic acids produced after dehalogenation are a carbon source or energy source for other types of microorganisms (Yang et al. 2019). Some studies have reported that an unnamed genus similar to Dehalococcoidia exists in the phylum Chloroflexi, and it has the potential to decompose carbohydrates, plant lignin, and cellulose in the sediment. Hence, diverse bacteria from the phylum Chloroflexi are widespread in the sediment (Hug et al. 2013).
Additionally, significantly different relative abundances of Sulfurovum were observed between the control (12.39%) and 8 mM sulfate-addition group (1%) (Fig. 5). Sulfurovum is a Gram-negative bacterium, chemoautotroph, and facultative anaerobe, and it uses elemental sulfur or thiosulfate as the electron donor (Inagaki et al. 2004; Mino et al. 2014). The 8 mM sulfate group is apt to convert the sulfate to negative-valence sulfur, which causes the relative abundance of Sulfurovum to be low due to the lack of electron donors (Mori et al. 2018).
Analysis of characteristics of target flora
In this study, target bacteria mainly included PSB (e.g., Bacillus, Flavobacterium, Streptomyces, and Micrococcus) and SRB (e.g., Desulfobacterium, Desulfurococcus, and Desulfuromonas of class Deltaproteobacteria). PSB are a group of bacteria related to P release. They produce organic acids, such as lactic acid, citric acid, succinic acid, acetic acid, and formic acid, as well as inorganic acids, such as nitric acid and sulfuric acid, to promote the dissolution of insoluble phosphorus fractions. For example, PSB are mainly responsible for the decomposition and release of D. HCl-P (Li et al. 2019). However, their ability to release positrons from Fe–Al compounds is much lower (Bautista-Cruz et al. 2019). The relative abundance of PSB was 1.13%, which is higher than that of control (0.69%). The dominant PSB genus in both groups was Thiobacillus, which is common sulfur-oxidizing denitrifying bacteria (Fig. 5). In the 8 mM sulfate group, there were more sulfide ions produced by sulfate reduction (Thode-Andersen and Jorgensen 1989). They were available for the process of denitrification of Thiobacillus who contributed to more D. HCl-P release.
SRB are chemotrophs and heterotrophs that can use sulfate as the electron acceptor and H2, lactate, fatty acid, ethanol, dicarboxylic acid, and aromatic compounds as the electron donor. They can reduce SO42− to H2S, and they are strictly anaerobic or facultative anaerobic (Cai et al. 2009; Feng and Ma 2005). SRB accounted for 9.23% of the total bacteria for the sulfate-addition group, which is higher than 4.92% in the control group (Fig. 5). This finding indicated that a high sulfate concentration was favorable for the growth of SRB, which could take more part in sulfate-driven phosphorus release.
Conclusions
These results presented in this study showed that sulfate reduction was closed related to the phosphorus release at sediment–water interface of the urban malodorous river. It was found that the phosphorus release was positively related to the level of sulfate, and the 8 mM Na2SO4-addition group demonstrated the maximum P release with the highest contents of TP and SRP (14.01 mg/L and 12.27 mg/L, respectively) in the overlying water. A clear transformation of NaOH-P and D. HCI-P to highly bioavailable Resin-P was observed for all sulfate-addition groups, which resulted in more release of phosphorus to the overlying water. It was also observed the relative abundance of PSB and SRB increased separately from 0.69 to 1.1% and 4.92 to 9.03% with the addition of sulfate. Taken together, the sulfate-driven release of endogenous P was mainly due to the competitive advantage of reductant sulfur for iron ions with phosphate. Sulfate reduction can produce the reductant sulfur (S2−) to react with FePO4 to replace PO43−. Further study is needed to clarify the above deduction by exploring the effects of different forms of sulfur on phosphorus release and fractions.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adnan M, Fahad S, Khan IA, Saeed M, Ihsan MZ, Saud S, Riaz M, Wang D, Wu C (2019) Integration of poultry manure and phosphate solubilizing bacteria improved availability of Ca bound P in calcareous soils. 3. Biotech 9(10):1–10
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater. AmericanPublic Health Association, Washington, DC
Baldwin DS, Mitchell A (2012) Impact of sulfate pollution on anaerobic biogeochemical cycles in a wetland sediment[J]. Water Res 46(4):965–974
Bautista-Cruz A, Antonio-Revuelta B, Gallegos VDM, Baez-Perez A (2019) Phosphate-solubilizing bacteria improve Agave angustifolia Haw growth under field conditions. J Sci Food Agric 99(14):6601–6607
Berner RA, Westrich JT (1985) Bioturbation and the early diagenesis of carbon and sulfur. Am J Sci 285(3):193–206
Cai J, Zheng P, Zhang L (2009) Sulfate-reducing bacteria and their metabolic pathway. Bull Sci Technol 25(4):427–431 (in Chinese)
Coleman Wasik JK, Engstrom DR, Mitchell CPJ, Swain EB, Monson BA, Balogh SJ, Jeremiason JD, Branfireun BA, Kolka RK, Almendinger JE (2015) The effects of hydrologic fluctuation and sulfate regeneration on mercury cycling in an experimental peatland. J Geophys Res Biogeosci 120(9):1697–1715
Dornblaser M, Anne EG, Brian F, Bruce JP (1994) Effects of sulfate concentration in the overlying water on sulfate reduction and sulfur storage in lake sediments. Biogeochemistry 24:129–144
Fan L, Hua Y, Yu F, Liu G, Zhu D (2014) Effect of external sulfate on phosphorus release, phosphate solubilizing microorganisms and phosphatase activity in Lake Donghu, Wuhan. Acta Sci Circumst 34(1):210–218 (in Chinese)
Feng J, Ma L (2005) Anaerobic bio-treatment of high-sulfate containing wastewater. Environ Protect Sci 31:23–26 (in Chinese)
He Y, Zhao Y, Chai X, Guo C, Zhou G (2008) Phosphorus fractions and activation in aged refuse from municipal solid waste dumpling site and landfill. Environ Chem 1:77–80 (in Chinese)
He Y, Chen Y, Zhang Y, Huang M (2013) Role of aerated turbulence in the fate of endogenous nitrogen from malodorous river sediments. Environ Eng Sci 30(1):11–16
Herlihy AT, Mills AL (1985) Sulfate reduction in freshwater sediments receiving acid mine drainage. Appl Environ Microbiol 49:179–186
Huang Q, Wang D, Wang C, Ma M, Wang Z (2003) Relation between phosphorus forms in sediments and lake eutrophication. China Environ Sci 23(6):583–586 (in Chinese)
Hug LA, Castelle CJ, Wrighton KC, Thomas BC, Sharon I, Frischkorn KR, Williams KH, Tringe SG, Banfield JF (2013) Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 1(1):22–39
Hupher M, Gachter R, Giovanoli R (1995) Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat Sci 57(4):305–324
Inagaki F, Takai K, Nealson KH, Horikoshi K (2004) Sulfurovum lithotrophicum gen. nov., sp nov., a novel sulfur-oxidizing chemolithoautotroph within the epsilon-Proteobacteria isolated from Okinawa Trough hydrothermal sediments. Int J Syst Evol Microbiol 54(5):1477–1482
Jan J, Borovec J, Kopacek J, Hejzlar J (2013) What do results of common sequential fractionation and single-step extractions tell us about P binding with Fe and Al compounds in non-calcareous sediments. Water Res 47:547–557
Jansen B, Nierop KG, Vestraten JM (2003) Mobility of Fe(II), Fe(III) and AI in acid forest soils mediated by dissolved organic matter influence of solution Ph and metal/ organic carbon ratios. Geoderma 113(3):323–430
Johnson NW, Mitchell CP, Engstrom DR, Bailey LT, Coleman Wasik JK, Berndt ME (2016) Methylmercury production in a chronically sulfate-impacted sub-boreal wetland[J]. Environ Sci Process Impacts 18(6):725–734
Li Y, Zhang J, Zhang J, Xu W, Mon Z (2019) Characteristics of inorganic phosphate-solubilizing bacteria from the sediments of a eutrophic Lake. Int J Environ Res Public Health 16(12):2041
Liu H, Hu L, Zhu M, Zhao L, Xu H, Zhou W, Shi P, Hang F, Ji P, Zhu G (2019) Applicability of bioavailable phosphorus in sediments to indicating trophic levels of lakes and reservoirs. Environ Sci 40(9):4023–4032
Loganathan P, Vigneswaran SK, Kandasamy J, Bolan NS (2014) Removal recovery of phosphate from water using sorption. Crit Rev Environ Sci Technol 44(8):847–907
Lu X, Zhang Y, Chen J (2010) Seasonal variation of water qualities and physical responses of nymphaea tetragonal under continuous aeration. Chin J Environ Eng 4(9):1978–1983 (in Chinese)
Mino S, Kudo H, Arai T, Sawabe T, Takai K, Nakagama S (2014) Sulfurovum aggregans sp nov., a hydrogenoxidizing, thiosulfate-reducing chemolithoautotroph within the Epsilonproteobacteria isolated from a deep-sea hydrothermal vent chimney, and an emended description of the genus Sulfurovum. Int J Syst Evol Microbiol 64(9):3195–3201
Mori K, Yamaguchi K, Hanada S (2018) Sulfurovum denitrificans sp. nov., an obligately chemolithoautotrophic sulfur-oxidizing epsilonproteobacterium isolated from a hydrothermal field. Int J Syst Evol Microbiol 68(7):2183–2187
Myrbo A, Swain EB, Engstrom DR, Coleman Wasik J, Brenner J, Shore MD, Peters EB, Blaha G (2017) Sulfide generated by sulfate reduction is a primary controller of the occurrence of wild rice (Zizania palustris) in shallow aquatic ecosystems. J Geophys Res Biogeosci 122(11):2736–2753
Nair RR, Silveira CM, Diniz MS, Almeida MG, Moura JJG, Rivas MG (2015) Changes in metabolic pathways of Desulfovibrio alaskensis G20 cells induced by molybdate excess. J Biol Inorg Chem 20(2):311–322
Norton SA, Coolidge K, Amirbahman A, Bouchard R, Kopáček J, Reinhardt R (2008) Speciation of Al, Fe, and P in recent sediment from three lakes in Maine, USA. Sci Total Environ 404:276–283
Oehm NJ, Luben TJ, Ostrofsky ML (1997) Spatial distribution of acid volatile sulfur in the sediments of Canadohta Lake PA. Hydrobiologia 345:79–85
Pant HK, Reddy KR (2001) Phosphorus sorption characteristics of estuarine sediments under different redox conditions. J Environ Qual 30:1474–1480
Pollman CD, Swain EB, Bael D, Myrbo A, Monson P, Shore MD (2017) The evolution of sulfide in shallow aquatic ecosystem sediments—an analysis of the roles of sulfate, organic carbon, iron and feedback constraints using structural equation modeling. J Geophys Res Biogeosci 122(11):2719–2735
Ren L, Song X, Jeppesen E, Xing P, Liboriussen L, Xu X, Wu Q (2018) Contrasting patterns of freshwater microbial metabolic potentials and functional gene interactions between an acidic mining lake and a weakly alkaline lake. Limnol Oceanogr 63:S354–S366
Rutterberg KC, Berner RA (1993) Authigenic apatite formation and burial in sediments from non-upwelling, continental margin environments. Geochim Cosmochim Acta 57(5):991–1007
Stagg CL, Schoolmaster DR, Krauss KW, Cormier N, Conner WH (2017) Causal mechanisms of soil organic matter decomposition: deconstructing salinity and flooding impacts in coastal wetlands. Ecology 98(8):2003–2018
Steinman AD, Rediske R, Reddy KR (2004) The reduction of internal phosphorus loading using alum in spring lake, Michigan[J]. J Environ Qual 33:2040–2048
Tang X, Wu M, Dai X, Chai P (2014) Phosphorus storage dynamics and adsorption characteristics for sediment from a drinking water source reservoir and its relation with sediment compositions. Ecol Eng 64:276–284
Thode-Andersen S, Jorgensen BB (1989) Sulfate reduction and the formation of 35 Slabeled FeS, FeS2, and S0 in coastal marine sediments. Limnol Oceanogr 34:793–806
Tiessen H, Moir JO (1993) Characterization of available P by sequential extraction. In: Cater MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. Talor and Francis, London, pp 75–86
van Houten RT, Hulshoff Pol LW, Lettinga G (1994) Biological sulphate reduction using gas-lift reactors fed with hydrogen and carbon dioxide as energy and carbon source. Biotechnol Bioeng 44(5):586–594
Wang P, Benoit G (2017) Modeling the biogeochemical role of photosynthetic sulfur bacteria in phosphorus cycling in a managed eutrophic lake. Ecol Model 361:66–73
Wang G, Liu J, Wang J, Yu J (2006) Soil phosphorus forms and their variations in depressional and riparian freshwater wetlands (Sanjiang Plain, Northeast China). Geoderma 13:259–274
Wang J, Chen J, Luo J, Zhang H, Yu P (2018) Comparative study on quantitative estimations of phosphorus release flux from Sediments of Lake Hongfeng , Guizhou Province, China. Earth Environ 46(1):1–6
Yang H, Chen J, Liu W, Wang J, Li J, Ji Y, Chen Y (2016) Distribution characteristics and controlling factors of total organic carbon, total nitrogen, and total phosphorus in sediments of Caohai Lake China. Earth Environ 44(3):297–303 (in Chinese)
Yang Y, Zhang Y, Li X, Zeng X, Song Y, Yan J (2019) Roles of Dehalococcodia class in the biogeochemical cycle of organohalides. Acta Sci Circumst 39(10):3207–3214 (in Chinese)
Yuan T, Hua Y, Zhu D, Zhao J, Cai J (2012) Response of phosphorus components in sediments from eutrophic lake to external sulfate. Environ Sci 33(7):0250–3301 (in Chinese)
Zhang J, Huang X (2007) Relative importance of solid-phase phosphorus and iron on the sorption behavior of sediments. Environ Sci Technol 41(8):2789–2795
Zhang W, Jin X, Liu D, Tang W, Shan B (2017) Assessment of the sediment quality of freshwater ecosystems in eastern China based on spatial and temporal variation of nutrients. Environ Sci Pollut Res 24(23):1–10
Zhu J, He Y, Wang J, Qiao Z, Wang Y, Li Z, Huang M (2017) Impact of aeration disturbances on endogenous phosphorus fractions and their algae growth potential from malodorous river sediment. Environ Sci Pollut Res 24:8062–8070
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was carried out with the financial support from the National Science and Technology Major Project for Water Pollution Control and Treatment (2018ZX07208008), National Natural Science Foundation of China (41877477), and Shanghai Science and Technology Development Funds (18DZ1203806).
Author information
Authors and Affiliations
Contributions
Guan Linchang and Xia Zhenyu: writing—original draft preparation, investigation, and data analysis. Jin Lili: investigation, data analysis, and visualization. Xu Yiwen: investigation and data analysis. He Yan: conceptualization, writing—review and editing, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible Editor: Philipp Gariguess
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guan, L., Xia, Z., Jin, L. et al. Influence of sulfate reduction on fraction and regeneration of phosphorus at sediment–water interface of urban malodorous river. Environ Sci Pollut Res 28, 11540–11548 (2021). https://doi.org/10.1007/s11356-020-11187-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11187-z