Abstract
Organochlorine pesticides (OCPs) are ubiquitous contaminants with high bioaccumulation and persistence in the environment; they can have adverse effects in humans and animals. This study examined residual concentrations in water, sediments, and fishes as well as the association between the health risks of OCPs and fish consumption in the Taiwanese population. Various water and sediment samples from Taiwanese aquaculture and fish samples from different sources were collected and analyzed through gas chromatography tandem mass spectrometry to determine the concentrations of 20 OCPs, namely, aldrin; cis-chlordane; trans-chlordane; dieldrin; endrin; alpha-endosulfan; beta-endosulfan; heptachlor; hexachlorobenzene; alpha-hexachlorocyclohexane; beta-hexachlorocyclohexane; lindane; mirex; pentachlorobenzene; o,p′-dichlorodiphenyltrichloroethane (DDT); p,p′-DDT; and DDT metabolites (o,p’-dichlorodiphenyldichloroethane [DDD]; p,p’-DDD; o,p’-dichlorodiphenyldichloroethylene [DDE]; and p,p’-DDE). None of the analyzed samples was positive for OCP contamination, suggesting no new input pollution from the land through washing into Taiwanese aquaculture environments. However, OCP residues were detected in fishes caught along the coast, namely, skipjack tuna and bigeye barracuda, and in imported fishes, such as codfish and salmon. DDT was the predominant pesticide. The contamination pattern of persistent organic pollutants was as follows: dieldrin > cis-chlordane > hexachlorobenzene, with average concentrations ranging from 0.09 to 2.74 ng/g. The risk was assessed in terms of the estimated daily intake (EDI) for potential adverse indices; the EDI of OCP residues was lower than 1% of the acceptable daily intake established by the Food and Agriculture Organization of the United Nations and World Health Organization. The assessed risk was negligible and considered to be at a safe level, suggesting no association between fish consumption and risks to human health in Taiwan. However, a continuous monitoring program for OCP residues in fishes is necessary to further assess the possible effects on human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persistent organochlorine pesticides (OCPs), such as aldrin, chlordane, dieldrin, endrin, and dichlorodiphenyltrichloroethane (DDT), are ubiquitous contaminants in the environment and toxic compounds of global concern. OCPs are typically persistent organic pollutants (POPs) and are characterized by high persistence, bioaccumulation through food webs, and long-range global transport (Chou et al., 2003). Because these compounds are lipophilic and hydrophobic in nature, they bioaccumulate in living and dead materials, specifically in marine organisms (Hellou et al. 1993). The application and production of these pesticides have been banned in most countries; however, some developing countries still use them, owing to their lower cost and ease of management during production. In Taiwan, OCPs were widely used from the 1950s to the 1980s, and since the 1970s, the Taiwanese government has banned the agricultural production and use of POPs—briefly, that of endrin in 1971, DDT in 1973, aldrin and dieldrin in 1975, and endosulfan in 2014. To date, all OCPs listed as POPs by the Persistent Organic Pollutants Review Committee under the Stockholm Convention are prohibited by agricultural and environmental sanitarians. However, various OCP residues have still been detected in sediments (Chang and Doong, 2006; Doong et al. 2008), water (Gbeddy et al., 2015; Ibigbami et al. 2015), aquatic species (Chou et al., 2003), and livestock and poultry products (Tsai, 2010) in Taiwan.
In recent decades, aquatic ecosystems have been widely contaminated by OCPs of agricultural and industrial origins (Ballesteros et al., 2014). Eventually, POPs may accumulate in the human body through the consumption of contaminated aquatic organisms, drinking water, and agricultural supplements. Moreover, marine animals, owing to their low capability to metabolize POPs, typically accumulate higher concentrations of POPs than do terrestrial animals (Martí et al., 2010). Notably, POPs can cause various adverse health effects in humans and animals, such as endocrine disruption, neurotoxic and carcinogenic effects, and reproductive and developmental disturbances (Martí et al., 2010; Zhang et al., 2014). Fish are a favorable indicator of toxic agents in aquatic environments because of their direct exposure to water pollutants through the gills and skin (Oka et al., 2009; Zhang et al., 2014). Therefore, analyses of pollutants accumulated in fish indicate not only OCPs in the environment but also those transferred via the trophic chain.
Taiwan’s geographical and archipelagic characteristics, particularly its subtropical location and temperate climate, render it ideal for culturing fish, shrimp, shellfish, and amphibians (Chang et al., 2016). The average annual revenue from aquaculture reached USD 11 billion between 2011 and 2015 (Chang, 2017). However, limited to land use, aquatic farms are close to agricultural areas and human residential sites. Several OCPs were formerly used as insecticides for controlling crop ectoparasites, introducing pesticides into the water and soil and consequently contaminating aquaculture products (Doong et al., 2008; Tsai, 2010). Such a breeding approach is susceptible to OCP residues, necessitating the monitoring of persistent agents for their prevention and treatment. Therefore, this study clarified the concentrations and accumulation levels of OCPs in fishes from different sources, including those cultured in inland Taiwan, caught in the offshore zone, and imported from other countries. OCPs in Taiwanese aquaculture environments were determined through water and sediment sample collection and analyses. Moreover, we estimated the effects of exposure to these OCPs on the health of the Taiwanese population by determining the estimated daily intake (EDI) of these contaminants through seafood consumption.
Materials and methods
Samples
Fish samples were collected from Taiwanese aquafarms, fishing ports (catch from the offshore zone), or supermarkets (fishes imported from Norway, Canada, and Iceland) between January 2013 and December 2014. In total, 95 samples (31 from aquafarms, 32 from the offshore zone, and 32 imported) were collected (Supplementary Fig.1). The aquafarm samples comprised 12 tilapia, 10 milkfish, and 9 perch. These fishes are bred on a large scale in Taiwan (Fisheries Agency of Taiwan, 2016). The samples from fishing ports comprised one each of cobia, gray mullet, blue mackerel, and yellowfin tuna; two each of beltfish and yellow seabream; three each of bigeye barracuda, marlin, brindle grouper, and skipjack tuna; five dolphin fish; and seven white-tipped mackerel. These species are caught at a high frequency along the Taiwanese coast. The import samples comprised 16 codfish and 16 salmon; these fishes are consumed at high rates in Taiwan.
To more comprehensively understand the pollutant behavior in the aquatic system, contaminant levels in surface water as well as surface bottom sediments (0–5 cm) were analyzed. Thirty samples of surface waters (n = 15) and surface bottom sediments (n = 15) were collected from the same Taiwanese aquafarms that served as the sources for the aforementioned 15 local species of fish. Furthermore, the water samples were collected in 1-L clean, amber glass bottles for physicochemical and contaminant analyses and were immediately transported to the laboratory (4 °C). The sediment samples were collected with a steel corer; these samples were air-dried at room temperature until they reached a constant weight. All fish and sediment samples were stored at − 20 °C in sealed glass containers until analyses were conducted.
Chemicals and reagents
A total of 20 OCP analytical standards, namely, aldrin (99.0%); cis-chlordane (99.7%); trans-chlordane (99.0%); heptachlor (99.5%); hexachlorobenzene (99.7%); alpha-hexachlorocyclohexane (99.5%); beta-hexachlorocyclohexane (98.5%); lindane (99.7%); mirex (99.0%); o,p’-DDD (99.5%); p,p’-DDD (99.0%); o,p’-DDE (97.5%); p,p’-DDE (98.5%); o,p’-DDT (99.5%);and p,p’-DDT (98.5%), were purchased from Dr. Ehrenstorfer GmbH (Ausburg, Germany). Dieldrin (99.5%) and endrin (99.5%) were obtained from Chem Service Inc. (West Chester, PA, USA). Alpha-endosulfan (99.0%) and beta-endosulfan (99.0%) were obtained from RdH Laborchemikalien GmbH (Seelze, Germany). Pentachlorobenzene (99.9%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). We determined the concentrations of ΣDDTs, which had been detected as DDT and DDT’s metabolites.
Chromatography-grade acetonitrile (ACN), acetone, dichloromethane, petroleum ether, and n-hexane were purchased from Merck (Darmstadt, Germany). Reagent-grade anhydrous sodium sulfate and sodium chloride (NaCl) were purchased from Sigma-Aldrich. Florisil solid-phase extraction (SPE) cartridges were obtained from JT Baker (Phillipsburg, NJ, USA). Quick easy cheap effective rugged safe (QuEChERS) extraction salt packet (Agilent SampliQ QuEChERS EN extraction kit, p/n 5982-5650; mixture containing 4 g of anhydrous magnesium sulfate, 1 g of NaCl, 1 g of sodium citrate, and 0.5 g of citric acid disodium salt) and 15-ml QuEChERS cleanup tubes (Agilent SampliQ QuEChERS EN fatty dispersive-SPE kit, p/n 5982-5156) were purchased from Agilent Technologies (Wilmington, DE, USA).
Instruments and analysis of GC/MS-MS
To analyze the herbicides, gas chromatography tandem mass spectrometry (GC/MS-MS) was performed on a GC system (Bruker 450-GC, Bruker Instruments, Sunnyvale, CA, USA) and a mass spectrometer (320-MS, Bruker Instruments) coupled with an Agilent JW Scientific VF-5MS capillary column (0.25 mm × 30 cm × 0.25 μm, Agilent Technologies, Santa Clara, CA, USA). The GC/MS-MS analyses were performed in positive and negative electron-impact ionization (EI) interface modes. Helium was used as the carrier gas in the Bruker gas chromatograph mass spectrometer at a constant flow rate of 1 mL/min. The injector and detector temperatures were set to 280 and 250 °C, respectively. The oven temperature was set at 90 °C, initially maintained isothermal for 2 min, subsequently increased to 300 °C at the rate of 7 °C/min, and finally maintained constant at 300 °C for 4 min. The transfer line and source temperature were set at 280 and 250 °C, respectively. The injection volume in the splitless mode was 1.0 μL. In the collision chamber (second quadrupole), these ions were collision-activated with argon at 1.5 mTorr.
Preparation of standard solutions
Stock solutions of individual pesticide standards were prepared by accurately weighing 100 mg of each analyte and dissolving them in 100 mL of acetone, ACN, or MeOH, depending on the solubility of analytes, in volumetric flasks. Working standard mixtures were prepared by combining each type of stock solution and diluting it to 1 mg/L. All solutions were stored at − 20 °C and brought to room temperature before each use. Using these working standard solutions, a series of calibration standards were prepared by serial dilution within the range of 0.5–500 ng/mL.
Extraction procedure and analysis
For detecting OCP residues in water, each sample was obtained and cleaned according to a modified version of the method reported by Li et al. (2003). Briefly, 50 mL of water sample was added into a flask containing 50 mL of acetone, 12.5 mL of 10% NaCl, and 37.5 mL of petroleum ether, before extraction with 50 mL of dichloromethane. Both organic layers were combined and dehydrated with 20 g of anhydrous sodium sulfate. The anhydrous organic phase was evaporated to dryness, and the residue was dissolved with 2 mL of n-hexane. Furthermore, 1 mL of sample solution was cleaned using a Florisil SPE cartridge. After elution with 15 mL n-hexane/dichloromethane (1:5, v/v), the collection was purged with N2 to dryness, and the residue was redissolved with 1.0 mL n-hexane for OCP analysis.
OCPs from the sediment samples were extracted in accordance with a modified version of the protocol recommended by the Taiwan Environmental Protection Administration (EPA) (2016a). Briefly, 20 g of soil sample was mixed with 20 g of anhydrous sodium sulfate. The samples were then Soxhlet-extracted with 120 mL of acetone/dichloromethane (1:1, v/v) in a 180 °C water bath for 3 h for extraction. The extracts were collected and filtered through 0.2-μm polyvinylidene fluoride filter (Whatman, Maidstone, UK) and then evaporated to dryness. Finally, the resultant dry extract was reconstituted in 1 mL of acetone and n-hexane and introduced into an autosampler vial for GC/MS-MS analysis.
OCP residues in fishes were analyzed according to the QuEChERS extraction procedure developed by the European Committee for Standardization (Shen et al., 2013). Each sample (10 g) was weighed into a 50-mL centrifuge tube, and 10 mL of ACN was added to it. The mixture was vigorously vortexed for 1 min, and QuEChERS extraction salt was added. Furthermore, the mixture was vigorously vortexed for 1 min and centrifuged at 3000×g for 5 min. After vigorous vortexing for 1 min, approximately 6 mL of the crude ACN extract was removed and transferred into QuEChERS cleanup tubes. The ACN layer was vigorously vortexed for 2 min and centrifuged for 5 min at 3000×g. Thereafter, 1 mL of the extracted solution was near completely dried in a nitrogen evaporator at 40 °C. Finally, the residue was redissolved with 1 mL of n-hexane/acetone (1:1, v/v), filtered through a 0.2-μm filter membrane, and introduced into an autosampler vial for GC/MS-MS analysis.
Quality assurance and validation
The proposed method was validated through estimation of recoveries, repeatability, linearity range, and limits of quantification (LOQs) (Shen et al., 2013). To determine the recoveries and repeatability, blank samples were spiked in triplicate with a standard mixture of analytes at two concentrations (lower and higher, 5 and 50 ng/g, respectively) for OCP analysis. These samples were extracted and treated according to a previously described protocol (Li et al., 2003; Shen et al., 2013; Environmental Protection Administration, Taiwan 2016b). The recoveries and repeatability (expressed as the percentage of relative standard deviation) of OCPs ranged from 70 to 107% (repeatability range 1–20%) in water samples, 67–112% (repeatability range 1–18%) in sediment samples, and 70–119% (repeatability range 2–15%) in fish samples (Supplementary Table 1). Linearity was evaluated through matrix-matched calibration by using blank sample extracts and by adding the corresponding amount of the working solution with the target compounds at a concentration of 2–500 ng/mL. The high linearity and reproducibility of calibration curves revealed that our analytical matrix-matched calibration achieved good correlation coefficients (R-squares) of > 0.990. The LOQs were determined to be the analyte concentrations that yielded peak signals of three and ten times the background noise from the chromatogram. The LOQs of dieldrin and endrin were 0.2 ng/mL in water samples and 4 ng/g in fish samples. Compared with these chemicals, other OCPs were confirmed by lower LOQs (0.1 ng/mL in water samples and 2 ng/g in fish samples). Moreover, the LOQ of the sediment samples in all OCP analytes was 0.3 ng/g (Supplementary Table 1); those samples with lower concentrations than these LOQs were considered undetectable. These LOQs of water and sediment samples for analyzing the residues of OCPs met the official certification requirement of the Taiwan EPA that the LOQs of environmental matrixes be below 5 ng/g for OCP residual analyses (Environmental Protection Administration, Taiwan 2016b).
Estimated daily intake
To assess human exposure to pesticide residues in fishes, the EDI was estimated from the residual concentrations of the analyzed OCPs and was compared with the acceptable daily intake (ADIs) values established by the Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO). The EDI was calculated using the following equation: EDI (ng/kg/day) = (daily fish consumption [g/day]) × (mean OCP concentration [ng/g])/(human body weight [kg]) (Takazawa et al., 2008). The daily fish consumption values for Taiwanese men and women (91.5 and 68.9 g, respectively) were obtained from the National Nutrition and Health Survey conducted by the Ministry of Health and Welfare (Wu et al., 1999). We used 60 kg as the mean Taiwanese body weight (Wu et al., 1999) and calculated the EDI based on the mean concentrations of OCP residues. The maximum EDI was derived from the maximum residues.
Results
OCP residues in water and sediment samples from aquafarms
OCP concentrations in the water samples did not show any peak signals on the chromatogram. However, in a sediment sample from a tilapia culture, the concentration of p,p′-DDD was 0.96 ng/g, which was lower than the LOQ. Thus, no considerable levels of OCP compounds were obtained in the present study.
OCP residues in various fish samples
Table 1 presents the violation rates and detection compounds of OCP residues in different fish sample sources. The samples from aquafarms did not contain OCPs. However, the rate of positive banned OCPs in the samples from the offshore zone was 6.25% (violation in 2 of 32 samples). One sample each of bigeye barracuda and skipjack tuna was positive for DDTs, including p,p′-DDD; p,p′-DDE; and p,p′-DDT. Moreover, violations were detected in 3 (2 codfish and one salmon) of the 32 imported fish samples, at a rate of 9.38%. One codfish was multiresidual for cis-chlordane, DDTs (p,p′-DDD; p,p′-DDE; and p,p′-DDT), and hexachlorobenzene. Another codfish sample was multiresidual for cis-chlordane and hexachlorobenzene. Conversely, a salmon sample contained p,p′-DDD and p,p′-DDE. Overall, 5.26% of all fish samples contained detectable residues of OCPs, and the violation rate was lower than 10% for all samples and different sampling sources.
Detection levels and rates of OCP residues in all samples
Among the 20 analyzed pesticides, cis-chlordane, DDTs, and hexachlorobenzene were detected in the fish samples (Table 2). The predominant residues were DDTs, (p,p′-DDD; p,p′-DDE; and p,p′-DDT). In all fish samples, the highest detection rate was of p,p′-DDD and p,p′-DDE (4.21%), followed by detectable residues of p,p′-DDT (3.16%). The levels of p,p′-DDD; p,p′-DDE; and p,p′-DDT were 2.51–34.04, 5.07–51.76, and 7.26–78.41 ng/g, respectively. Moreover, the average concentrations (derived from all samples, including detected and undetected samples) of p,p′-DDD; p,p′-DDE; and p,p′-DDT were 0.59, 1.10, and 1.06 ng/g, respectively. Cis-chlordane, dieldrin, and hexachlorobenzene, followed by DDTs, were the minor detectable residues, with a detection rate of 2.11%. The present results revealed that the rates of cis-chlordane, dieldrin, and hexachlorobenzene residues were 4.76–5.75, 4.61–6.82, and 2.03–7.04 ng/g, respectively. Moreover, the average concentrations of cis-chlordane, dieldrin, and hexachlorobenzene were approximately 0.11, 0.12, and 0.09 ng/g, respectively. Altogether, the maximum detection levels ofΣDDTs were as high as 164.22 ng/g, and the maximum average concentration was 2.74 ng/g at a higher detection rate (4.21%).
EDIs of OCPs in fish samples on Taiwanese adults
The EDIs extrapolated from the average levels of OCP residues obtained for chlordane, dieldrin, DDTs, and hexachlorobenzene were 0.17, 0.18, 4.18, and 0.15 ng/kg body weight/day, respectively, in Taiwanese men and 0.13, 0.14, 3.15, and 0.12 ng/kg body weight/day, respectively, in Taiwanese women (Table 3). As shown in Table 3, these EDI values were much lower than the ADIs of chlordane, dieldrin, and DDTs recommended by the FAO/WHO joint meeting on pesticide residue (Yohannes et al. 2014). Contrastingly, the ADI of hexachlorobenzene was withdrawn in 1978 by the FAO/WHO. Therefore, the EDI of hexachlorobenzene in our study was lower than the ADI of this compound suggested by the FAO/WHO in 1975 (Stuetz et al., 2001)]. For Taiwanese men and women, the EDI values in the percentage of ADI for OCP residues were lower by 0.2%. Altogether, fish consumption resulted in the highest risk of exposure to dieldrin, with ADIs of 0.18 and 0.14% for male and female adults, respectively.
Discussion
In the present study, 20 banned OCPs, namely, aldrin; alpha-endosulfan; beta-endosulfan; alpha-hexachlorocyclohexane; beta-hexachlorocyclohexane; cis-chlordane; trans-chlordane; o,p′-DDD; p,p′-DDD; o,p′-DDE; p,p′-DDE; o,p′-DDT; p,p′-DDT; dieldrin; endrin; heptachlor; hexachlorobenzene; lindane; mirex; and pentachlorobenzene, were analyzed in 95 fish samples collected from different sources in Taiwan. Moreover, to confirm their presence in the environmental matrixes, water and sediment samples from the aquaculture areas in Taiwan were analyzed. The Taiwan Food and Drug Administration (TFDA) does not specify recommended maximum residue limits (MRLs) for OCPs in fishes. Therefore, the present study detected residues of these banned compounds in fishes, which was sufficient to confirm the presence of the substances.
OCPs may be washed in runoff from the land into water sources and be retained in these environmental matrixes because of their high affinity for soil (Fu et al., 2003). In aquaculture areas, OCPs may be carried from marine sediments by rainfall, causing significant water pollution in tropical and subtropical countries (Abdullah, 1995; Chau, 2006). The relevance of the sediment phase in the fate and accumulation of contaminants is established in environmental monitoring. Here, our analyses of surface sediments in the 30 samples showed no significant residual concentrations. Few studies have analyzed OCP residues in agricultural ecosystems, particularly aquafarm soils in Asian countries. Studies have reported the concentrations of various POPs in marine sediments in China (Fu et al., 2003; Chau, 2006; Zhang et al., 2014; Cui et al., 2015), India (Sarkar et al., 2008), Vietnam (Minh et al., 2007), Malaysia (Ibrahim, 2007), Indonesia and Thailand (Todd et al., 2010), and Japan (Kim et al., 2007). The POPs were determined to be chlordane, dieldrin, endrin, hexachlorocyclohexane, hexachlorobenzene, heptachlor, lindane, and DDTs (DDT and its metabolites, DDD and DDE). In addition, Taiwan’s river sediment concentrations displayed a rapid decrease from 1973 to 1976, but although there were variations among rivers, the sediment concentrations of aldrin, dieldrin, DDT, DDE, hexachlorocyclohexane, and lindane were the predominant pesticides (at part-per-billion levels) in 2000 (Wang and Liu, 2000; Doong et al. 2008). Notably, p,p′-DDD was detected in one sediment sample in this study, although its residual level was lower than the LOQ of DDD. Therefore, p,p′-DDD in the sediment of Taiwan’s aquatic environment may have been associated with DDT contamination during the early 1970s because of its past use or residues deposited by water through runoff and erosion.
In general, the levels of OCP residues in water are lower than those in sediments, as observed in the present results, because of water drift or surface runoff; this affects the abundance and diversity of distribution and causes complex effects in ecosystems (Barni et al., 2016). Studies have reported OCP residues in Taiwan’s rivers from two surveys conducted during 1973–1976 (Ku, 1979) and 1998–1999 (Wang and Liu 2000). These studies reported that the rivers were affected by dieldrin, DDTs, endrin, lindane, and heptachlor, but the OCP level in water was relatively low (< 0.02 ng/g). The detection rate (8.33%) of a survey conducted in 1998–1999 was lower than that of one conducted in 1973–1976 (> 25.0%). Although organochlorine insecticide mean concentrations in river water were detected from 1973 to 1976, the residual levels have rarely been detected and have declined since the 1990s (Wang and Liu, 2000; Doong et al. 2008; Tsai 2010). This finding is in concordance with those of other studies reporting aldrin, dieldrin, DDTs, endrin, heptachlor, and chlordane as the predominant residues (Zhang et al., 2003; Zhang et al., 2014; Gbeddy et al., 2015; Ibigbami et al. 2015; Barni et al., 2016). However, no studies have directly investigated OCP contamination in water from Taiwanese aquafarms. Therefore, the results of these surveys, in addition to the present findings, revealed that OCP residues in water bodies decreased from the 1970s to 2010s. However, the present study indicated no significant OCP contamination in the aquafarm waterbodies and showed that the banned OCPs may be present in trace levels and below the LOQs of the analyzed method. Thus, future studies must enhance the LOQ in water matrixes. Furthermore, the ecological risk of OCPs in the Taiwanese aquatic environment is negligible.
A previous study in Taiwan indicated that OCP residues in cultured fishes and OCP have a distribution pattern of DDTs > hexachlorocyclohexane > dieldrin in the 1970s (Jeng and Sun, 1974) (Supplementary Table 2). Another study conducted between 2001and 2003 had a 22.95% detection rate for DDTs in marine products from fish farms (Sun et al., 2006). The order of DDTs was as follows: p,p′-DDD > p,p′-DDE > p,p′-DDT, suggesting the DDT metabolites, including p,p′-DDD and p,p′-DDE, were the early major residues of OCPs in cultured fishes in Taiwan. Similarly, DDT metabolites have been found to be abundant in cultured fishes in China (Kong et al., 2005) and Brazil (Botaro et al., 2011). The frequency of DDT might be expected to be higher in areas where this insecticide has been widely used; this compound was largely withdrawn after the 1970s but is still used for controlling disease vectors (Roberts et al. 1997; Takazawa et al., 2008). However, the present result did not detect DDT contamination in the fish samples from Taiwanese aquaculture farms. Altogether, the sediment, water, and farm fish samples reported inadequate OCPs and no bioaccumulation of these compounds in the 2010s. Thus, Taiwan’s contemporary aquaculture environment has been suggested to have relatively fewer levels of OCP residues compared with those in the 1970s and early twentieth century. The risk of high levels of OCPs in various aquatic organisms, such as fishes, in a large area of Taiwanese aquacultural farms was confirmed to be low.
All 64 locally caught and imported samples, analyzed through GC/MS-MS, were positive for cis-chlordane, DDTs, dieldrin, and hexachlorobenzene. The trend of the detected OCPs was as follows: DDTs > dieldrin > cis-chlordane > hexachlorobenzene. These compounds are banned for use in agriculture, environment, public health, and food-producing animals in Taiwan. DDT and its metabolites were the most abundant pesticides in fish muscle, particularly in the offshore catch. Taiwan’s inshore aquaculture farms were considered to be composed of the surrounding waters of China’s eastern coast and the path of the Kuroshio Current along the west coast of Taiwan (Yang. 1975). Because of its availability and low cost, some aquaculture farmers continue to illegally use DDT in China; as a result, DDT contamination has been reported in the major estuaries of China’s rivers (Peng et al. 2005; Zhang et al., 2003; Chau 2006; Bao et al., 2012). The timing of DDT input (i.e., whether it was recent) can be determined by comparing its concentration and metabolites. The rate of DDD and DDE to total DDT can be used as an indicator of the long-term weathering (> 0.5) or recent introduction (< 0.5) of DDTs into the environment (Jeng and Sun, 1974). In the present study, the rate of (DDE + DDD)/ΣDDT was 1.54, suggesting that the detected DDTs had been in continuous use over the past decade for agricultural and public health programs (Muralidharan et al., 2009). The lack of fresh input might be because of the DDT ban in many developed countries. Simultaneously, the present study estimated the residues of two DDT isomers as o,p′-DDT and p,p′-DDT but only detected p,p′-DDT. The predominance of p,p′-DDT over o,p′-DDT suggested the nonapplication of dicofol in the catchment areas, because the rate of o,p′-DDT/p,p′-DDT can be also used to distinguish DDT pollution caused by technical DDT from that caused by dicofol (Yohannes et al., 2013).
OCP contamination because of cis-chlordane, dieldrin, DDTs, and hexachlorobenzene was observed in two codfish and one salmon. Many studies have demonstrated heavy OCP contamination in codfish from the Baltic Sea (Falandysz, 1986), northern Atlantic sea (Angerhöfer et al., 1999), and Adriatic Sea (Storelli et al., 2004) as well as in salmon from northern Japan (Oka et al., 2009), Norway, Maine, Canada, Norwegian, Alaska, and the Aleutian Islands (Shaw et al., 2006). In those studies, DDT and its metabolites chlordane, hexachlorocyclohexane, and hexachlorobenzene were the most abundant pesticides, with total DDTs (> 40 ng/g) accounting for the predominant pesticides in cultured salmon from Norwegian samples associated with contaminated feed. Moreover, chlordane, dieldrin, and hexachlorobenzene were commonly used as insecticides in the past and are still widely distributed in the environment (Takazawa et al., 2008; El-Kady et al., 2017). Emission sources may be assumed to be present in most developed countries. Although the detailed sources and sizes of import fish samples were unobtainable, the higher concentrations and detection rate of OCPs in codfish and salmon likely indicate the long-range atmospheric biotransport and bioaccumulation of these compounds and their ubiquity in marine food chains (Iwata et al., 1994; Shaw et al., 2006). Therefore, it was possible to determine the biomagnification of persistent OCPs through the benthic and pelagic food webs from small aquatic prey species to older and larger individuals at the top of the webs (Braune et al., 2014).
Of the analytes investigated in the present study, OCPs were detected and were multiresidual in 5.26% of the 95 samples. Few studies have directly analyzed the contamination of sanitary OCPs in marine products in the 2010s. However, the present findings differed from those of the surveys conducted by Ling in 2000 (Ling, 2004) and the TFDA in 2002 (Chou et al., 2003). Compared with these reports, the violation rates for OCP residues were 46.67% in 30 samples in 2000 (Ling, 2004) and 6.78% in 59 samples in 2002 (Chou et al., 2003). These positive results were observed after the samples were screened for aldrin, DDTs, and endosulfan. The differences can be partly explained by sample size. Moreover, these detection rates of OCPs were lower than those for fish species sampled from Asian countries, namely, Thailand (Kumblad et al., 2001), Hong Kong (Cheung et al., 2007), India (Muralidharan et al., 2009), China (Zhou et al., 2012), and Indonesia (Shoiful et al., 2013). In the present study, OCP contamination was mainly observed in the locally caught and imported fish samples, not in marine culture products. Therefore, these categories of OCP contaminants in congener profiles indicate the same contamination sources, even with varying metabolisms among species.
Some parameter guidelines facilitate the assessment of risk in organisms such as humans for conditions such as ADI, as well as excess cancer risk (ECR), provisional tolerable weekly intake, and target hazard quotients (THQ) (Gu et al., 2015; Gu et al., 2017; Ke et al., 2017). One is the ADI, formulated by the FAO/WHO. However, the ADI does not consider differences in eating habits and consumption rates (Yang et al. 2006; Li et al., 2008). THQ is defined as an assessment of the health risk of noncarcinogenic harmful effects (Gu et al., 2017). The other is the risk, which considers the effects of chronic and carcinogenic factors. This new, more accurate estimate of chronic dietary intake is called the EDI and has been proposed by the FAO/WHO Expert Committee on Food Additives (Vragović et al., 2011) and US EPA (Zhang et al. 2014). The present findings concluded that the daily exposure did not exceed the ADI. Because of the lower residual values of OCPs, the estimated EDI values of chlordane, dieldrin, DDTs, and hexachlorobenzene in the Taiwanese population were below the ADI. Furthermore, compared with the ADI in this study, the EDI values indicated no health risk associated with fish consumption. The assessed risk is negligible considering that the EDI is less than 1% of the ADI (Vragović et al., 2011). Thus, it can be concluded that the OCP levels in Taiwanese food products do not adversely affect human health.
In conclusion, the OCP composition profiles showed no contamination in the water and sediment samples from Taiwanese aquaculture environments. Moreover, no OCP residues were observed in the cultured tilapia, milkfish, and perch evaluated in this study. In fishes from the locally caught and imported samples, DDT and its metabolites were the most abundant pesticides, with DDE being the predominant metabolite. Levels of dieldrin, chlordane, and hexachlorobenzene, followed by DDTs, were remarkable for the other main detectable residues, suggesting contamination in these sample source areas. However, the OCP residues detected in this study did not present a risk to human health because the EDI was below the ADI established by the FAO/WHO.
References
Abdullah AR (1995) Environmental pollution in Malaysis: trends and prospects. Trends Anal Chem 14(5):191–198. https://doi.org/10.1016/0165-9936(95)91369-4
Angerhöfer D, Kimmel L, Koske G, Fingerling G, Burhenne J, Parlar H (1999) The role of biotic and abiotic degradation processes during the formation of typical toxaphene peak patterns in aquatic biota. Chemosphere 39(4):563–568. https://doi.org/10.1016/S0045-6535(99)00121-6
Ballesteros ML, Miglioranza KS, Gonzalez M, Fillmann G, Wunderlin DA, Bistoni MA (2014) Multimatrix measurement of persistent organic pollutants in Mar Chiquita, a continental saline shallow lake. Sci Total Environ 490:73–80. https://doi.org/10.1016/j.scitotenv.2014.04.114
Bao LJ, Maruya KA, Snyder SA, Zeng EY (2012) China’s water pollution by persistent organic pollutants. Environ Pollut 163:100–108. https://doi.org/10.1016/j.envpol.2011.12.022
Barni MF, Ondarza PM, Gonzalez M, Da Cuña R, Meijide F, Grosman F, Sanzano P, Lo Nostro FL, Miglioranza KS (2016) Persistent organic pollutants (POPs) in fish with different feeding habits inhabiting a shallow lake ecosystem. Sci Total Environ 550:900–909. https://doi.org/10.1016/j.scitotenv.2016.01.176
Botaro D, Torres JP, Malm O, Rebelo MF, Henkelmann B, Schramm KW (2011) Organochlorine pesticides residues in feed and muscle of farmed Nile tilapia from Brazilian fish farms. Food Chem Toxicol 49(9):2125–2130. https://doi.org/10.1016/j.fct.2011.05.027
Braune BM, Gaston AJ, Elliott KH, Provencher JF, Woo KJ, Chambellant M, Ferguson SH, Letcher RJ (2014) Organohalogen contaminants and total mercury in forage fish preyed upon by thick-billed murres in northern Hudson Bay. Mar Pollut Bull 78(1-2):258–266. https://doi.org/10.1016/j.marpolbul.2013.11.003
Chang SM, Doong RA (2006) Concentration and fate of persistent organochlorine pesticides in estuarine sediments using headspace solid-phase microextraction. Chemosphere 62(11):1869–1878. https://doi.org/10.1016/j.chemosphere.2005.07.023
Chang GR, Chen HS, Lin FY (2016) Analysis of banned veterinary drugs and herbicide residues in shellfish by liquid chromatography-tandem mass spectrometry (LC/MS/MS) and gas chromatography-tandem mass spectrometry (GC/MS/MS). Mar Pollut Bull 113(1-2):579–584. https://doi.org/10.1016/j.marpolbul.2016.08.080
Chau KW (2006) Persistent organic pollution characterization of sediments in Pearl River estuary. Chemosphere 64(9):1545–1549. https://doi.org/10.1016/j.chemosphere.2005.11.060
Cheung KC, Leung HM, Kong KY, Wong MH (2007) Residual levels of DDTs and PAHs in freshwater and marine fish from Hong Kong markets and their health risk assessment. Chemosphere 66(3):460–468. https://doi.org/10.1016/j.chemosphere.2006.06.008
Chou JP, Chen HC, Chen CS, Chang PC, Chou SS (2003) Survey on the organochlorine pesticide residues in fish and shellfish in Taiwan. Ann Rept BFDA Taiwan 21:266–291
Cui L, Ge J, Zhu Y, Yang Y, Wang J (2015) Concentrations, bioaccumulation, and human health risk assessment of organochlorine pesticides and heavy metals in edible fish from Wuhan, China. Environ Sci Pollut Res Int 22(20):15866–15879. https://doi.org/10.1007/s11356-015-4752-8
Doong RA, Lee SH, Lee CC, Sun YC, SC W (2008) Characterization and composition of heavy metals and persistent organic pollutants in water and estuarine sediments from Gao-ping River, Taiwan. Mar Pollut Bull 57(6-12):846–857. https://doi.org/10.1016/j.marpolbul.2007.12.015
El-Kady AA, Wade TL, Sweet ST, Sericano JL (2017) Distribution and residue profile of organochlorine pesticides and polychlorinated biphenyls in sediment and fish of Lake Manzala, Egypt. Environ Sci Pollut Res Int 24(11):10301–10312. https://doi.org/10.1007/s11356-017-8714-1
Environmental Protection Administration, Taiwan (2016a) Standard analysis method for evaluating organochlorine pesticides in soil, sediments and solid waste, Method NIEA M618.05C. Environmental Analysis Laboratory, EPA, Taiwan (2016). Available at http://gazette.nat.gov.tw/EG_FileManager/eguploadpub/eg022140/ch07/type3/gov60/num21/images/Eg01.pdf. Accessed 28 Dec 2017
Environmental Protection Administration, Taiwan (2016b) The national implementation plan of Taiwan under the Stockholm Convention on persistent organic pollutants. pp 38. Available at https://pops.epa.gov.tw/Ncesoft/105Nip/NIP(2016年修訂版-定稿).pdf. Accessed 28 Dec 2017
Falandysz J (1986) Organochlorine pesticides and polychlorinated biphenyls in cod from the southern Baltic, 1983. Z Lebensm Unters Forsch 182(2):136–139. https://doi.org/10.1007/BF01454247
Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SM, Ishizuka M (2014) Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a Rift Valley lake-Lake Ziway, Ethiopia. Ecotoxicol Environ Saf 106:95–101
Chang (2017) Surveys on banned veterinary drugs residues in marine bivalves and gastropods in Taiwan between 2010 and 2015: a mini review. J Aquat Pollut Toxicol 1:1–5
Fu J, Mai B, Sheng G, Zhang G, Wang X, Peng P, Xiao X, Ran R, Cheng F, Peng X, Wang Z, Tang UW (2003) Persistent organic pollutants in environment of the Pearl River Delta, China: an overview. Chemosphere 52(9):1411–1422. https://doi.org/10.1016/S0045-6535(03)00477-6
Gbeddy G, Glover E, Doyi I, Frimpong S, Doamekpor L (2015) Assessment of organochlorine pesticides in water, sediment, African cat fish and Nile tilapia, consumer exposure and human health implications, Volta Lake, Ghana. J Environ Anal Toxicol 5:1–8
Gu YG, Lin Q, Wang XH, FY D, ZL Y, Huang HH (2015) Heavy metal concentrations in wild fishes captured from the South China Sea and associated health risks. Mar Pollut Bull 96:508–512. https://doi.org/10.1016/j.marpolbul.2015.04.022
Gu YG, Lin Q, Huang HH, Wang LG, Ning JJ, FY D (2017) Heavy metals in fish tissues/stomach contents in four marine wild commercially valuable fish species from the western continental shelf of South China Sea. Mar Pollut Bull 114:1125–1129. https://doi.org/10.1016/j.marpolbul.2016.10.040
Hellou J, Warren WG, Payne JF (1993) Organochlorines including polychlorinated biphenyls in muscle, liver, and ovaries of cod, Gadus morhua. Arch Environ Contam Toxicol 25(4):497–505. https://doi.org/10.1007/BF00214339
Ibigbami OA, Aiyesanmi AF, Adeyeye EI, Adebayo AO (2015) Persistent organochlorine pesticide residues in water, sediments and fish samples from Ogbese River. Environ Nat Resource J 5:28–36
Ibrahim MS (2007) Persistent organic pollutants in malaysia. Devel. Environ Sci 7:629–655
Iwata H, Tanabe S, Ueda K, Tatsukawa R (1994) Geographical distribution of persistent organochlorines in air, water, sedi ments, and soil from Asia and Oceania, with their implications for global redistribution from lower latitudes. Environ Pollut 85(1):15–33. https://doi.org/10.1016/0269-7491(94)90234-8
Jeng SS, Sun LT (1974) Organochlorine pesticide residues in cultured fishes of Taiwan. Bull Inst Zoo Academia Sinica 13:37–45
Ke CL, YG G, Liu Q, Li LD, Huang HH, Cai N, Sun ZW (2017) Polycyclic aromatic hydrocarbons (PAHs) in wild marine organisms from South China Sea: occurrence, sources, and human health implications. Mar Pollut Bull 117(1-2):507–511. https://doi.org/10.1016/j.marpolbul.2017.02.018
Kim YS, Eun H, Katase T, Fujiwara H (2007) Vertical distributions of persistent organic pollutants (POPs) caused from organochlorine pesticides in a sediment core taken from Ariake bay, Japan. Chemosphere 67(3):456–463. https://doi.org/10.1016/j.chemosphere.2006.09.063
Kong KY, Cheung KC, Wong CKC, Wong MH (2005) The residual dynamic of polycyclic aromatic hydrocarbons and organochlorine pesticides in fishponds of the Pearl River delta, South China. Water Res 39(9):1831–1843. https://doi.org/10.1016/j.watres.2005.02.011
Ku TY (1979) Chlorinated hydrocarbon insecticide residues in the agricultural environment of Taiwan. Plant Protection Center, Taichung, Taiwan. Technical Report, pp 1-118. Plant Protection Center, Taichung
Kumblad L, Olsson A, Koutny V, Berg H (2001) Distribution of DDT residues in fish from the Songkhla Lake, Thailand. Environ Pollut 112(2):193–200. https://doi.org/10.1016/S0269-7491(00)00118-4
Li HP, Li GC, Jen JF (2003) Determination of organochlorine pesticides in water using microwave assisted headspace solid-phase microextraction and gas chromatography. J Chromatogr A 1012(2):129–137. https://doi.org/10.1016/S0021-9673(03)00916-6
Li XM, Gan YP, Yang XP, Zhou J, Dai JY, MQ X (2008) Human health risk of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in edible fish from Huairou Reservoir and Gaobeidian Lake in Beijing, China. Food Chem 109(2):348–354. https://doi.org/10.1016/j.foodchem.2007.12.047
Ling YC (2004) Persistent organic pollutants (POPs) in food in Taiwan. Taiwan Watch 6:8–12
Martí M, Ortiz X, Gasser M, Martí R, Montaña MJ, Díaz-Ferrero J (2010) Persistent organic pollutants (PCDD/Fs, dioxin-like PCBs, marker PCBs, and PBDEs) in health supplements on the Spanish market. Chemosphere 78(10):1256–1262. https://doi.org/10.1016/j.chemosphere.2009.12.038
Minh NH, Minh TB, Kajiwara N, Kunisue T, Iwata H, Viet PH, Cam TNP, Tuyen BC, Tanabe S (2007) Pollution sources and occurrences of selected persistent organic pollutants (POPs) in sediments of the Mekong River delta, South Vietnam. Chemosphere 67(9):1794–1801. https://doi.org/10.1016/j.chemosphere.2006.05.144
Muralidharan S, Dhananjayan V, Jayanthi P (2009) Organochlorine pesticides in commercial marine fishes of Coimbatore, India and their suitability for human consumption. Environ Res 109(1):15–21. https://doi.org/10.1016/j.envres.2008.08.006
Oka M, Arai T, Shibata Y, Miyazaki N (2009) Concentrations of persistent organic pollutants in masu salmon, Oncorhynchus masou. Bull Environ Contam Toxicol 83(3):393–397. https://doi.org/10.1007/s00128-009-9785-6
Peng X, Zhang G, Zheng L, Mai B, Zeng S (2005) The vertical variations of hydrocarbon pollutants and organochlorine pesticide residues in a sediment core in Lake Taihu, East China. Geochem Explor Environ Anal 5(1):99–104. https://doi.org/10.1144/1467-7873/03-038
Roberts DR, Laughlin LL, Hsheih P, Legters LJ (1997) DDT, global strategies, and a malaria control crisis in South America. Emerg Infect Dis 3(3):295–302. https://doi.org/10.3201/eid0303.970305
Sarkar SK, Bhattacharya BD, Bhattacharya A, Chatterjee M, Alam A, Satpathy KK, Jonathan MP (2008) Occurrence, distribution and possible sources of organochlorine pesticide residues in tropical coastal environment of India: an overview. Environ Int 34(7):1062–1071. https://doi.org/10.1016/j.envint.2008.02.010
Shaw SD, Brenner D, Berger ML, Carpenter DO, Hong CS, Kannan K (2006) PCBs, PCDD/Fs, and organochlorine pesticides in farmed Atlantic salmon from Maine, eastern Canada, and Norway, and wild salmon from Alaska. Environ Sci Technol 40(17):5347–5354. https://doi.org/10.1021/es061006c
Shen YR, Cheng MW, BS W, Yang KC, Chang YH, Tseng SH, Kao YM, Chiueh LC, Shih DYC (2013) Developement of the method for analysis of multiple pesticide residues in animal matrices by QuEChERS method. Taiwanese J Agric Chem Food Sci 51:148–160
Shoiful A, Fujita H, Watanabe I, Honda K (2013) Concentrations of organochlorine pesticides (OCPs) residues in foodstuffs collected from traditional markets in Indonesia. Chemosphere 90(5):1742–1750. https://doi.org/10.1016/j.chemosphere.2012.10.022
Storelli MM, D'Addabbo R, Marcotrigiano GO (2004) Temporal trend of persistent organic pollutants in codfish-liver from the Adriatic Sea, Mediterranean Sea, 1993–2003. Bull Environ Contam Toxicol 73(2):331–338. https://doi.org/10.1007/s00128-004-0432-y
Stuetz W, Prapamontol T, Erhardt JG, Classen HG (2001) Organochlorine pesticide residues in human milk of a Hmong hill tribe living in Northern Thailand. Sci Total Environ 273(1-3):53–60. https://doi.org/10.1016/S0048-9697(00)00842-1
Sun F, Wong SS, Li GC, Chen SN (2006) A preliminary assessment of consumer’s exposure to pesticide residues in fisheries products. Chemosphere 62(4):674–680. https://doi.org/10.1016/j.chemosphere.2005.04.112
Takazawa Y, Tanaka A, Shibata Y (2008) Organochlorine pesticides in muscle of rainbow trout from a remote Japanese lake and their potential risk on human health. Water Air Soil Pollut 187:31–40
Todd PA, Ong X, Chou LM (2010) Impacts of pollution on marine life in southeast asia. Biodivers Conserv 19(4):1063–1082. https://doi.org/10.1007/s10531-010-9778-0
Tsai WT (2010) Current status and regulatory aspects of pesticides considered to be persistent organic pollutants (POPs) in Taiwan. Int J Environ Res Public Health 7(10):3615–3627. https://doi.org/10.3390/ijerph7103615
Vragović N, Bazulić D, Njari B (2011) Risk assessment of streptomycin and tetracycline residues in meat and milk on Croatian market. Food Chem Toxicol 49(2):352–355. https://doi.org/10.1016/j.fct.2010.11.006
Wang CH, Liu C (2000) Dissipation of organochlorine insecticide residues in the environment of Taiwan, 1973-1999. J Food Drug Anal 8:149–158
Wu SJ, Chang YH, Fang CW, Pan WH (1999) Food sources of weight, calories, and three macro-nutrients-NAHSIT 1993-1996. Nutr Sci J 24:41–58
Yang RT (1975) Skipjack tuna fisheries in the waters around Taiwan. J Fisheries Soc Taiwan 4:93–110
Yang N, Matsuda M, Kawano M, Wakimoto T (2006) PCBs and organochlorine pesticides (OCPs) in edible fish and shellfish from China. Chemosphere 63(8):1342–1352. https://doi.org/10.1016/j.chemosphere.2005.09.029
Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SM, Ishizuka M (2013) Occurrence, distribution, and ecological risk assessment of DDTs and heavy metals in surface sediments from Lake Awassa–Ethiopian Rift Valley Lake. Environ Sci Pollut Res Int 20(12):8663–8671. https://doi.org/10.1007/s11356-013-1821-8
Zhang ZL, Hong HS, Zhou JL, Huang J, Yu G (2003) Fate and assessment of persistent organic pollutants in water and sediment from Minjiang River Estuary, Southeast China. Chemosphere 52(9):1423–1430. https://doi.org/10.1016/S0045-6535(03)00478-8
Zhang G, Pan Z, Bai A, Li J, Li X (2014) Distribution and bioaccumulation of organochlorine pesticides (OCPs) in food web of Nansi Lake, China. Environ Monit Assess 186(4):2039–2051. https://doi.org/10.1007/s10661-013-3516-5
Zhou P, Zhao Y, Li J, Wu G, Zhang L, Liu Q, Fan S, Yang X, Li X, Wu Y (2012) Dietary exposure to persistent organochlorine pesticides in 2007 Chinese total diet study. Environ Int 42:152–159. https://doi.org/10.1016/j.envint.2011.05.018
Acknowledgements
The author is grateful to Ms. Li-Ju Lee and Feng-Yi Lin for sample collection and extraction. This manuscript was edited by Wallace Academic Editing.
Funding
This work was supported by grants (103AS-11.3.2-PI-P1 and 103AS-11.3.2-PI-P1) from the Council of Agriculture, Executive Yuan, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
Supplementary Fig. 1
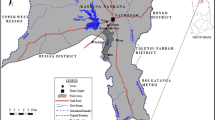
Location of sampling sites in Taiwan. The import samples were purchased from supermarkets in four areas (S1, S3, and S37–38). Three sampling sites at fishing ports were included for the caught-fish samples from the offshore zone along the Taiwanese coast (S2, S15, and S29). The aquafarm fish, surface water, and sediment samples were collected from the same Taiwanese aquafarms (S4, S7, S9, S11–13, S18–21, S25, S28, and S33–35). The other aquafarm fish samples were obtained from areas S5–6, S8, S10, S14, S16–17, S22–S24, S26–27, S30–32, and S36. (JPEG 1714 kb)
ESM 1
(DOCX 352 kb)
Rights and permissions
About this article
Cite this article
Chang, GR. Persistent organochlorine pesticides in aquatic environments and fishes in Taiwan and their risk assessment. Environ Sci Pollut Res 25, 7699–7708 (2018). https://doi.org/10.1007/s11356-017-1110-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-1110-z