Abstract
Calcium nitrate and a lanthanum-modified bentonite (Phoslock®) were investigated for their ability to control the release of phosphorus from contaminated sediment. Their effectiveness and mode of action were assessed using microcosm experiments by monitoring the variation of physiochemical parameters and phosphorus and nitrogen species over time following the treatment for 66 days. Phoslock® was more effective reducing phosphorus in overlaying water and controlling its release from sediment. Calcium nitrate improved redox condition at the sediment-water interface and temporally reduce phosphorus in overlaying water but phosphorus level returned back in a long run. Phosphorus fractionation suggested that Phoslock® converted mobile phosphorus to more stable species while calcium nitrate increased the fractions of mobile phosphorus species. Phoslock® generally showed no effect on nitrogen species. Whereas calcium nitrate temporally increased nitrate, nitrite, and ammonium concentrations but their concentrations quickly reduced likely due to the denitrification process. Results suggested that Phoslock® can be more effective in controlling the release of phosphorus from sediment than calcium nitrate. However, calcium nitrate can improve the redox condition at the sediment-water interface, which may provide other benefits such as stimulating biodegradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Increases of nutrient concentrations in aquatic ecosystem are the main reason for eutrophication (Smith 2003). Eutrophication leads to the impairment of water quality and deterioration of aquatic ecosystem, resulting in biodiversity decline and economic losses (Dodson et al. 2000; Smith 2003). As a result, many research focus on nutrients reduction to mitigate the eutrophication problem (Carpenter 2008; Jarvie et al. 2013). Although many measures are carried out to cut off the external loading, nutrient concentrations might still be high in water due to internal release from the contaminated sediment (Jeppesen et al. 2005; Søndergaard et al. 2007). Therefore, internal nutrient loadings need to be properly controlled for a successful restoration attempt.
Many restoration techniques have been developed and used in the past decades to control internal loadings. Sediment dredging is a simple way to remove internal nutrients but the outcomes were found controversial (Jing et al. 2013; Liu et al. 2015; Zhang et al. 2010). In addition, high cost and problems associated with the treatment and disposal of the dredged sediment prevent the wide application of sediment dredging (Murphy et al. 1999). In situ treatment, which can considerably reduce the cost and cause less disturbance to the sediment, becomes a more attractive solution.
In situ treatment with chemical modifiers can be an effective way to control internal nutrient loadings. Chemical modifiers such as aluminum, iron salts, nitrate, and Phoslock® have been studied in previous research (Hansen et al. 2003; Liu et al. 2009; Meis et al. 2013). Aluminum and iron salts were able to inhibit the release of phosphorus (P) from sediment by combining with soluble reactive phosphorus (SRP) to form insoluble precipitants (Liu et al. 2009). However, aluminum can be toxic to aquatic organisms (Pessot et al. 2014), and the effect of iron salt is sensitive to redox and pH conditions (Burley et al. 2001; Immers et al. 2013).
Nitrate application was firstly tested by Ripl (1976), which achieved a drastic reduction of the nutrient levels and an improvement of the redox condition. Research had shown that nitrate addition could not only eliminate black-odorous and suppress internal phosphorus loading in the contaminated sediment but also successfully stimulate indigenous microorganisms for in situ bioremediation (Hemond and Lin 2010; Xu et al. 2014; Yamada et al. 2012). Phoslock®, a new lanthanum-modified bentonite, was developed in the 1990s by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) of Australia (Robb et al. 2003), and had succeeded in reducing phosphorus in both laboratory and field trials (Meis et al. 2012; Meis et al. 2013; Lürling and van Oosterhout 2013). Phoslock® had also been used to remove phosphorus from wastewater and to control phosphate mobilization in soils (Geurts et al. 2011; Haghseresht et al. 2009).
However, the phosphorus immobilization mechanisms by these two modifiers and their effects on nitrogen (N) cycling in sediment has not been well characterized. Therefore, this work aims to (1) examine the changes of phosphorus in water and sediment over time following the treatment with calcium nitrate and Phoslock®, (2) to evaluate their potential impacts on physicochemical properties of the sediment and on N cycling, and (3) to assess and compare the modes of action of these two modifiers.
2 Materials and Methods
2.1 Materials and Chemicals
Sediment used in this work was collected from Donghu Lake (30° 33′ N, 114° 23′ E), the largest urban lake in China located in the city of Wuhan with an area of 33 km2 and an average depth of 2.5 m. Over 100 small-scale enterprises; 11 hospitals; and 200,000 residential homes are located around the lake (Wang et al. 2002). Due to the discharge of municipal wastewater, the phosphorus dynamics showed an upward trend with a peak period in the mid-1980s in the lake accompanied by an outbreak of cyanobacteria blooms (Xie and Xie 2002). The input of wastewater has been considerably reduced in recent years as a result of the city sewer system upgrade; however, the internal P load will continue to threat the water quality of the lake. Sediment sample was collected from the lake (30° 32′ 54.91″ N, 114° 21′ 15.61″ E) with a stainless steel grab sampler, homogenized, and sieved to <0.18 mm to remove the coarse particles (to avoid damage to the microelectrode). Water content, total organic carbon (TOC), total Kjeldahl nitrogen (TKN), and total phosphorus (TP) of the sediment was 68.34 %, 1.67 %, and 3.89 mg N/g and 2.32 mg P/g, respectively. The concentrations of NH4 +–N, NO3 −–N, and NO2 −–N were 0.23, 0.21, and 0.07 mg N/g, respectively.
Calcium nitrate (Ca(NO3)2·4H2O) is analytical reagent grade and was provided by Sinopharm Chemical Reagent Co., Ltd. Phoslock® (lanthanum-modified bentonite containing 5 % lanthanum) was supplied by the Phoslock® Water Solutions Ltd. All other chemicals used in the experiment are analytical reagent grade and were purchased from Sinopharm Chemical Reagent Co., Ltd.
2.2 Microcosm Experiment
Microcosms were setup in 1-L beakers with a height of 19 cm and a diameter of 10 cm. Each beaker was filled with 800-g sediment and 600-mL water, and the heights of sediment and the overlaying water were about 10 and 8 cm, respectively. In treatment 1, calcium nitrate was added to the surface of the sediment at a dose of 61 g N/m2. In treatment 2, 4 g of Phoslock® (equal to approximately 510 g/m2) was added by sprinkling the solid evenly onto the surface of the sediment. In control, no modifier was added. The application rate was selected by reference to the literature. Previous research by Foy (1986) showed that the addition of 61 g N/m2 of nitrate to the sediment delayed and reduced phosphorus release, causing complete suppression. Intact core experiment performed by Meis et al. (2013) indicated that 510 g Phoslock®/m2 is required to control P release under anaerobic condition.
Microcosms were maintained at 24 °C in dark. Three beakers were retreated from each treatment and control on days 1, 15, 30, and 66. Overlaying water was siphoned into a sampling bottle, and the sediment was sliced into 2-cm sections to a depth of 8 cm using a column sampler. The remaining sediment in the beaker was homogenized for the measurement of water content. The sliced sediment samples from each layer from each treatment and control were mixed to form composited samples.
On days 1, 5, 30, and 66, a microelectrode (Unisense, Denmark) was used to analyze the dissolved oxygen (DO) at the sediment-water interface. After about an hour of polarization and calibration, the electrode was emplaced onto a micromanipulator (MM 33, Unisense). The electrode was 1 cm above the surface of the sediment. Motor controller (MC-232, Unisense) drove the electrode down 500 μm every 5 s to a depth of 7 mm below the sediment surface.
2.3 Chemical Analysis
Water pH was measured by a Hach miniLab H135 pH meter. Water samples were filtered through 0.45-μm cellulose acetate membranes. Total nitrogen (TN) and TP of the overlaying water were measured using the alkaline persulfate oxidation and persulfate oxidation method, respectively. NH4 +–N was determined by Nessler’s colorimetric, NO2 −–N and soluble reactive phosphorus (SRP) were determined by N-(1-Naphthalene)-diaminoethane and ammonium molybdate methods, and NO3 −–N was determined by the UV spectrophotometry method (MEPC 2002).
Water content of each sample was measured from the weight loss by drying at 105 °C to a constant weight. Total Kjeldahl nitrogen (TKN) of the sediment was determined by the automatic Kjeldahl nitrogen analyzer (S5, Behr, Germany). TKN includes ammonia and organic N which could be transformed to ammonia via sulfuric acid digestion. NH4 +–N and NO2 −–N were measured by spectrophotometric method following potassium chloride extraction. NO3 −–N was determined by phenol disulfonic acid spectrophotometric method. TN was calculated as the sum of TKN, NO2 −–N, and NO3 −–N. TP was measured as described by Ruban et al. (1999).
The sequential extraction scheme of Psenner et al. (1984) with slightly modifications was used for P fractionation. P was separated into loosely sorbed P (NH4Cl-P), reductant-soluble P (BD-P), metal oxide bound P (NaOH-SRP), organic bound P (NaOH-NRP), calcium bound P (HCl-P), and residual-P. NaOH-NRP was calculated as NaOH-TP minus NaOH-SRP, and residual-P was calculated as TP substrate all the extractable P species.
2.4 Data Analysis
Results of the overlaying water samples were presented as the means and standard deviations of three replicates. Results of the sediment samples were reported on dry weight basis. Differences between treatments and control for NO3 −–N, NO2 −–N, NH4 +–N, TP, and SRP in overlaying water were tested using one-way ANOVA by a LSD post hoc test using PASW Statistics 18 (SPSS Inc.). A confidence level of 95 % was used.
3 Results and Discussion
3.1 The Influences of the Modifiers on Physical and Chemical Conditions
3.1.1 Effects on pH and Water Content
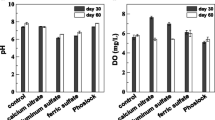
Chemical treatment (e.g., Al or Fe salts) may cause a decline in pH, which may cause a re-release of the phosphorus. In our study, the pH in the overlaying water in calcium nitrate treatment was slightly lower than the control (p < 0.05) (Table 1), and this might be due to the release of hydrogen ion during the oxidation of reducible agents (e.g., Fe salts) by nitrate (Ottley et al. 1997). Possible reactions involved in this process are as follows:
The Phoslock® treatment has a slightly higher pH (p < 0.05) in the first few days but showed no difference (p > 0.05) at the end compared with the control (Table 1). Ross et al. (2008) found that Phoslock® was most effective at pH values between 5 and 7, and the adsorption capacity decreased above pH 9. Therefore, the slight change of pH here wouldn’t affect the adsorption capacity of Phoslock®. A significant increase (p < 0.05) in water content of the nitrate-treated sediment was also observed after day 15 (Table 1). This was likely attributed to the formation of N2 and N2O in denitrification processes, which made the sediment loose (Zhang et al. 2009). The denitrification processes can be represented by the following two equations:
3.1.2 DO at the Sediment-Water Interface
The sediment-water interface was divided into aerobic layer (from the sediment surface and above), transition layer, and anaerobic layer (from the place where DO drops to 0 and below). As presented in Fig. 1, the addition of nitrate had a great influence on DO content. At the beginning, the application of nitrate caused a decline in DO in the aerobic and transition layers on day 1 and 5. However, similar trend in two treatments and control was observed on day 30. On day 66, DO in calcium nitrate treatment was even higher than that in control in the aerobic layer, and the boundary between the transition lay and anaerobic layer increased from 2 mm below the sediment surface on day 1 to 6 mm.
The addition of calcium nitrate could promote denitrification process in the sediment (Eqs. (9) and (10)), resulting in a release of Fe2+ due to the oxidation of metal sulfide to sulfate (Shao et al. 2009). The mobilized Fe (II) diffused into the sediment-water interface, and when they contact with oxygen or nitrate, Fe (II) was oxidized (Schauser et al. 2006). Nitrate can easily penetrate into the sediment, it was possible that most of Fe (II) was oxidized to Fe (III) by nitrate before they entered the water from sediment. So, there might be other responsible reasons for this result. Dissolved organic carbon (DOC) might enter the overlaying water from sediment due to the loose of the sediment. Yamada et al. (2012) found that nitrate addition could increase the DOC in both interstitial water and overlaying water a few days after treatment. Therefore, it was possible that the DO depletion was due to the oxidation of DOC. Over time, DO in the overlaying water increased gradually as the decrease of DO consumption. Nitrate addition increased the redox potential of the sediment. Therefore, less DO might be consumed during the diffusion to deeper layers. As a result, the depth of the transition layer increased.
DO in aerobic layer was slightly higher in Phoslock® treatment than in control on days 5 and 66. Phoslock® transformed SRP into highly recalcitrant P forms and controlled P release from sediment (Meis et al. 2013). This process can reduce the bioavailable P and as a consequence, might reduce microbial respiration (Ramírez et al. 2003). Therefore, the increase of DO concentration of Phoslock® treatment might relate to the decreased DO consumption by microbes. These results indicated that both nitrate and Phoslock® can improve the redox condition in sediment. However, the effect of calcium nitrate was more pronounced.
3.2 Effects of the Modifiers on Phosphorus
3.2.1 Effects on Phosphorus in the Overlaying Water
Variation of SRP and TP in the overlaying water during the experiment is presented in Fig. 2. SRP in Phoslock® treatment decreased on day 1 and maintained at low concentration during the experiment. SRP in calcium nitrate treatment decreased on day 1 but increased gradually thereafter, and at the end of the experiment, SRP was comparable to its initial concentration, which was different from the results from other researchers (Ripl 1976; Yamada et al. 2012). Release of SRP and TP from porewater might have occurred in calcium nitrate treatment as a result of water content increase due to gas production from denitrification in the sediment. In control, SRP increased first and then dropped to even lower than the initial concentration. TP showed a similar trend as SRP in Phoslock® treatment and control. Whereas TP decreased in days 1 and 15 and then increased on day 30 and then returned back. Overall, Phoslock® showed a better effect in removing P from water and controlling P release from sediment.
Phoslock® could reduce SRP by formation of highly insoluble lanthanum–phosphate complex (Eq. (11)), and then striped it from water column to sediment (Haghseresht et al. 2009; Reitzel et al. 2013).
The addition of calcium nitrate could transform Fe (II) to Fe (III) (Eqs. (1)–(4)), which can bind SRP from water (McAuliffe et al. 1998; Yamada et al. 2012). In addition, calcium ion could also bind with P. This might be why SRP and TP decreased in the first few days. However, subsequent increase in SRP and TP might be related to the mineralization of organic materials. In addition, P can be released into the overlaying water during the release of gases produced during the denitrification processes in the sediment.
3.2.2 Effects on Phosphorus in the Sediment
Changes of P species in the sediment profile over time are presented in Fig. 3. Shift in P species was observed following calcium nitrate and Phoslock® application. Phoslock® application mainly affected the top layer of the sediment (0~2 cm), while nitrate treatment can have a deeper influence, mainly the first three layers (0~6 cm). Slight change in P species in control was also observed during the experiment, mainly in the top layer of the sediment.
NH4Cl-P represents loosely sorbed P, which includes P in porewater, CaCO3-associated P, or P leaching from decaying cells of bacterial biomass in deposited phytodetrital aggregates (Kaiserli et al. 2002). NH4Cl-P in the control increased slight over time in the top layer. NH4Cl-P in the Phoslock® treatment showed little variation over time and was lower than the control in the top layer, while NH4Cl-P increased over time and was higher than the control in almost all layers (Fig. 3a). The most drastic change in nitrate treatment occurred in the top layer, in which NH4Cl-P increased from 4.08 mg P/kg on day 1 to 39.9 mg P/kg on day 66. Increase of NH4Cl-P in nitrate treatment was likely attributed to the increase of P releasing from decaying cells of bacterial biomass in deposited phytodetrital aggregates as nitrate addition might increase the microbial activity in the sediment (Xu et al. 2014).
BD-P represents the redox-sensitive P forms, mainly bound to Fe and Mn compounds (Kozerski and Kleeberg 1998; Schauser et al. 2006). BD-P showed a similar trend as NH4Cl-P. In the top layer, BD-P increased in two treatments and control but increase was more drastic in calcium nitrate treatment, in which BD-P increased from 0.29 mg P/g on day 1 to 0.92 mg P/g day 66 (Fig. 3b). The change of BD-P might be attributed to oxidation of Fe (II) to Fe (III) (Eqs. (1)–(4)) as a result of improved redox condition as observed in Fig. 1. Previous research showed that addition of nitrate promoted autotrophic denitrification, leading to the oxidation of iron sulfide and release of Fe (II) into water (Yamada et al. 2012). Fe (II) was then oxidized and the formation of Fe oxide-hydroxide provided the adsorption sites for P, leading to the increase of BD-P.
NaOH-SRP represents P bound to metal oxides, mainly of Al and Fe, which is exchangeable again with OH− and inorganic P compounds soluble in alkaline condition (Kozerski and Kleeberg 1998). NaOH-NRP represents P in microorganisms including poly-P, organic P in detritus, P bound in humic compounds (Hupfer et al. 1995). Nitrate application decreased NaOH-SRP, and slightly increased NaOH-NRP on days 15 and 30, while Phoslock® application slightly decreased NaOH-SRP in the top layer (Fig. 3c, d). This result suggests that calcium nitrate addition can transform NaOH-SRP into other P species, especially BD-P, which increased most. Anion exchange between nitrate and phosphate may be responsible for the NaOH-SRP decrease. The increase of NaOH-NRP on days 15 and 30 might be due to the increase of microbial biomass stimulated by N addition. With the decrease of N in the sediment as a result of denitrification, NaOH-NRP decreased as well. In Phoslock® treatment, part of NaOH-SRP may be transformed into HCl-P, which is more stable.
HCl-P represents P bound to carbonates, apatite-P, and traces of hydrolyzed organic P (Hupfer et al. 1995). HCl-P increased from 0.38 mg P/g on day 1 to 0.57 mg P/g on day 66 due to the addition of Phoslock®, and this increase was mainly observed in the top layer of the sediment (Fig. 3e). Whereas, calcium nitrate showed minor influence on HCl-P. This result suggests that P can be released from lanthanum–phosphate complex at acid condition. But at typical environmental conditions, release of HCl-P is unlikely to happen. Therefore, HCl-P can be considered as non-bioavailable.
Residual-P is refractory P, which is difficult to release, and can be treated as an inert P-fraction (Psenner and Pucsko 1988). Residual-P increased in nitrate treatment and control (Fig. 3f). It was likely that NaOH-SRP was partially transformed into residual-P pool in calcium nitrate treatment, which might be related to the microbial processes.
NH4Cl-P, BD-P, and NaOH-SRP were considered as mobile P fractions and can be potentially released under certain environment conditions (Hupfer et al. 1995; Meis et al. 2012). Whereas, NaOH-NRP, HCl-P, and residual-P were generally non-bioavailable and have a low release potential (Hupfer et al. 1995; Gonsiorczyk et al. 1998; Kaiserli et al. 2002). Our results showed that the application of Phoslock® transformed mobile P into un-bioavailable P. Calcium nitrate application transformed NaOH-SRP into NH4Cl-P, BD-P, and residual-P. Therefore, the effects of calcium nitrate in P control are more sensitive to the variation of environmental conditions.
3.3 Effects of the Modifiers on N Cycling
3.3.1 Effects on the N Cycling in the Overlaying Water
The addition of Phoslock® showed minor effect on N species. Only NH4 +–N showed a significant increase on day 1 (p < 0.05). Following the addition of calcium nitrate, the overlaying water showed a sharp rise in NO3 −–N concentration (Fig. 4a). The highest NO3 −–N concentration appeared on day 1 and was 718.20 mg/L. However, it decreased over time and dropped to 42.40 mg/L by the end of the experiment, which reduced by 94 % compared to the highest level. Nitrate addition significantly increased (p < 0.05) the concentration of NH4 +–N and NO2 −–N in the overlaying water. The highest concentration of NO2 −–N was observed on day 15 with a value of 2.60 mg/L (Fig. 4b), and the highest NH4 +–N concentration was observed on day 1 with a value of 0.93 mg/L (Fig. 4c). However, NO2 −–N and NH4 +–N decreased over time after peaking with no significant different from the control at the end of the experiment.
The decline of nitrate and increase of NO2 −–N was mainly attributed to denitrification, and the increase of NH4 +–N might be related to the dissimilatory nitrate reduction to ammonia process (DNRA). The added nitrate acted as electron acceptor, leading to the enhancement of two biological processes including sulfide-oxidizing and organics-oxidizing (Chen et al. 2013). In sulfide-oxidizing process, nitrate might cause the development of nitrate-reducing, sulfide-oxidizing bacterium (NR-SOB) thus enhanced denitrification (García de Lomas et al. 2006). The increase of NH4 +–N and NO2 −–N in nitrate treatment was temporary with the consumption of DO at the beginning of the experiment as described in Section 3.1.2, NH4 +–N can be oxidized to nitrate by facultative heterotrophic microorganisms. NO2 −–N increased in the first few days as an intermediate product of DNRA and denitrification processes. With the consumption of nitrate, the concentration of NO2 −–N decreased as well.
Accumulate of NH4 +–N in Phoslock® treatment on day 1 might be related to the suppression of the nitrification process caused by the capping of bentonite on the sediment surface (Gibbs et al. 2011). However, this influence seems to be transient, with the improvement of redox condition, NH4 +–N was soon oxidized.
3.3.2 Effects on the N Cycling in the Sediment
The addition of Phoslock® showed minor effects on NH4 +–N, NO2 −–N, and NO3 −–N in sediments. NO3 −–N concentrations were low during the whole experiment period, which ranged from 0 to 1.60 mg N/kg. Calcium nitrate treatment showed influence on NO2 −–N and NO3 −–N. NO3 −–N content declined gradually with depth in the sediment. The highest NO3 −–N concentration appeared in the top layer on day 1 with a value of 2023 mg N/kg. By the end of the experiment, it declined by 98 % to 35.59 mg N/kg (Fig. 5a).
As an intermediate product in NO3 −–N reduction process, NO2 −–N in the sediment also increased before day 15 but decreased thereafter (Fig. 5b). The increase of NO2 −–N was observed in the bottom layer, which suggested that the nitrate addition can have an influence on deeper layers. However, the influence weakened with depth.
Similar trends were observed for NH4 +–N, which decreased over time, in both treatments and control (Fig. 5c). This might be related to the improvement of redox condition in the sediment.
According to Camargo and Alonso (2006), high levels of NH4 +–N, NO2 −–N, and NO3 −–N can impair the ability of aquatic animals to survive, grow, and reproduce, resulting from the toxic effects of the nitrogenous compounds. Our data indicated that calcium nitrate addition temporally increased the concentration of NO2 −–N and NO3 −–N but quickly recovered while Phoslock® addition had negligible effects. Therefore, both treatments are unlikely to cause nitrogenous associated risks.
4 Conclusions
Calcium nitrate application temporally decreased dissolved oxygen in overlaying water but improved the redox condition in sediment in a long run. Whereas Phoslock® only slightly increased the DO in overlaying water. Both treatments reduced SRP and TP in water but their concentrations returned back overtime in calcium nitrate treatment while stayed low in Phoslock® treatment. Phosphorus fractionation in the sediment profile suggested that calcium nitrate addition increased mobile phosphorus fractions (NH4Cl-P and BD-P), which are potentially bioavailable. Whereas Phoslock® addition increased HCl-P, which is considered non-bioavailable and have a low release potential. Calcium nitrate increased the rate of autotrophic denitrification, causing a short-term elevation of nitrite in water and sediment and ammonia in water. The added nitrate was consumed rapidly overtime with only 2 % left at the end of the experiment, likely due to the denitrification process. Nitrite and ammonia in water and nitrite in sediment also declined remarkably following the consumption of nitrate. Phoslock® showed minor influence on nitrogen species. Our results suggested that Phoslock® is a better choice for phosphorus control in eutrophicated lakes, while the effectiveness of calcium nitrate can be more sensitive to environmental conditions. However, calcium nitrate can improve redox condition of the sediment and stimulate indigenous microbial activities, which can provide other benefits for remediation. Therefore, calcium nitrate might be used together with Phoslock® for a better in situ treatment result.
References
Burley, K. L., Prepas, E. E., & Chambers, P. A. (2001). Phosphorus release from sediments in hardwater eutrophic lakes: the effects of redox‐sensitive and‐insensitive chemical treatments. Freshwater Biology, 46(8), 1061–1074.
Camargo, J. A., & Alonso, Á. (2006). Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environment International, 32(6), 831–849.
Carpenter, S. R. (2008). Phosphorus control is critical to mitigating eutrophication. Proceedings of the National Academy of Sciences, 105(32), 11039–11040.
Chen, L., Wang, L., Liu, S., Hu, J., He, Y., Zhou, H., & Zhang, X. (2013). Profiling of microbial community during in situ remediation of volatile sulfide compounds in river sediment with nitrate by high throughput sequencing. International Biodeterioration & Biodegradation, 85, 429–437.
Dodson, S. I., Arnott, S. E., & Cottingham, K. L. (2000). The relationship in lake communities between primary productivity and species richness. Ecology, 81(10), 2662–2679.
Foy, R. (1986). Suppression of phosphorus release from lake sediments by the addition of nitrate. Water Research, 20(11), 1345–1351.
García de Lomas, J., Corzo, A., Gonzalez, J. M., Andrades, J. A., Iglesias, E., & Montero, M. J. (2006). Nitrate promotes biological oxidation of sulfide in wastewaters: experiment at plant-scale. Biotechnology and Bioengineering, 93(4), 801–811.
Geurts, J. J. M., van de Wouw, P. A. G., Smolder, A. J. P., Roelofs, J. G. M., & Lamers, L. P. M. (2011). Ecological restoration on former agricultural soils: feasibility of in situ phosphate fixation as an alternative to top soil removal. Ecological Engineering, 37(11), 1620–1629.
Gibbs, M. M., Hickey, C. W., & Özkundakci, D. (2011). Sustainability assessment and comparison of efficacy of four P-inactivation agents for managing internal phosphorus loads in lakes: sediment incubations. Hydrobiologia, 658(1), 253–275.
Gonsiorczyk, T., Casper, P., & Koschel, R. (1998). Phosphorus-binding forms in the sediment of an oligotrophic and an eutrophic hardwater lake of the Baltic Lake District (Germany). Water Science and Technology, 37(3), 51–58.
Haghseresht, F., Wang, S., & Do, D. D. (2009). A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Applied Clay Science, 46(4), 369–375.
Hansen, J., Reitzel, K., Jensen, H. S., & Andersen, F. Ø. (2003). Effects of aluminum, iron, oxygen and nitrate additions on phosphorus release from the sediment of a Danish softwater lake. Hydrobiologia, 492(1–3), 139–149.
Hemond, H. F., & Lin, K. (2010). Nitrate suppresses internal phosphorus loading in an eutrophic lake. Water Research, 44(12), 3645–3650.
Hupfer, M., Gachter, R., & Giovanoli, R. (1995). Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquatic Sciences, 57(4), 305–324.
Immers, A. K., Van der Sande, M. T., Van der Zande, R. M., Geurts, J. J. M., Van Donk, E., & Bakker, E. S. (2013). Iron addition as a shallow lake restoration measure: impacts on charophyte growth. Hydrobiologia, 710(1), 241–251.
Jarvie, H. P., Sharpley, A. N., Withers, P. J., Scott, J. T., Haggard, B. E., & Neal, C. (2013). Phosphorus mitigation to control river eutrophication: murky waters, inconvenient truths, and “postnormal” science. Journal of Environmental Quality, 42(2), 295–304.
Jeppesen, E., Søndergaard, M., Jensen, J. P., Havens, K. E., Anneville, O., Carvalho, L., et al. (2005). Lake responses to reduced nutrient loading—an analysis of contemporary long‐term data from 35 case studies. Freshwater Biology, 50(10), 1747–1771.
Jing, L. D., Wu, C. X., Liu, J. T., Wang, H. G., & Ao, H. Y. (2013). The effects of dredging on nitrogen balance in sediment-water microcosms and implications to dredging projects. Ecological Engineering, 52, 167–174.
Kaiserli, A., Voutsa, D., & Samara, C. (2002). Phosphorus fractionation in lake sediments—Lakes Volvi and Koronia, N. Greece. Chemosphere, 46(8), 1147–1155.
Kozerski, H. P., & Kleeberg, A. (1998). The sediments and Benthic-Pelagic exchange in the shallow Lake Müggelsee (Berlin, Germany). International Review of Hydrobiology, 83(1), 77–112.
Liu, G., Ye, C., He, J., Qian, Q., & Jiang, H. (2009). Lake sediment treatment with aluminum, iron, calcium and nitrate additives to reduce phosphorus release. Journal of Zhejiang University Science A, 10(9), 1367–1373.
Liu, C., Shen, Q., Zhou, Q., Fan, C., & Shao, S. (2015). Precontrol of algae-induced black blooms through sediment dredging at appropriate depth in a typical eutrophic shallow lake. Ecological Engineering, 77, 139–145.
Lürling, M., & van Oosterhout, F. (2013). Case study on the efficacy of a lanthanum-enriched clay (Phoslock®) in controlling eutrophication in Lake Het Groene Eiland (The Netherlands). Hydrobiologia, 710(1), 253–263.
McAuliffe, T. F., Lukatelich, R. J., McComb, A. J., & Qiu, S. (1998). Nitrate applications to control phosphorus release from sediments of a shallow eutrophic estuary: an experimental evaluation. Marine and Freshwater Research, 49(6), 463–473.
Meis, S., Spears, B. M., Maberly, S. C., O’Malley, M. B., & Perkins, R. G. (2012). Sediment amendment with Phoslock® in Clatto Reservoir (Dundee, UK): investigating changes in sediment elemental composition and phosphorus fractionation. Journal of Environmental Management, 93(1), 185–193.
Meis, S., Spears, B. M., Maberly, S. C., & Perkins, R. G. (2013). Assessing the mode of action of Phoslock® in the control of phosphorus release from the bed sediments in a shallow lake (Loch Flemington, UK). Water Research, 47(12), 4460–4473.
MEPC. (2002). Standard methods for examination of water and wastewater (4th ed.). Beijing: Chinese Environmental Sciences Press.
Murphy, T., Lawson, A., Kumagai, M., & Babin, J. (1999). Review of emerging issues in sediment treatment. Aquatic Ecosystem Health & Management, 2(4), 419–434.
Ottley, C. J., Davison, W., & Edmunds, W. M. (1997). Chemical catalysis of nitrate reduction by iron (II). Geochimica et Cosmochimca Acta, 61, 1819–1828.
Pessot, C. A., Atland, A., Liltved, H., Lobos, M. G., & Kristensen, T. (2014). Water treatment with crushed marble or sodium silicate mitigates combined copper and aluminium toxicity for the early life stages of Atlantic salmon (Salmo salar L.). Aquacultural Engineering, 60, 77–83.
Psenner, R., & Pucsko, R. (1988). Phosphorus fractionation: advantages and limits of the method for the study of sediment P origins and interactions. Archiv für Hydrobiologie–Beiheft Ergebnisse der Limnologie, 30, 43–59.
Psenner, R., Pucsko, R., & Sager, M. (1984). Die Fraktionierung organischer und anorganischer Phosphorverbindungen von Sedimenten–Versuch einer Definition ökologisch wichtiger Fraktionen. Archiv für Hydrobiologie, Supplement, 70, 111–155.
Ramírez, A., Pringle, C. M., & Molina, L. (2003). Effects of stream phosphorus levels on microbial respiration. Freshwater Biology, 48(1), 88–97.
Reitzel, K., Lotter, S., Dubke, M., Egemose, S., Jensen, H. S., & Andersen, F. Ø. (2013). Effects of Phoslock® treatment and chironomids on the exchange of nutrients between sediment and water. Hydrobiologia, 703(1), 189–202.
Ripl, W. (1976). Biochemical oxidation of polluted lake sediment with nitrate: a new lake restoration method. Ambio, 5(3), 132–135.
Robb, M., Greenop, B., Goss, Z., Douglas, G., & Adeney, J. (2003). Application of Phoslock™, an innovative phosphorus binding clay, to two Western Australian waterways: preliminary findings. The Interactions between Sediments and Water (pp. 237–243). Springer.
Ross, G., Haghseresht, F., & Cloete, T. E. (2008). The effect of pH and anoxia on the performance of Phoslock®, a phosphorus binding clay. Harmful Algae, 7(4), 545–550.
Ruban, V., López-Sánchez, J., Pardo, P., Rauret, G., Muntau, H., & Quevauviller, P. (1999). Selection and evaluation of sequential extraction procedures for the determination of phosphorus forms in lake sediment. Journal of Environmental Monitoring, 1(1), 51–56.
Schauser, I., Chorus, I., & Lewandowski, J. (2006). Effects of nitrate on phosphorus release: comparison of two Berlin lakes. Acta Hydrochimica et Hydrobiologica, 34(4), 325–332.
Shao, M., Zhang, T., & Fang, H. H. (2009). Autotrophic denitrification and its effect on metal speciation during marine sediment remediation. Water Research, 43, 2961–2968.
Smith, V. H. (2003). Eutrophication of freshwater and coastal marine ecosystems—a global problem. Environmental Science and Pollution Research, 10(2), 126–139.
Søndergaard, M., Jeppesen, E., Lauridsen, T. L., Skov, C., Van Nes, E. H., Roijackers, R., Lammens, E., & Portielje, R. (2007). Lake restoration: successes, failures and long‐term effects. Journal of Applied Ecology, 44, 1095–1105.
Wang, H., Wang, C., Wu, W., & Wang, Z. (2002). Persistent organic pollutants (POPs) in surface sediments of Donghu Lake, Wuhan, Hubei, China. Journal of Environmental Science and Health, Part A, 37(4), 499–507.
Xie, L., & Xie, P. (2002). Long-term (1956–1999) dynamics of phosphorus in a shallow, subtropical Chinese lake with the possible effects of cyanobacterial blooms. Water Research, 36, 343–349.
Xu, M. Y., Zhang, Q., Xia, C. Y., Zhong, Y. M., Sun, G. P., Guo, J., Yuan, T., Zhou, J., & He, Z. (2014). Elevated nitrate enriches microbial functional genes for potential bioremediation of complexly contaminated sediments. ISME Journal, 8, 1932–1944.
Yamada, T., Sueitt, A., Beraldo, D., Botta, C., Fadini, P., Nascimento, M., Faria, B. M., & Mozeto, A. A. (2012). Calcium nitrate addition to control the internal load of phosphorus from sediments of a tropical eutrophic reservoir: microcosm experiments. Water Research, 46(19), 6463–6475.
Zhang, M., Zhang, T., Shao, M. F., & Fang, H. (2009). Autotrophic denitrification in nitrate-induced marine sediment remediation and Sulfurimonas denitrificans-like bacteria. Chemosphere, 76, 677–682.
Zhang, S. Y., Zhou, Q. H., Xu, D., Lin, J. D., Cheng, S. P., & Wu, Z. B. (2010). Effects of sediment dredging on water quality and zooplankton community structure in a shallow of eutrophic lake. Journal of Environmental Sciences-China, 22(2), 218–224.
Acknowledgments
The authors would like to thank the support from the National Major Science and Technology Projects for Pollution Control and Management (2012ZX07104-002-005, 2012ZX07101-007-002), the Major Scientific and Technological Innovation Projects of the Hangzhou City (20131813A04), and the Science and Technology Project of the Ministry of Housing and Urban–rural Development of China (2014-K7-014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, J., Qiu, P., Yan, X. et al. Effectiveness and Mode of Action of Calcium Nitrate and Phoslock® in Phosphorus Control in Contaminated Sediment, a Microcosm Study. Water Air Soil Pollut 226, 330 (2015). https://doi.org/10.1007/s11270-015-2590-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2590-4