Abstract
The levels, distribution patterns, ecological risk and human health risk of fourteen organochlorine pesticides in surface water, sediment, and fish (Tilapia zilli and Clarias gariepinus) collected from a massive rice field in Illushi, were monitored. Samples obtained were extracted and analyzed using gas chromatography equipped with electron capture detector while risk assessment was carried out using standard models (risk quotient and Chronic daily intake). Pesticide concentration in water samples ranged from ND to 1.65 µg/l, while the concentration of pesticide residues in sediment, C. gariepinus, and T. zilli ranged from ND to 8.45 µg/kg dw, 0.03 to 1.57 µg/kg/ww, and 0.03 to 0.85 µg/kg/ww, respectively. There was a clear dominance of the organochlorine; Hexachlorocyclohexane (α-HCH, γ-HCH, β-HCH) in all matrices. Risk quotient estimates for heptachlor, heptachlor epoxide, aldrin, dieldrin, DDT, endosulfan I, endosulfan II, and endosulfan aldehyde showed risk to aquatic organisms under extreme conditions. Health risk estimations showed that there is a potential risk to humans exposed to contaminated water, sediment, and fishes through ingestion, inhalation, and dermal routes of exposures. Cumulative hazard quotient estimated for pesticide mixtures in each matrix further confirmed that there was potential risk to human health especially children. Furthermore, the risk of non-carcinogenic effects followed the following sequence; biota > sediment > water. This study presents evidence of the multiple contamination of water, sediment, and biota from the Illushi River Basin which would possibly lead to non-cancer effects in humans especially children when exposed. Therefore, water bodies draining agricultural farmlands should be monitored regularly to prevent the contamination of the aquatic environment by toxic and banned pesticides, while efforts should be intensified to regulate the sales, application, and disposal of pesticide products in Nigeria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is concern that lowland and upland rice production systems are the major non-point sources of pesticide pollution of surface and groundwater, both of which are used for domestic purposes (Ecobichon 2001). Rice, as the main staple of the developing countries (including Nigeria), is the most important cereal crop in Nigeria and its consumption is increasing rapidly because of urbanization, relative ease of preparation, and convenience in storage (Ekeleme et al. 2008). Hence there are massive farms for the indigenous cultivation of rice. However, in recent times, there has been low yields in rice production due to the impact of adverse weather conditions (floods, drought, typhoons, etc.) and pest epidemics. Reports have shown that the yield potential of rice cultivated in West Africa including Nigeria is continually challenged by chronic pest infestation and outbreaks (Nwilene et al. 2011). In a bid to improve rice production by combating pest epidemics and outbreaks, rice farmers in Edo state and Nigeria at large resort to the use of various pesticides to protect their crops. By design, pesticides are toxic, bioaccumulative and due to their vertebrate and non-vertebrate toxicity can affect non target organism and human health (Ize-Iyamu et al. 2007; Lamers et al. 2011; Masia et al. 2013). These pesticides are used without proper training on application and warning of the harmful effect on the environment. More worrisome is the fact that reports have shown that many of the pesticides sold to rural farmers are banned and illegal stockpiles (Zhou et al. 2006; Nwilene et al. 2011; Masia et al. 2013). Pesticides applied during rice cultivation are transported in the environment through various pathways. However, water has been reported to be the primary pathway of pesticides residues into the environment (Schulz 2004). These residues of pesticides might ultimately pass unto humans through the routine consumption of drinking water, fish, contact with contaminated sediment, and inhalation of pesticide dust trapped in sediments (Zhou et al. 2006; Qu et al. 2014). In Nigeria, pesticide contamination levels in various environmental compartments (water, sediment and several aquatic species) has been reported (Ize-Iyamu et al. 2007; Ezemonye et al. 2008a, b, 2009; Okeniyia et al. 2009; Adeboyejo et al. 2011; Adeyemi et al. 2011; Upadhi and Wokoma 2012; Williams 2013). Unfortunately, these studies have concentrated on monitoring the levels of pesticide residues in these compartments without going a step further in determining the risk associated with the presence of these concentrations to both non-target organisms and humans. This has prompted recent studies by members of the pesticide research group at the laboratory for ecotoxicology and environmental forensics (LECTOX), University of Benin to include the use of risk assessment models/indices to project the potential effects of pesticide contamination on non-target organisms including humans (Ezemonye et al. 2015a, b; Ogbeide et al. 2015a, b; Tongo and Ezemonye 2015; Tongo et al. 2015). Estimation of potential risk of pesticide exposure to non-target organisms involves the use of the risk quotient (RQ) deterministic method. This is the ratio of the measured environmental concentration (exposure) and the toxicant reference value (Faggiano et al. 2010). For human health risk estimations, two exposure routes are considered: dietary and non-dietary exposures. Dietary exposures include: 1. the consumption of pesticide contaminated food stuffs here, health risk is predicted using the estimated daily intake (EDI) (Ezemonye et al. 2015a, b), and 2. The ingestion of contaminated water where health risks are estimated using estimated chronic daily intake (ECDI) (Papadakis et al. 2015). Non-dietary exposures include: 1. accidental ingestion of contaminated sediments, 2. inhalation of pesticide dust, and 3. dermal contact. For these exposure routes, health risk estimates are obtained using the chronic daily intake (CDI) (Huang et al. 2014). Non-Cancer effects that could arise from dietary and non-dietary exposures to pesticides can also be predicted by dividing EDI/CDI/ECDI with the corresponding reference dose (RfD) for each pesticide (Tongo and Ezemonye 2015) while cancer effects are predicted by multiplying EDI/CDI/ECDI with the cancer slope factor for each pesticide. To the best of authors’ knowledge, this is the first study to evaluate the concentration of pesticide residues in the Illushi River. Therefore, the objectives of this study were to monitor 14 organochlorine pesticides in water, sediment, and biota collected from the Illushi River Basin, project the ecological risk associated with the presence of these pesticides in the environment and finally estimate the human health risk that may arise through dietary intake (the consumption of contaminated fish species and water) and non-dietary intake (direct contact with contaminated sediments) of these pesticides.
Materials and Methods
Site Description and Sampling
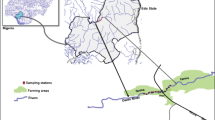
Edo State is typically an agrarian state, with various agro-ecological zones suitable for the production of several important food crops. The Illushi River is located in the Illushi River basin (N: 06o45′40″; E: 005o46′07.4″) (Fig. 1). The river basin is a flood plain, very close to the River Niger, a coastal region of Edo State, Nigeria. This terrain is known for large-scale rice farming. The river serves as a drainage for these massive rice farms located around it. Large amount of pesticides are used in preparing the land for farming and also used in routine maintenance of these rice farms. These farms are regularly inundated by overflows from the river leading to continuous contamination of the river. Samples used for analysis were collected for 18 months (January 2012 to June 2013). Water samples (n = 216) were collected from 0.3 m below the water surface with a pre-cleaned glass bottle using hydrobios sample based on the method described by Ezemonye et al. (2008a, b). Sediment samples (n = 216) were also collected using methods described by Ezemonye et al. (2008a, b). Samples were taken from the positions where an accumulation of fine-texture substrate took place. The upper 2 cm of bed sediment at each site was collected with a Teflon-coated spoon and wrapped in aluminum foil. Samples of C. gariepinus and T. zilli (n = 30) each were captured randomly using a fishing net, while 24 samples of C. gariepinus and T. zilli were bought from fishermen around each river (Ize-Iyamu et al. 2007). Overall, a total of 54 samples of C. gariepinus and T. zilli (each) were obtained for this study.
C. gariepinus and T. zilli were selected because they are major aquatic resources of the Illushi River basin and they are also important elements in the diet of the population residing in this region. These species of fish are the most widely consumed freshwater fishes in Edo State, Nigeria, based on its large size at maturity, affordability, nutritional benefits, and rapid growth (Akinwumi 2003; Olaifa and Ayodele 2004). Ethical clearance has been obtained from the University of Benin Ethics Committee on handling of the fish species.
Extraction and Analysis
Sediment samples were extracted and cleaned up according to the method described by Hladik and McWayne (2012). Edible portions of C. gariepinus and T. zilli samples were extracted and cleaned up according to methods described by Steinwandter (1992), while water samples were extracted and cleaned up based on methods described by Osibanjo and Adeyeye (1997) and USEPA (2007). The cleaned up extracts were analyzed for pesticides (α-HCH, γ-HCH, β-HCH, heptachlor, heptachlor epoxide, aldrin, dieldrin, endrin, DDT, endosulfan I, endosulfan II, endosulfan aldehyde, endosulfan sulfate). Results were obtained by Hewlett-Packard (hp) 5890 Series II gas chromatography (GC) equipped with 63 Ni electron capture detector (ECD) of activity 15 mCi with an auto sampler. The chromatographic separation was done using a VF-5 ms of 30 mm capillary column with 0.25 mm internal diameter and 0.25 µm film thicknesses and equipped with 1 m retention gap (0.53 mm, deactivated). The GC conditions were as follows: The oven temperature programme: Initial temperature was set at 60 °C for 2 min and ramped at 25 °C/min to 300 °C for 5 min and allowed to stay for 15 min giving a total run time of 58 min. The injector setting was a pulsed spit less mode with a temperature of 250 °C at a standard pressure. The injection volume was 1.5 µg/l. The detector temperature was 320 °C (held for 5 min). Helium was used as a carrier gas while Nitrogen gas (N2) was used as the makeup gas, maintained at a constant flow rate of 29 ml/min. The efficiency of the analytical method (the extraction and clean-up methods) was determined by recoveries of an internal standard. The recoveries of observed pesticides were greater than 85 %. Peak identifications were conducted by comparing the retention time of standards and those obtained from the extracts. Concentrations were calculated using a four-point calibration curve. Method detection limits (MDLs) ranged from 0.01 µg/g/dw for pesticides in biota. Method detection limits (MDLs) ranged from 0.005 µg/l for pesticides in water and 0.01 µg/g/dw for pesticides in sediment and biota.
Risk Assessment of Pesticide Residues
Ecological Risk Assessment
Risk quotient (RQ) method was used to determine risk of pesticide exposure to non-target aquatic organisms. RQ is ratio of the measured environmental concentration (MEC) to the predicted no effect concentration (PNEC). The predicted no effect concentration (PNEC) was obtained by multiplying the LC50 with an assessment factor (AF) of 100. The assessment factor takes into account the uncertainty in extrapolation from laboratory toxicity tests for a limited number of species to the real environment (Sangchan et al. 2012). The LC50 was obtained from Munn et al. (2006).
(Papadakis et al. 2015).
Health Risk Estimations
To assess the health risk associated with exposures to pesticide residues through consumption of fish, drinking contaminated water (dietary intake), and contact with contaminated sediment samples (non-dietary intake); the guidelines for potential risk assessment drawn up by the US EPA were used .
Human Health Risk Assessment of Pesticide Residues in Biota (Dietary)
To estimate the carcinogenic and non-carcinogenic risk of detected pesticides to humans, using two population groups (young children and adults) the estimated acceptable daily intake (EADI) was used. EADI was obtained by multiplying the residual pesticide concentration (μg/kg) in each fish species by the consumption rate in Nigeria (l/day or kg/day) and dividing the product by the body weight (kg) (WHO 1997; Fianko et al. 2011). The hazard quotient (HQ) was then obtained from the ratio of EADI and reference dose. The reference dose (RFD) of each pesticides is the exposure that is likely to be without an appreciable risk of deleterious effects and was provided by the USEPA (1996). The food and agricultural organization (FAO 2011) quotes the per capita consumption of fishes in Nigeria as 9 kg. The following formula was used to estimate the dietary intake.
(WHO 1997; Fianko et al. 2011).
EADI is the estimated average daily intake, C is the concentration of pesticide residues, CR represents consumption rate of each fish species while BW represents the body weight of age group. The food and agricultural organization (FAO 2011) quotes the per capita consumption of fishes in Nigeria as 9 kg. While body weight was set at 70 kg for adult population group.
Hazard quotient (HQ): Hazards quotients were obtained by dividing the EADI by their corresponding reference dose (RfD).
(WHO 1997; Fianko et al. 2011).
Hazard index (HI): using the hazard quotient derived from Eq. 3, the hazard index (HI) was obtained. Hazard index is used to assess the risk involved in exposure to mixtures of the detected pesticides belonging to the same chemical group (organochlorines).
(Tsakiris et al. 2011).
Human Health Risk Assessment of Pesticides in Water Samples
To estimate the risk of detected pesticides to humans (young children and adults) through drinking/ingestion of contaminated water, the hazard quotient (HQ) method was employed (Papadakis et al. 2015).
where ECDI is the estimated chronic daily intake. This was obtained by using the formula
where C w is the concentration of water samples, I R (water) is the ingestion rate of water, E F is the exposure frequency, E D is the exposure duration, B W is the body weight, and A T is the average life Span. The values for each of these parameter are provided in Table 1.
Human Health Risk Assessment of Pesticides in Sediment Samples (Non-Dietary)
This can be regarded as the human exposure to pesticide residues through other routes apart from diet. Non-dietary intake can be estimated using the chronic daily intake (CDI). Three routes of exposures were considered which include dermal contact, ingestion, and inhalation. Chronic daily intake is a model, designed by US Environmental Protection Agency (USEPA) (1992, 1997) to estimate the non-carcinogenic risks for adults and children from non-dietary exposure to contaminants. The CDI was estimated using the following formulae adapted from Huang et al. 2014 (Eqs. 7–9).
where C S is the concentration of sediment samples, I R (sediment) is the ingestion rate of sediment, C F is the carcinogenic slope factor, (E F) is the exposure frequency, E D is the exposure duration, B W is the body weight, F E is the dermal exposure ratio, A F is the dermal surface factor, ABS is the dermal absorption factor, A T is the average life span, S A is the surface area and PEF is the particle emission factor. The values for each of these parameter are provided in Table 1.
Results and Discussion
Spatial Distribution of Pesticides in Water
The concentration of pesticides in surface water from the Illushi River ranged from ND to 1.65 µg/l (Fig. 2). Fourteen (14) pesticides and their metabolic products were detected in this river (Table 2; Fig. 2) with α-HCH having the highest mean concentration (0.2772 µg/l) in water samples, while endrin had the lowest mean concentration (0.02 µg/l). Percentage distribution of pesticide residues in water samples shows that α-HCH, β-HCH, and γ-HCH were 11, 10, and 8 %, respectively, collectively making up 29 % of the total pesticide residues. Seasonal variations in pesticide distribution in water samples from the Illushi River showed that the highest concentrations of pesticides were measured during the peak of the rainy season where runoffs were highest. Runoffs have been reported to carry pesticides applied in farms into rivers (Ezemonye et al. 2008a; Papadakis et al. 2015). The presence of these pesticides residues in water samples from the Illushi River is indicative for the contamination of the river through various routes of entry from farms located close by. With the full stretch of the river surrounded by rice farms, the proliferation of pesticide residues in rivers has become a common feature due to an increase in the use of pesticides during agricultural activities. Several studies have reported the presence of pesticide residues in surface water close to agricultural areas, within and outside this region. Some of these studies include Doong et al. (2002), Konstantinou et al. (2006), Zhou et al. (2006), Ize-Iyamu et al. (2007), Ezemonye et al. (2008a), Begum et al. (2009), Okeniyia et al. (2009), Muhayimana and Shihua (2009), Phillips et al. (2010), Adeboyejo et al. (2011), Adeyemi et al. (2011), Hu et al. (2011), Falahudin and Munawir (2012), Fatemeh et al. (2012), Upadhi and Wokoma (2012), Darko and Babayo (2013), Okoya et al. (2013), Stamatis et al. (2013), Yang et al. (2005), and Williams (2013). Furthermore, pesticide residues observed in this study were found to be higher than maximum residual limits (MRL) of pesticides in fresh water bodies set by European Union (EU) (0.01 µg/l) and the National Environmental Standard and Regulation Enforcement Agency (NISERA) (0.1 µg/l).
Spatial Distribution of Pesticides in Sediment
Sediment contamination with pesticide residues has become a common feature in areas with intense agricultural activities. Pesticides used during agriculture activities find their way to river systems which as a drain for adjacent farms. Concentrations of pesticides in investigated sediment samples are shown in Table 2 and Fig. 3, and concentrations ranged from ND to 8.45 µg/kg/dw. All fourteen (14) pesticides were detected in sediment samples with β-HCH having the highest mean concentration (2.17 µg/kg/dw), while Endosulfan aldehyde had the lowest mean concentration (0.5 µg/kg/dw). As observed in water samples, HCH and its isomers had the highest percentage distribution (25 %). Furthermore, temporal distribution of pesticide residues showed seasonal variations. High concentrations were observed during the dry season, when there was a remarkable decrease in runoff allowing for sedimentation. Sediments have been reported to be the sink of aquatic ecosystems with large proportion of pesticide residues binding to suspended particles and settling at the bottom of the river, leading to a compromise in the sediment quality (Aktar et al. 2009; Hellar-Kihampa et al. 2013). When compared with water samples, pesticide concentration in sediments was highest, which could be attributed to the hydrophobic nature of each pesticide detected (Ezemonye et al. 2008a, 2009; Williams 2013). The distribution patterns of HCH in sediment samples showed that the unstable α-HCH had the highest concentration, reflecting the recent use of technical HCH within these areas. This is further confirmed by the ratio of α-HCH and γ-HCH. In the investigated sediment samples obtained from the Illushi River, α-HCH/γ-HCH ratio was below 3 which indicates a fresh use of technical HCH. It has been reported that a ratio less than 3 indicates that the source of HCH in the environment is fresh input of technical HCH (Chen et al. 2009). This trend also corroborates the findings of Olatunbosun et al. (2011), Doong et al. (2002), and Chen et al. (2009) who reported high concentrations of α-HCH compared with other isomers in sediment samples.
Spatial Distribution of Pesticide in Biota
All samples of C. gariepinus and T. zilli obtained from the Illushi River were observed to contain varying levels of pesticides residues (Table 2; Figs. 4, 5). The presence of pesticides in C. gariepinus and T. zilli could be attributed to the uptake of pesticide residues either through bioconcentration (from water through the gills or epithelial tissues) and through bioaccumulation (through water/food) leading to biomagnification (Murty 1986). In C. gariepinus, β-HCH had the highest concentration (1.57 µg/kg/ww), while DDT had the lowest (0.03 µg/kg/ww) concentration (Fig. 4). 45 % of pesticide residues in C. gariepinus were HCH and its isomers, with β-HCH having the highest percentage distribution of 19 %. On the other hand, distribution of pesticide residues in T. zilli showed the dominance of ∑HCH, with γ-HCH (0.85 µg/kg/ww) having the highest pesticide concentration (Fig. 5). Higher concentration of pesticide residues was observed C. gariepinus when compared with T. zilli. This difference could be attributed to the feeding mode, age, and mobility of the fish species (Mwevura et al. 2002). Also C. gariepinus habituates levels closest to the sediments where it gets most of its food; hence, there is the likelihood of exposure to pesticides bound in sediment particles (Biego et al. 2010). Kidwell et al. (1990) and Murano et al. (1997) equally add that pesticide accumulation in fish is influenced by their lipid content, implying that the high lipid content in C. gariepinus allows more pesticide residues to be trapped in their lipid stores compared to T. zilli.
Ecological Risk Assessment of Pesticide Residues
Risk assessment using the risk quotient deterministic method, where MEC (measured environmental concentration) was divided by the PNEC (probable no effect concentration), is presented in Table 3. The measured pesticide concentration comprised of the median and maximum detected water concentrations in other to reflect general and worst case scenario. It was observed that dieldrin, endosulfan I, endosulfan II, endosulfan aldehyde, heptachlor, heptachlor epoxide, aldrin, and DDT had risk quotient (RQ) values greater than 1. This is due to their high toxicity on fishes and aquatic invertebrates including algae. The implication of this is that concentrations of these pesticides in water from the Illushi River under medium and extreme conditions could pose high risk to non-target organisms in the river. It has been reported that when RQ ≥ 1, there is high risk, while 0.1 ≤ RQ ≤ 1 indicates medium risk, and 0.01 ≤ RQ ≤ 0.1 indicates low risk (Sanchez-Bayo et al. 2002; Fatemeh et al. 2012). Risk assessment in this study agrees with similar studies by Hela et al. (2005), Vryzas et al. (2009) and Fatemeh et al. (2012).
Human Health Risk Assessment of Pesticides in Water Samples
The hazard quotient (HQ) or risk of non-cancer effect estimated for organochlorines pesticides residues in water samples is presented in Table 4. It was observed that HQ estimated for pesticide exposure for adults were lower than 1, indicating no potential health risk/non-cancer effects while HQ estimated for pesticide exposure for children were lower than one apart from heptachlor epoxide which was 1.21, indicating potential risk. These results implies that there is a high potential for non-cancer effects through the ingestion of pesticide contaminated water from the Illushi River by children. It has been reported that when the HQ is greater than 1, then adverse health effects are possible (Jiang et al. 2005). Cumulative risk assessment (hazard index) was calculated using a simplified additive approach by summing the individual HQ posed by each OCP; however, the synergistic, antagonistic or other interaction effects of pesticide mixtures were not considered. For adults, hazard index (0.66) was less than 1 indicating that the river water is relatively safe for portable consumption by adults. However, hazard index (2.28) estimated for pesticide exposures in children was above the threshold of 1. This implies that the river water is not safe for portable consumption by children. Results indicate that children were at higher risk compared to adults as a result of exposure to the contaminated river water. There has been no report of non-carcinogenic risk assessment of pesticide residues in Nigerian waters subjected to constant pesticide contamination; however, results from this study corroborate studies by Hu et al. (2011) and Papadakis et al. (2015).
Human Health Risk Assessment of Pesticides in Sediment Samples
Three routes of exposures were considered to determine the human health risk of exposure to contaminated sediments. The routes include ingestion of contaminated sediment, direct contact with skin, and inhalation of dust from sediments. Estimates were obtained using formulas 7–9 while results are presented in Table 5. For non-dietary exposures, potential human health risk is projected by comparing CDI estimates with the respective reference dose for each OCP. According to the USEPA’s standards, when the estimated chronic daily intake (CDI) for a contaminant is more than the reference dose (RfD) of the contaminant via each exposure route, the contaminant level would exert an adverse human health effect (Huang et al. 2014). In this study, CDI estimates for all exposure routes were below the reference dose for each pesticides. This suggests that these contaminants under nondietary exposure to sediments are unlikely to pose any adverse health effects on individuals. Furthermore, CDI estimates were used to determine the hazard quotient (HQ) for each pesticide in other to project the risk of non-cancer effects. Results show all HQ calculated for each pesticide for all exposure routes where below 1 with exceptions to heptachlor epoxide (1.15) and Aldrin (1.48). Subsequently, cumulative risk assessment (hazard index) calculated for ingestion, dermal, and inhalation routes of exposures for adult were below the threshold (1). However, HI calculated for ingestion and dermal routes of exposure for children were above the threshold (1), implying that there is the high risk of non-cancer effects for children upon exposure to contaminated sediments. Model projections also showed that children were a higher risk population group, compared to adults while the risk of non-cancer effects is highest when the route of exposure is through the accidental ingestion of contaminated sediments.
Human Health Risk Assessment of Pesticide Residues in Biota
Clarias gariepinus and T. zilli are commercial aquatic products from the Illushi Region of Edo state (Ezemonye et al. 2015a, b). As shown in Tables 6 and 7, the estimated daily intake (EADI) and the hazard quotient (HQ) for each pesticide in each fish species were calculated using two population groups with varying body weights. Estimated average daily intake (EADI) for heptachlor epoxide, aldrin, dieldrin, and endrin in C. gariepinus were higher than their respective reference dose (RfD) for children and adults (Table 6). Consequently, hazard quotient (HQ) estimated for heptachlor epoxide (5.38), aldrin (9.11), dieldrin (2.93), and endrin (3.44) were above one (1) suggesting that there is the potential for non-cancer effects through the consumption of pesticide contaminated C. gariepinus. On the other hand, human health risk estimates for OCPs in T. zilli, presented in Table 7 showed that EADI for aldrin and heptachlor epoxide in T. zilli were higher than the acceptable daily intake for all weight groups suggesting that there is the potential for non-cancer effects through the consumption of pesticide contaminated T. zilli. This is further confirmed by hazard index (HI) calculations for C. gariepinus and T. zilli which showed that mixtures of OCPs were above one (1) for all the population groups implying that there could be risk (Tsakiris et al. 2011). Therefore, results from this study suggest a great potential for chronic toxicity through the consumption of pesticide contaminated C. gariepinus and T. zilli obtained from the Illushi. It was also observed that children were at greater risk of non-cancer effects when compared with the adult population group. This finding agrees with studies by Darko and Akoto (2008), Fianko et al. (2011), Andoh et al. (2013), and Sohair et al. (2013).
Conclusion
This study presents the first evidence of pesticide contamination of water, sediment, and fish species, arising from rice cultivation in the Illushi River Basin, Illushi town Edo State. Overall, sediment samples had the highest concentrations of pesticides residues, with the concentration of pesticide residues in the following order; Sediment > Clarias gariepinus > Tilapia zilli > water. Though the observed concentrations were minute, ecological risk assessment showed that there is a high potential of toxic effects to aquatic organisms upon exposure to organochlorine pesticides. Risk projections for humans from dietary and non-dietary intake also revealed that there is the potential for non-cancer effects. Projections showed that children were at higher health risk compared to adults. This calls for the need for the continuous monitoring of water bodies that drain agricultural fields because continuous exposure to pesticide contaminated resources obtained from the Illushi River (water, sediment, and fish) could affect the health of the population especially children. Thus, more efforts should be geared at reducing the indiscriminate and illegal use of pesticides (banned or approved). Orientation exercises for farmers to ensure proper application of pesticides, procurement of appropriate instrument of application, should be conducted regularly.
References
Adeboyejo OA, Clarke EO, Olarinmoye MO (2011) Organochlorine pesticides residues in water, sediments, fin and shell-fish samples from Lagos Lagoon complex, Nigeria. Researcher 3(3):38–45
Akinwumi FO (2003) Food and feeding habits of Tilapia zilli (Pisces: Cichlidae) in Ondo State University fish farm. In: 16th Annual Conference of the Fisheries Society of Nigeria (FISON), 4–9 November 2001, Maiduguri, pp 195–198
Adeyemi D, Anyakora C, Ukpo G, Adedayo A, Darko G (2011) Evaluation of the levels of organochlorine pesticide residues in water samples of Lagos Lagoon using solid phase extraction method. J Environ Chem Ecotoxicol 3:160–166
Aktar W, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2(1):1–12
Andoh H, Akoto O, Darko G (2013) Health risk assessment of pesticide residues in maize and cowpea from Ejura, Ghana. In: Paper presented at 5th international toxicology symposium in Africa. Jointly Hosted by College of Science, Kwame Nkrumah University of Science and Technology, Ghana and Hokkaido University, Japan sponsored by Japan Society for Promotion of Science 2013
Begum A, Harikrishna S, Khan I (2009) A Survey of persistent organochlorine pesticides residues in some streams of the Cauvery River, Karnataka, India. Int J Chem Technol Res 1(2):237–244
Biego GHM, Yao KD, Ezoua P, Kouadio LP (2010) Assessment of organochlorine pesticides residues in fish sold in Abidjan markets and fishing sites. Afr J Food Agric Nutr Dev 10:3
Chen W, Jing M, Bu J, Burnet JE, Qi S, Song Q, Ke Y, Miao J, Liu M, Yang C (2009) Organochlorine pesticides in the surface water and sediments from the Peacock River Drainage Basin in Xinjiang, China: a study of an arid zone in Central Asia. Environ Monit Assess 177:1–21
Darko G, Akoto O (2008) Dietary intake of organophosphorus pesticide residues through vegetables from Kumasi, Ghana. Food Chem Toxicol 46:3703–3706
Darko G, Babayo AU (2013) Studies of persistent organic and inorganic pollutants in water and fish samples around irrigation sites and its environs in north eastern part of Nigeria. In: Paper presented at 5th international toxicology symposium in Africa. Jointly hosted by College of Science, Kwame Nkrumah University of Science and Technology, Ghana and Hokkaido University, Japan sponsored by Japan Society for Promotion of Science 2013
Doong RA, Peng CK, Sun YC, Liao L (2002) Composition and distribution of organochlorine pesticide residues in surface sediments from the Wu-Shi River estuary, Taiwan. Mar Pollut Bull 45:246–253
Ecobichon DJ (2001) Pesticide use in developing countries. Toxicology 160:27–33
Ekeleme F, Kamara AY, Omoigui LO, Tegbaru A, Mshelia J, Onyibe JE (2008) Guide to rice production in Borno State, Nigeria. IITA, Ibadan
Ezemonye LIN, Ikpesu TO, Tongo I (2008a) Distribution of diazinon in water, sediment and fish from Warri River, Niger Delta, Nigeria. Jordan J Biol Sci 1(2):77–83
Ezemonye LIN, Ikpesu TO, Tongo I (2008b) Distribution of Lindane in Water, Sediment, and Fish from the Warri River of the Niger Delta, Nigeria. Arch Ind Hyg Toxicol 59:261–270
Ezemonye LIN, Ikpesu TO, Tongo I (2009) Distribution of Propoxur in water, sediment and fish from Warri River, Nigeria Delta, Nigeria. Turk J Biochem 34(3):121–127
Ezemonye L, Ozekeke O, Isioma T (2015a) Distribution and ecological risk assessment of pesticide residues in surface water, sediment and fish from Ogbesse River Edo State Nigeria. J Environ Chem Ecotoxicol 7(2):20–30
Ezemonye L, Ogbeide O, Tongo I, Enuneku A, Ogbomida E (2015b) Pesticide contaminants in Clarias gariepinus and Tilapia zilli from three rivers in Edo state, Nigeria; implications for human exposure. Int J Food Contamin 2:3
Faggiano L, de Zwart D, Garcia- Benthou E, Lek S, Gevrey M (2010) Patterning ecological risk of pesticide contamination at the river basin scale. Sci Total Environ 408(11):2319–2326. doi.10.1016/j.scitotenv.2010.02.002
Falahudin D, Munawir K (2012) Distribution and sources of persistent organochlorine pesticides in seawater and sediments in transitional season from Banten Bay. J Coast Dev 15(2):142–153
Fatemeh K, Moattar F, Farshchi P, Savari A, Parham H (2012) ERA: suitable method for estimation of ecological effects of pesticide contamination on aquatic species. J Persian Gulf 3(8):67–73
Fianko RJ, Augustine D, Samuel TL, Paul OY, Eric TG, Theodosia A, Augustine F (2011) Health risk associated with pesticide contamination of fish from the Densu River Basin in Ghana. J Environ Prot 2:115–123
Food Agriculture Organization (FAO) (2011) Fishery and aquaculture statistics. 2009. Statistics and Information Service of the Fisheries and Aquaculture Department/Service. 2009. FAO, Rome/Roma
Hela DG, Lambropoulou DA, Konstantinou IK, Albanis TA (2005) Environmental monitoring and ecological risk assessment for pesticide contamination and effects in Lake Pamvotis, north western Greece. Environ Toxicol Chem 24:1548–1556
Hellar-Kihampa H, Wael KD, Lugwisha E (2013) Spatial monitoring of organohalogen compounds in surface water and sediments of a rural–urban river basin in Tanzania. Sci Total Environ 447:186–197
Hladik ML, McWayne MM (2012). Methods of analysis—determination of pesticides in sediment using gas chromatography/mass spectrometry: U.S. Geological Survey Techniques and Methods 5–C3. Available via http://pubs.usgs.gov/tm/tm5c3
Hu WY, Huang B, Zhao YC, Sun WX, Gu ZQ (2011) Organochlorine pesticides in soils from a Typical Alluvial Plain of the Yangtze River Delta region, China. Bull Environ Contam Toxicol 87(5):561–566
Huang T, Guo Q, Tian H, Mao X, Ding Z, Zhang G, Li J, Ma J, Gao H (2014) Assessing spatial distribution, sources, and human health risk of organochlorine pesticide residues in the soils of arid and semiarid areas of northwest China. Environ Sci Pollut Res 21:6124–6135. doi:10.1007/s11356-014-2505-8
Ize-Iyamu OK, Asia IO, Egwakhide PA (2007) Concentrations of residues from organochlorine pesticide in water and fish from some rivers in Edo State Nigeria. Int J Phys Sci 2(9):237–241
Jiang QT, Lee TKM, Chen K, Wong HL, Zheng JS, Giesy JP, Lo KKW, Yamashita N, Lam PKS (2005) Human health risk assessment of organochlorines associated with fish consumption in a Coastal City in China. Environ Pollut 136:155–165
Kidwell JM, Phillips LJ, Birchard GF (1990) Comparative analysis of contaminants levels in bottom feeding and predatory fish using the national contaminants bio-monitoring program data. Bull Environ Contam Toxicol 55(6):919–923
Konstantinou IK, Hela DG, Albanis TA (2006) The status of pesticide pollution in surface waters (rivers and lakes) of Greece. Part I. Review on occurrence and levels. Environ Pollut 141(3):555–570
Lamers M, Anyusheva M, La N, Nguyen VV, Streck T (2011) Pesticide pollution in surface- and groundwater by paddy rice cultivation: a case study from Northern Vietnam. CLEAN Soil Air Water 39(4):356–361
Masia A, Ibanez M, Blasco C, Sancho JV, Pico Y, Hernandez F (2013) Combined use of liquid chromatography triple quadruple mass spectrometry and liquid chromatography quadruple time-of-flight mass spectrometry in systematic screening of pesticides and other contaminants in water samples. Anal Chim Acta 2013(761):117–127
Maurano F, Guida M, Melluso G, Sansone G (1997) Accumulation of pesticide residues in fishes and sediments in the River Sele (South Italy). J Prev Med Hyg 1997(38):3–4
Muhayimana AS, Shihua QI (2009) Occurrence, distribution and sources of organochlorine pesticides (OCPs) in Karst Cave. J Am Sci 5(1):35–43
Munn MD, Gilliom RJ, Moran PW, Nowell LH (2006) Pesticide toxicity index for freshwater aquatic organisms, 2nd Edition: U.S. Geological Survey Scientific Investigations Report 2006; 5148
Murty AS (1986) Toxicity of pesticides to fish, vol II. LRC Press, Boca Roton
Mwevura H, Othman CO, George LM (2002) Organochlorine pesticide residues in edible biota from the coastal area of Dar es Salaam City. Pesticide residues in Coastal Dar Western Indian Ocean. J Mar Sci 1(1):91–96
Nwilene FE, Togola A, Oyetunji OE, Onasanya A, Akinwale G, Ogah E, Abo E, Ukwungwu M, Youdeowei A, Woin N (2011) In: Stoytcheva M (ed) Is pesticide use sustainable in lowland rice intensification in West Africa? Pesticides in the modern world—risks and benefits, ISBN: 978-953-307-458
Ogbeide O, Tongo I, Ezemonye L (2015a). Human health risk of pesticide residues in sediments through non-dietary exposures. In: Peer reviewed conference proceedings of the 7th international toxicology symposium in Africa. Jointly hosted by University of Johannesburg and North Western University South Africa, and Hokkaido University in Japan. Sponsored by Japan Society for the Promotion of Science, pp. 111–112
Ogbeide O, Isioma Tongo, Ezemonye L (2015b) Risk assessment of agricultural pesticides in water, sediment and fish from Owan river Edo state. Nigeria. Environ Monit Assess 187:1–16. doi:10.1007/s10661-015-4840-8
Okeniyia SO, Eqwikhide PA, Akporhonore EE, Obaze IE (2009) Distribution of organochlorine and polychlorinated pesticides residues in water bodies of some rivers in northern Nigeria. Electron J Environ Agric Food Chem 8(11):1269–1274
Okoya AA, Ogunfowokan AO, Asubiojo OI, Torto N (2013) Organochlorine pesticide residues in sediments and waters from cocoa producing areas of Ondo State, Southwestern Nigeria. International Scholarly Research Network (ISRN) Soil Science, New York
Olaifa FE, Ayodele IA (2004) Presence of hydrocarbon and heavy metals in some fish species in the cross river, Nigeria. J Livest Ext 3:90–95
Olatunbosun SS, Sojinu O, Sonibare E, Eddy O, Zeng Y (2011) Occurrence of organochlorine pesticides (OCPs) in surface sediments of the Niger Delta, Nigeria. J Appl Sci Res 7(8):1299–1305
Osibanjo O, Adeyeye A (1997) Organochlorine pesticide residues in foodstuffs of animal origin in Nigeria. Bull Environ Contam Toxicol 58:206–221
Papadakis EN, Zisis V, Athena K, Katerina K, Konstantinos CM, Euphemia PM (2015) A pesticide monitoring survey in rivers and lakes of northern Greece and its human and ecotoxicological risk assessment. Ecotoxicol Environ Saf 116:1–9
Phillips JP, Lisa HN, Robert JG, Naomi N, Karen RM, Carolyn VA (2010) Composition, distribution, and potential toxicity of organochlorine mixtures in bed sediments of streams. Sci Total Environ 408:594–606
Qu C, Shihua Q, Dan Y, Huanfang H, Jiaquan Z, Wei C, Habtom KY, Edward HS, Junhua Y, Xinli X (2014) Risk assessment and influence factors of organochlorine pesticides (OCPs) in agricultural soils of the hill region: a case study from Ningde, southeast China. J Geochem Explor. doi:10.1016/j.gexplo.2014.11.002
Sanchez-Bayo F, Baskaran S, Kennedy IR (2002) Ecological relative risk (EcoRR): another approach for risk assessment of pesticides in agriculture. Agr Ecosyst Environ 91:37–57
Sangchan W, Hugenschmidt C, Ingwersen J, Thavornyutikarn P, Pansombat K, Streck T (2012) Short-term dynamics of pesticide concentrations and loads in a river of an agricultural watershed in the outer tropics. Agric Ecosyst Environ 158(1):1–14
Schulz R (2004) Field studies on exposure, effects, and risk mitigation of aquatic nonpoint-source insecticide pollution: a review. J Environ Qual 2004(33):419–448
Sohair A, Gad A, Mohsen MA, Mohamed AA, Wasfi MT (2013) Dietary intake of pesticide residues in some Egyptian fruits. J Appl Sci Res 9(1):965–973
Stamatis N, Hela D, Triantafyllidis V, Konstantinou I (2013) Spatiotemporal variation and risk assessment of pesticides in water of the lower catchment basin of Acheloos River, Western Greece. Sci World J 2013. doi:10.1155/2013/231610
Steinwandter H (1992) Development of micro extraction methods in residue analysis. In: Cairns T, Sherma J (eds) Emerging strategies for pesticide analysis. CRC, Boca Raton, pp 3–50
Tongo I, Ezemonye L (2015) Human health risks associated with residual pesticide levels in edible tissues of slaughtered cattle in Benin City, Southern Nigeria. Toxicol Rep 2:1117–1135
Tongo I, Ogbeide O, Ezemonye L (2015) PAH Levels in smoked fish species from selected markets in Benin City, Nigeria: potential risk to human health. In: Peer reviewed conference proceedings of the 7th international toxicology symposium in Africa. Jointly hosted by University of Johannesburg and North Western University South Africa, and Hokkaido University in Japan. Sponsored by Japan Society for the Promotion of Science, pp 69–70
Tsakiris IN, Toutoudaki M, Kokkinakis M, Paraskevi M, Aristides TA (2011) In: Stoytcheva M (ed) Risk assessment study of greek population dietary chronic exposure to pesticide residues in fruits, vegetables and olive oil, pesticides—formulations, effects, fate, ISBN: 978-953-307-532-7
United State Department of Energy (USDoE) (2011) U.S. Department of Energy’s Oak Ridge Operations Office (ORO). The Risk Assessment Information System (RAIS)
United States Environmental Protection Agency (USEPA) (1996) USEPA, integrated risk information system. United States Environmental Protection Agency, Office of Health and Environmental Assessment, Washington, DC
United States Environmental Protection Agency (USEPA) (2007) Pesticides in water, soil, sediment, biosolids, and tissue by HRGC/HRMS. EPA method 1699. EPA-821-R-08-001. U.S. Environmental Protection Agency, Office of Water, Washington, DC
Upadhi F, Wokoma OAF (2012) Examination of some pesticide residues in surface water, sediment and fish tissue of Elechi Creek, Niger Delta, Nigeria. Res J Environ Earth Sci 4(11):939–944
US Environmental Protection Agency (USEPA) (1992) Guidelines for exposure assessment. Risk Assessment Forum and U.S. Environmental Protection Agency, Washington, DC
US Environmental Protection Agency (USEPA) (1997) Exposure factors handbook. US Environmental Protection Agency, Washington, DC
US Environmental Protection Agency (USEPA) (2002) Supplemental guidance for developing soil screening levels for superfund sites OSWER 9355. US Environmental Protection Agency, Washington, DC, pp 4–24
Vryzas Z, Vassiliou G, Alexoudis C, Papadopoulou-Mourkidou E (2009) Spatial and temporal distribution of pesticide residues in surface waters in north-eastern Greece. Water Res 43(1):1–10
WHO (World Health Organization) (1997) Guidelines for predicting dietary intake of pesticide residues (revised) global environmental programme (GEMS/Food) in collaboration with Codex Committee on pesticide residues. Progr Food Saf Food Aid, pp 1–44
Williams BA (2013) Levels and distribution of chlorinated pesticide residues in water and sediments of Tarkwa Bay, Lagos Lagoon. J Res Environ Sci Toxicol 2(1):1–8
Yang RQ, Lu AH, Shi JB, Jaing GB (2005) The level and distribution of organochlorine pesticides (OCPs) in sediments from the Haihe River, China. Chemosphere 61:347–354
Zhou R, Zhu L, Yang K, Chang Y (2006) Distribution of organochlorine pesticide in surface water and sediment from Qiantang River, East China. J Hazard Mater A 2006(137):68–78
Acknowledgments
The authors would like to appreciate the Japan Society for Promotion of Science and the National center for energy and environment, University of Benin for providing travel grants and funds for pesticide analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogbeide, O., Tongo, I., Enuneku, A. et al. Human Health Risk Associated with Dietary and Non-Dietary Intake of Organochlorine Pesticide Residues from Rice Fields in Edo State Nigeria. Expo Health 8, 53–66 (2016). https://doi.org/10.1007/s12403-015-0182-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-015-0182-6