Abstract
Sediment and fish (Oreochromis niloticus) samples collected from Lake Manzala were analyzed to assess the spatial distribution of OCPs and 96 PCBs. Relatively higher concentrations of chlorpyrifos, ∑DDT, and HCB were found, particularly at the Bahr Al-Baqar drain station, which has uncontrolled inputs of untreated domestic, agricultural, and industrial wastes. The concentrations of ∑PCBs ranged from 19 to 69 ng/g dw and from 7.4 to 29 ng/g dw for sediment and fish samples, respectively. Ratios of DDT to its metabolites suggest that the source of ∑DDT is from past usage of technical DDT in the regions surrounding the lake. Sediment quality guidelines were exceeded in 88, 75, and 42% of sediments for the Effects Range Low (ERL) for ∑PCBs, ∑DDT, and 4,4′-DDE, respectively. Sediment from the Bahr Al-Baqar drain exceeded the Probable Effects Level (PEL) for DDT isomers 2,4′ and 4,4′. All fish samples from Lake Manzala were well below action and tolerance levels set by US EPA for ∑DDT, chlordane, dieldrin, heptachlor, mirex, and PCBs.
Highlights
-
Distributions of OCPs and PCBs in sediment and tilapia from Lake Manzala were investigated.
-
Chlorpyrifos, ∑DDT, and HCB sediment concentrations were spatially variable and relatively elevated.
-
∑96PCBs, ∑DDT, and 4,4′-DDE exceeded the Effects Range Low in 88, 75, and 42% of the sediments, respectively.
-
The major input from Cairo, the Bahr Al-Baqar drain, exceeded the Probable Effects Level for DDT isomers in sediments.
-
OCP and PCB concentrations in tilapia were below action and tolerance levels set by the US-EPA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorinated pesticides (OCPs) and polychlorinated biphenyls (PCBs) were routinely used in large quantities for agricultural and industrial purposes. Due to their persistence, bioaccumulation, and biomagnification, they are ubiquitous in the environment and have been a global environmental concern for decades (Fleming et al. 2006; Sheng et al. 2013). Approximately 1,000,000 metric tons (MT) of OCPs were used in Egypt between 1952 and 2003 (Mansour 2004). Three major pesticides, namely, DDT (13,500 MT, 1952–1971), lindane (11,300 MT, 1952–1978), and endrin (10,500 MT, 1961–1981), represented about 35% of the total organochlorine pesticide used (El-Sebae et al. 1993). Between 1952 and 1990, these chemicals, with the addition of toxaphene, constituted 41% of the total insecticide used on cotton fields (El-Sebae et al. 1993). Annual commercial pesticide use in Egypt decreased from 26,000 MT during the 1970s to 24,369 MT in the 1980s and to 17,000 MT during the 1990s (−6.3 and −30%, respectively, Mansour 2004). DDT and lindane were banned in Egypt in the early 1980s. Despite the ban, the import of 80 pesticides, including DDT and lindane, continued but their use was restricted in 1996 based on a Ministerial Decree (Barakat et al. 2013a).
In Egypt, few studies have been conducted to examine the contamination levels of PCBs. These studies were limited to specific locations such as the Red Sea Coast (Khaled et al. 2004), River Nile (El-Kady et al. 2007), Lakes Manzala and Maryut (Barakat et al. 2012a, b), and Lake Qarun (Barakat et al. 2013a). Concerns about the persistence and toxicity of PCBs led to a worldwide ban in the late 1970s (Breivik et al. 2007); however, PCBs can still be formed, as unintentional by-products, through the incineration of wastes and other industrial processes (Liu et al. 2009).
Adsorption processes play an effective role in transporting OCPs and PCBs to sediments due to the large surface area of sediment particles, ionic and hydrogen bonding, ligand exchange, and van der Waal forces (Tomlin 1994; Gevao et al. 2000; Xue et al. 2006). Fish at the top of the aquatic food web are most likely to be adversely affected as they biomagnify these contaminants to higher concentrations (Bervoets and Blust 2003; Wang et al. 2011). Fish production in Egypt is an important industry constituting about 6.9% of total agricultural production. Egypt is the 8th largest aquaculture producer in the world with a total production of 986,820 t (Elmenofi et al. 2014; FAO 2013). Nile tilapia is the most popular fish due to its low cost. The yearly consumption rate of fish in Egypt has grown from 8.5 kg/person in 1996 to 15.4 kg/person in 2008 (81%; Macfadyen et al. 2012). Lake Manzala, the largest of the Nile Delta lakes, produces 30% of all the fish consumed in Egypt. Lake Manzala receives untreated sewage, agricultural, and industrial wastes raising human health concerns (Osfor et al. 1998).
The objective of this study was to determine the concentration and spatial distribution of OCPs and PCBs in sediment and fish samples collected from Lake Manzala. Another objective is to compare the measured concentrations to Sediment Quality Guidelines (Effects Range Low (ERL) and the Effects Range Median (ERM)) in order to assess the quality of the Lake Manzala aquatic ecosystem. The study documents contaminant concentrations that may be of concern and that will allow for informed management decisions to be made.
Materials and methods
Study area description
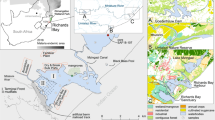
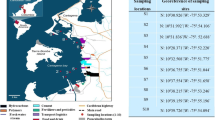
Lake Manzala is located on the northeastern edge of the Nile Delta, 170 km away from Cairo and 15 km from Port Said (Fig. 1). Over the past several decades, the surface area of Lake Manzala has decreased by nearly 50% due to continuous land reclamation projects with more reclamation activity projected in the future unless action is taken to stop this trend (United Nations 1998). Lake Manzala is a long shallow and eutrophic environment with depth from 1.2 to 1.5 m (El-Kholy et al. 2012).
Sampling locations in Lake Manzala, Egypt (adapted from El-Kady et al. 2015)
Reagents and chemicals
Certified standards were purchased from Sigma-Aldrich (St. Louis, MO, USA) with purities greater than 95%. Working solutions were prepared by dilution to appropriate concentration. Hydrochloric acid (EM science), anhydrous sodium sulfate (J.T. Baker), Aldrich alumina oxide (∼150 mesh), Aldrich silica gel (100–200 mesh), granular copper (20–30 mesh), hexane, methylene chloride, methanol, and pentane (pesticide grade) were used for the extraction and purification of these samples.
Sampling
Sediment and Nile tilapia (Oreochromis niloticus) samples (24 each) were collected from the eight stations (Fig. 1) in May, June, and July of 2011. Stations were chosen to provide spatial coverage including stations that could be used to assess input sources. The sampling sites cover several locations receiving agricultural, domestic, and industrial wastes. The south and southeast parts of the Lake receive freshwater from five drains (Ayache et al. 2009) while the east part of the Lake receives wastewater from Port Said Governorate (Gilpin 1995). One site (S2) covers the connection between the Lake and the Mediterranean Sea. Surface sediments (0 to 10 cm) were collected using a stainless steel manual corer and placed into pre-cleaned Teflon containers. Sediment samples from each monthly sampling were analyzed separately. Sediments were dried at room temperature, homogenized using a mortar and pestle, and stored at −20 °C until analysis. Fish samples were obtained from fishermen at the same locations as the sediment samples. Fish samples were frozen before removing scales and skin. Fish samples from each location were pooled into composite samples, homogenized, freeze dried, and kept at −20 °C until analysis. The pooled fish samples from each station for each month were analyzed separately.
Extraction and cleanup
Surrogate standards 4,4-dibromooctafluorobiphenyl (DBOFB), PCB103, and PCB198 were added to the samples prior to extraction and used for quantification of analytes (Wade et al. 1993). Extraction of OCPs and PCBs was carried out on 5 g of dried sediment or fish samples using an accelerated solvent extractor (Dionex, ASE 200). Activated copper was added to the sediment extracts to remove elemental sulfur, and the extracts were then purified from co-extracted materials by alumina/silica column chromatography (see Barakat et al. 2012b for details). Tissue sample extracts were purified using alumina/silica column chromatography followed by gel permeation chromatography, as a further cleanup step.
Instrumental analysis
The OCPs and PCBs were analyzed by gas chromatography in splitless mode using an electron capture detector (ECD). A 30 m × 0.25 mm i.d. fused silica column with a 0.25-μm DB-5 bonded phase (J&W Scientific, Inc.) provided component separation. The oven temperature was held for 1 min at 100 °C and then increased to 250 °C at 5 °C min−1, held for 1 min, and further to 300 °C at 10 °C min−1 with a final hold of 5 min. The internal standard, 2,4,5,6-tetrachloro-m-xylene (TCMX), was added prior to GC/ECD analysis to determine surrogate recoveries. The concentrations of target analytes were calculated based on the response of calibration solutions injected at four different concentrations while a standard solution containing only PCBs was used to confirm the identification of PCB congeners.
Analytical quality assurance
Each analytical batch of 20 samples included a procedural blank, a duplicate sample, a matrix spiked sample, and standard reference material to evaluate overall accuracy, sample homogeneity, and analytical variability of the procedures used for sample preparation and analysis. Surrogate recoveries in sediment and tissue samples ranged from 63 to 119% and from 68 to 119%, respectively. Recoveries of target analytes in a sediment standard reference material [National Institute of Standards and Technology (NIST) SRM 1944] ranged from 77 to 97% of the certified concentrations. For tissue samples, the recoveries for NIST SRM 1947 (Lake Michigan Fish Tissue) ranged from 102 to 131% of the certified concentration. Recoveries of target analytes in matrix spike samples ranged from 78 to 102% and from 90 to 106% in sediment and tissue samples, respectively.
Results and discussions
Sediment samples
PCB and OCP average concentrations (reported as ng/g on a dry weight basis), standard deviations, relative percent standard deviation (RPSD), and percent detection frequency in sediment samples are reported (Table 1). PCBs were detected at all sites with concentrations ranging from 19 ng/g (Al-Boghaz site) to 69 ng/g (Bahr Al-Deshdy site). The mean PCB concentrations were similar across the lake with a range for the eight stations from 36 to 56 ng/g with the highest mean concentration at the Bahr Al-Baqar drain. The RPSD ranged from 7 to 52% for PCBs. This heterogeneity for sediment samples collected for one station and multiple sites has been noted in other studies (Wade et al. 2008) where the RPSD was 45% for sediment samples analyzed for PAH. Compared with other studies in Egypt, PCB levels in sediment samples from Lake Manzala were lower than concentrations reported for Alexandria harbor and Lake Maryut (Table 2, Barakat et al. 2002, 2012b). In addition, the total concentration of 29 PCB congeners in Lake Qarun was higher than the levels reported in this study for 96 PCB congeners. Indicator PCBs (congeners 28, 52, 101, 138, 153, and 180) are very persistent and found in most environmental samples. Together, they account for nearly 50% of the commercial mixture of PCB formulations (Arnich et al. 2009). For indicator PCBs, the Bahr Al-Baqar drain contained the highest mean level followed by West of Bashteer. Sediment samples collected from other locations in Lake Manzala were comparable with average concentrations between 9.3 and 11 ng/g. Indicator PCBs constituted 22 to 32% of the total amount of 96 PCBs with an average of 28% for all sediment samples. PCB 28 represented 32 to 85% of the total amount of indicator PCBs with an average of 57% for all sediment samples. The high percentage of PCB 28 was also reported for sediment samples from the Nile River where it represented 26 to 45% of the sum of the same indicator PCBs (El-Kady et al. 2007).
This may reflect the commercial PCB mixture used in Egypt. PCB chlorination level congener distribution in sediments was generally in the order of di- (32%) > tri- (24%) > penta- (18%) > tetra- (13%), and they constituted to be 75 to 92% of the total amount of PCBs (Fig. 2). In addition to the fairly uniform distribution of PCB concentrations in Lake Manzala sediments, the predominance of lower-chlorinated biphenyls in sediment samples over the entire lake suggests that long-range atmospheric transport may be a major source of PCBs to the lake (Park et al. 2001, 2002; Dunnivant et al. 2002). The PCB concentrations in 88% of the sediment samples exceeded the ERL while none of them exceeded the Probable Effects Levels (PEL) or the Effects Range Median (ERM). This indicates that most of the sites have a low, although possible, probability of experiencing adverse effects from the PCBs.
Determination of DDT and its metabolites, DDD and DDE, included both 4,4′ and 2,4′ isomers. The 2,4′ isomers were not reported individually as their concentrations were below or near the detection limit. DDTs were detected in all samples. The mean concentrations of ∑DDTs ranged from 0.56 to 15 ng/g. These concentrations are comparable to Egyptian locations from Lakes Manzala and Qarun (Barakat et al. 2012a, 2013a) and those along the Egyptian Mediterranean Sea Coast (El Nemr and El-Sadaawy 2016) (Table 2). However, the concentrations reported here are low compared to data reported in samples from Alexandria harbor and Lake Maryut, Egypt (Barakat et al. 2002), and high compared to sediment samples from lakes Edku and Burullus, two of the Mediterranean coastal lakes (Barakat et al. 2013b).
In Lake Manzala sediments, 4,4′-DDE and 4,4′-DDD constituted 47 to 78 and 4.0 to 41%, respectively, of the total DDT (Fig. 3a). With the exception of samples collected at Al-Boghaz, Al-Deshdy, Al-Hamra, and Al-Ginka, 4,4′-DDT was mostly below the limit of detection with frequency of detection of 25% (Table 1).
All sediment samples had detectable concentrations of 4,4′-DDE in agreement with data reported in sediments from Lake Maryut, Alexandria, Egypt (Barakat et al. 2012b) while 4,4′-DDD, 2,4′-DDT, and 4,4′-DDT were found above the detection limit in 71, 29, and 25% of the samples, respectively. The predominance of DDE and, to a lesser degree, DDD in these samples is consistent with the past history of DDT applications in Egypt which were forbidden nearly 40 years ago. 4,4′-DDE was also detected with the highest frequency in sediment samples from Lake Qarun (97%, Barakat et al. 2013a) and Lake Manzala (88%, Abbassy et al. 2003). DDE occurs as an impurity in technical grade of DDT, but it can be produced by degradation, under aerobic conditions, or by abiotic dehydrochlorination of DDT isomers (Li et al. 2008; Guo et al. 2009). Estimates for the half-life in sediment of 4,4′-DDE is 5–7 years (Thomas et al. 2008; Toan et al. 2009). The frequency of past DDT applications to the soil suggests that the levels of DDE may persist for more than two decades (Thomas et al. 2008).
DDT derivative ratios, such as those proposed by Hong et al. (1999) [(4,4′-DDE+ 4,4′-DDD)/ 4,4′-DDT] and Strandberg et al. (1998) (4,4′-DDT/ 4,4′-DDE), are generally used to indicate recent or past usage of DDT; ratios >0.5 and <0.5, respectively, indicate old application of DDT. Hong’s ratios calculated for samples with detectable 4,4′-DDT ranged from 2.01 to 13.7 while Strandberg’s ratios in these samples ranged from 0.0 to 0.39 suggesting that both ratios indicate no major current use of technical DDT in the region surrounding Lake Manzala.
Between 1947 and 1988, in Egypt, usage of technical hexachlorocyclohexane (HCH) was 0.047 MT, placing it in the top ten countries for usage (Li 1999). For the technical grade, α-HCH constitutes about 55–80% of HCHs followed by β-(5–14%), γ-(12–14%), and then δ-HCH (2–10%) (Buser and Muller 1995). The detection frequency of HCH isomers was 96% for one or more of the HCH isomers. The isomer relative abundances were γ-HCH > β-HCH > δ-HCH > α-HCH. The concentrations of ∑HCHs in sediment samples ranged from <0.044 to 9.0 ng/g (Table 1). The ranges of γ-HCH and β-HCH were detected in 79 and 71% of the samples, respectively, and at concentrations ranging from <0.098–6.6 and <0.046–2.9 ng/g, respectively. The δ-HCH and α-HCH isomers were rarely detected. Lindane (γ-HCH) was used extensively in Egypt as a fumigant and to treat lice and scabies in animals (Willett et al. 1998). Between 1952 and 1990, nearly 11,300 MT of lindane was used in Egypt for pest control for cotton (El-Sebae et al. 1993). β-HCH is only a trace component of technical HCH but is hydrophobic, has a low vapor pressure, and is highly resistant to microbial degradation, so it is considered as the most stable HCH isomer (Lee et al. 2001; Doong et al. 2002; Yang et al. 2005). The concentrations of HCHs detected in sediment samples from Lake Manzala were higher compared to sediment values from Alexandria Harbor (Barakat et al. 2002) and lakes Edku and Burullus, Egypt (Barakat et al. 2013b). The highest concentrations reported in Lake Qarun (100.6 ng/g, Barakat et al. 2013a) and in the Mediterranean Sea coast (45.8 ng/g, El Nemr and El-Sadaawy 2016) were 10 and 5 times higher than the highest concentration found in the current study (9.0 ng/g, Table 2).
Endrin was detected in only 12.5% of the samples collected mainly along the northern part of the lake with concentrations ranging from <0.05 to 0.77 ng/g. Concentrations of dieldrin and aldrin ranged from <0.017 to 2.8 ng/g and from <0.020 to 3.6 ng/g, respectively. These concentrations were significantly lower compared to studies conducted in Lake Qarun (Barakat et al. 2013a) and higher than those reported in samples from the Mediterranean coastal lakes (Barakat et al. 2013b). Interestingly, aldrin and endrin were more prevalent in sediments reported previously from Lake Manzala (Barakat et al. 2012a). Comparatively, El Nemr and El-Sadaawy (2016) reported aldrin at concentrations as high as 110 ng/g in sediment samples from the Mediterranean Sea coast; to our knowledge, this would be the highest level detected in the Egyptian aquatic environment.
Chlorpyrifos been extensively used in Egypt since its approval for application on 64 crops (El-Marsafy 2004). Chlorpyrifos is still used intensively as an insecticide, particularly for cotton pest control (Farahat et al. 2011). Chlorpyrifos was found in 96% of the sediment samples with concentrations ranging from <0.25 ng/g (Al-Hamra site) to 30 ng/g (Bahr Al-Baqar drain site). The detection frequency of chlorpyrifos in the current study was higher than those reported in sediment samples collected from lakes Edku and Burullus (85 and 71%, respectively), Alexandria Harbor (26%), and Lake Maryut (62%) and a previous study for Lake Manzala (54%). The current concentrations for chlorpyrifos are higher than those reported for lakes Edku and Burullus as well as previously in Lake Manzala and are comparable with levels found in Lake Maryut. Pentachloroanisole (PCA) was detected at 87.5% of the sites at concentrations ranging from <0.046 ng/g (West of Bashteer site) to 0.69 ng/g (Bahr Al-Baqar drain site). PCA concentrations agree with previous studies in Lake Manzala (Barakat et al. 2012a); however, the concentrations detected in lakes Edku and Burullus (Barakat et al. 2013b) were lower than levels encountered in the current study.
HCB and other chlorobenzene compounds (i.e., 1,2,3,4-tetrachlorobenzene) were found with high frequencies in these samples (79 and 100%, respectively). The highest concentration of ∑CBs was found in the Bahr Al-Baqar drain while Al-Temsah had the lowest concentration (8.6 and 0.46 ng/g, respectively, Table 1). These concentrations are similar to those reported for Lake Maryut, Alexandria, Egypt (Barakat et al. 2012b), but higher than levels reported for lakes Edku and Burullus (Barakat et al. 2013b). Comparatively, Lake Qarun had higher HCB concentrations (Barakat et al. 2013a, Table 2).
Among chlordane-related compounds (CHLs), α-chlordane represented up to 96% of the ∑CHLs and ranged from 0.51 ng/g (Al-Hamra site) to 2.4 ng/g, (Bahr Al-Baqar drain site). γ-Chlordane was only detected in 29% of the samples with concentrations ranging from <0.034 to 0.62 ng/g. The Bahr Al-Baqar drain had the highest average concentration of the ∑CHLs, while sites located in the northern part of the lake (Al-Boghaz, Al-Temsah, and Al-Hamra) had the lowest mean concentrations (Table 1). This distribution might reflect the use of technical grade of chlordane in Egypt as α-chlordane is one of the main three components (Dearth and Hites 1991). Heptachlor and heptachlor epoxide were only found in 12 and 17% of the samples, respectively. The detection of heptachlor epoxide in these samples is lower than those found in Lake Qarun (91%, Barakat et al. 2013a) and, previously, in Lake Manzala (55%, Barakat et al. 2012a). South, southeast, and west portions of the Lake receive freshwater from the main five drains called El-Sherw, El-Gammaliah, Hadous, Ramsis, and Bahr El-Baqar (Ayache et al. 2009; Rasmussen et al. 2009). The combined inflow from these drains represents 32 to 47% of the Lake’s volume (Thompson et al. 2009). The east part of the Lake receives wastewater from lagoons serving the Governorate of Port Said (Gilpin 1995). The water discharged into the Lake is the major sources of micro-pollutants, which derived from agricultural, industrial, and domestic sources (Barakat 2004). On the other hand, Barakat (2004) stated that the drainage of organochlorine compounds (OCCs) in the coastal lagoon of Lake Manzala increased from the early 1960s to a maximum recorded in the 1980s due to the sedimentation rates in the Lake. After that, the levels of OCCs have declined due to the restrictions of their usage.
Effects Range Low (ERL) and Effects Range Median (ERM) suggested by Long et al. (1995) and Probable Effects Levels (PEL) suggested by the Canadian Council of Ministers of the Environment (CCME 2002) were used to assess Manzala Lake’s sediments according to their ecotoxicological risks. The ERL and ERM are 10th and 50th percentiles, respectively, using the concentrations in sediments associated with any adverse biological effects (Long et al. 1995). The current results illustrated that the concentration of OCPs in all sediment samples was much lower than that of the ERM established by Long et al. (1995). However 75 and 42% of sediment samples were above the Effects Range Low (ERL) for total DDTs (1.58 ng/g) and 4,4′-DDE (2.2 ng/g), respectively (Table 1). Endrin was above the ERL (0.02 ng/g) in 12% of the samples. With the exception of sediments collected from the Bahr Al-Baqar drain, which had a concentration of 2,4′ + 4,4′-DDE (6.75 ng/g), all the sediment samples were below the PEL set by the Canadian Council of Ministers of the Environment (Table 1).
Fish samples
PCB and OCP concentrations are reported on a nanogram per gram dry weight basis; their detection frequencies, size, and weight sample ranges are presented in Table 3. The mean concentration of ∑96 PCBs in fish collected from Lake Manzala ranged from 7.4 to 29 ng/g and was detected in all samples collected. The RPSD for these triplicate PCB analyses ranged from 16 to 46% indicating individual variability. Based on our data, this variability was not the result of differing length or weight of the fish analyzed. The mean concentration of ∑96 PCBs in fish collected from Lake Manzala was 12 ± 4.9 ng/g, at West of Bashteer, to 22 ± 4.2 ng/g, at Legan and Al-Ginka, while the mean concentrations of indicator PCBs ranged from 1.8 ± 0.85 ng/g (West of Bashteer) to 3.6 ± 0.84 ng/g (Bahr Al-Baqar drain). Detection frequencies for indicator PCB congeners (i.e., 28, 52, 101, 138, 153, and 180) were 75, 88, 96, 96, 100, and 100%, respectively. The indicator PCBs constituted 16% of the total amount of ∑96 PCBs in these samples. The mean concentrations of indicator PCBs in tilapia from Lake Manzala (1.8–3.6 ng/g) are slightly lower than values previously reported for the same species collected from the Nile River (3.2–5.8 ng/g; El-Kady et al. 2007). On average, the pattern of PCB congener distribution in tilapia was similar to the pattern observed in sediment samples and in the order di- (38%) > tri- (18%) > penta- (12%) > tetra- (11%); they constituted 71 to 85% of the total amount of PCBs (Fig. 2). In addition to indicator PCBs, congener nos. 56, 60, 66, 105, and 118 were also found to be dominant in the studied fish samples. These PCB congeners contain chlorine atoms in the 2,4-; 2,3,4-; 2,4,5-; 3,4,5-; and 2,3,4,5- positions, which result in a higher resistance to biotransformation (Brown and Lawton 2001; Megson et al. 2013).
Distribution of DDTs in fish was similar to the sediment samples (Fig. 3b) with 4,4′-DDE contributing up to 86% of the total DDT concentrations followed by 4,4′-DDD (7.3–20%) and 4,4′-DDT (3.4–28%). Both 4,4′-DDE and 4,4′-DDD were detected in every sample while 4,4′- and 2,4′-DDT were found in 83 and 12% of the samples, respectively. The concentrations of ∑DDTs in fish from Lake Manzala ranged from 0.26 to 3.7 ng/g which are lower than the concentrations previously reported for tilapia from lakes Edku (110–250 ng/g), El-Burullus (6.0–38 ng/g), Maryut (19–135 ng/g) (Badawy and El Dib 1984), the Nile River, and Lake Manzala (156–280 and 120–280 ng/g, respectively; Abou-Arab et al. 1995); a fish farm in Lake Manzala and fish ponds in Suez (265–330 and 12–17 ng/g, respectively, Shereif and Mancy 1995); and a market in Assuit City (ND-9.15 ng/g; Yahia and Elsharkawy 2014). In comparison, the DDT concentrations reported in samples collected from the Nozha Hydrodrome, a small shallow lake, (0.11–0.48 ng/g; Hilmy et al. 1983) were lower than the concentrations found in this study.
Lindane and β-HCH were detected in 50 and 67% of the samples analyzed with overall concentrations ranging from <0.015 to 0.81 ng/g and from <0.038 to 5.0 ng/g, respectively, while α and δ HCH were not detected. The predominance of β-HCH can be explained by its higher stability and resistance for microbial degradation (Yang et al. 2005). This finding was supported by Szlinder-Richert et al. (2008) who noticed that the proportion of β-HCH increased while γ- and α-HCHs decreased in Baltic fish. The same distribution pattern of HCH isomers was reported by Wang et al. (2013) with an average of β-HCH of 68% of total HCHs in Lake Taihu, China. The concentrations of γ-HCH in the present study (<0.015–0.81 ng/g) are lower than those detected in fish samples from lakes Edku (<0.1–50 ng/g), El-Burullus (<0.1–9.1 ng/g), and Maryut (6.0–55 ng/g) (Badawy and El Dib 1984), the Nile River and Lake Manzala (ND-11 and 9.5–16 ng/g, respectively; Abou-Arab et al. 1995); farms in Lake Manzala and ponds in Suez (65–85 and ND-1.8 ng/g, respectively; Shereif and Mancy 1995); and Assuit City (2.0–5.4 ng/g; Yahia and Elsharkawy 2014).
Aldrin and dieldrin were detected in 38 and 13% of the samples, respectively, at concentrations lower than previously reported. In the present study, aldrin concentrations (<0.045–0.22 ng/g) were lower than concentrations reported for Nile River and Lake Manzala (5.1–14 and 18–75 ng/g, respectively, Abou-Arab et al. 1995) and fish from a market in Assuit City (0.8–4.8 ng/g; Yahia and Elsharkawy 2014). This may be the result of a temporal decrease of Aldrin concentration between these two studies. Aldrin is converted quickly to dieldrin which is more stable than aldrin (Montgomery 2000). Endrin was not detected.
Chlorpyrifos was detected in all fish samples collected from Lake Manzala with concentrations ranging from 0.96 to 3.3 ng/g, West of Bashteer and the Bahr Al-Baqar drain, respectively. No clear trends can be observed regarding concentration distribution within the lake. Pentachloroanisole was found in 92% of the samples with concentrations ranging between <0.020 and 0.58 ng/g.
∑Chlorobenzene concentrations in the lake (0.060–1.9 ng/g) were dominated by 1,2,4,5-tetrachlorobenzene >1,2,3,4-tetrachlorobenzene > pentachlorobenzene > hexachlorobenzene (52, 27, 13, and 8%, on average, respectively). Chlordane compounds were detected at very low concentrations in fish collected from Lake Manzala (<0.022–0.27 ng/g).
To protect humans from foods contaminated with pesticides, PCBs, and other contaminants, the US Food and Drug Administration established action and tolerance levels for fish (US EPA 2000). Based on these action levels, concentrations in all tilapia collected and analyzed from Lake Manzala were well below levels of concern set for ∑DDT, chlordane, dieldrin, heptachlor epoxide, mirex, and PCBs. The concentrations of indicator PCBs in fish muscle were also found to be much lower (0.22–0.98 ng/g w.w.) than the tolerance limit recommended by the European Union for fish muscle (75 ng/g w.w.) [Commission Regulation (EU) No. 1259/2011].
In general, the concentration means in fish samples were lower by a factor of 2 for PCB, DDT, and CHLs in fish tissue when compared to the sediment which indicates that biomagnification is not an important process affecting tilapia muscle tissue.
Conclusions
Concentrations of 96 PCBs ranged from 19 to 69 ng/g dw for sediments and from 7.4 to 29 ng/g dw for tilapia fish. Indicator PCBs represented 28 and 16% of the total 96 PCBs in sediments and fish samples, respectively. Lower chlorinated biphenyls were predominant, suggesting a long-range atmospheric transport of PCBs to the Lake. DDT levels were between 0.56 and 15 ng/g in sediment and between 0.26 and 3.7 ng/g in fish. DDT ratios indicated that DDT is from past usage. Chlorpyrifos was detected in almost all sediment and fish samples with concentrations ranging from <0.25 to 30 ng/g and from 0.96 to 3.3 ng/g, respectively. It would be prudent to continue monitoring of this pesticide that is currently in use to see if it increases in the future. HCHs were found at concentrations from <0.044 to 9.0 ng/g and from <0.086 to 5.8 ng/g in sediment and fish, respectively. HCH isomeric abundance was dominated by γ-HCH in sediments and by β-HCH in tissues. The concentrations of total PCB, total DDTs, and 4,4′-DDE in 88, 75, and 42% of sediments exceed the ERL suggesting that the biota may be adversely effected at those locations. Tilapia collected from Lake Manzala showed similar PCB and DDT distribution patterns to the sediment. The concentrations of OCPs and PCBs in tilapia tissue were below the action and tolerance levels set by US EPA and the European Union. Generally, the highest mean concentrations are found at the Bahr Al-Baqar drain where stress by inputs of domestic and untreated wastewater from the densely populated Greater Cairo area enters the lake. Agricultural wastes from cultivated areas located in the Nile Delta (Rasmussen et al. 2009) impact the lake. The results provide evidence that Lake Manzala has been impacted by some OCP and PCB periods. Studies of other potential pollutants should be undertaken.
References
Abbassy MS, Ibrahim HZ, Abdel-Kader HM (2003) Persistent organochlorine pollutants in the aquatic ecosystem of Lake Manzala, Egypt. Bull Environ Contam Toxicol 70:1158–1164
Abou-Arab AAK, Gomaa MNE, Badawy A, Naguib K (1995) Distribution of organochlorine pesticides in the Egyptian aquatic ecosystem. Food Chem 54:141–146
Arnich N, Tard A, Leblanc J, Le Bizec B, Narbonne J, Maximilien R (2009) Dietary intake of non-dioxin-like PCBs (NDL-PCBs) in France, impact of maximum levels in some foodstuffs. Regul Toxicol Pharmcol 54:287–293
Ayache F, Thompson JR, Flower RJ, Boujarra A, Rouatbi F, Makina H (2009) Environmental characteristics, landscape history and pressures on three coastal lagoons in the Southern Mediterranean Region: Merja Zerga (Morocco), Ghar El Melh (Tunisia) and Lake Manzala (Egypt). Hydrobiologia 622:15–43
Badawy MI, El-Dib A (1984) Residues of organochlorine pesticides in fish from the Egyptian delta lakes. Environ Int 10:3–8
Barakat AO (2004) Assessment of persistent toxic substances in the environment of Egypt. Environ Int 30:309–322
Barakat AO, Kim M, Qian Y, Wade TL (2002) Organochlorine pesticides and PCB residues in sediments of Alexandria Harbour, Egypt. Mar Poll Bull 44:1421–1434
Barakat AO, Mostafa A, Wade TL, Sweet ST, El Sayed NB (2012a) Assessment of persistent organochlorine pollutants in sediments from Lake Manzala, Egypt. Mar Pollut Bull 64:1713–1720
Barakat AO, Mostafa AR, Wade TL, Sweet ST, El-Sayed NB (2012b) Spatial distribution and temporal trends of persistent organochlorine pollutants in sediments from Lake Maryut, Alexandria, Egypt. Mar Pollut Bull 64:395–404
Barakat AO, Khairy M, Aukaily I (2013a) Persistent organochlorine pesticide and PCB residues in surface sediments of Lake Qarun, a protected area of Egypt. Chemosphere 90:2467–2476
Barakat AO, Mostafa A, Wade TL, Sweet ST, El Sayed NB (2013b) Distribution and ecological risk of organochlorine pesticides and polychlorinated biphenyls in sediments from the Mediterranean coastal environment of Egypt. Chemosphere 93:545–554
Bervoets L, Blust R (2003) Metal concentrations in water, sediment and gudgeon (Gobio gobio) from a pollution gradient: relationship with fish condition factor. Environ Pollut 126:9–19
Breivik K, Sweetman A, Pacyna JM, Jones KC (2007) Towards a global historical emission inventory for selected PCB congeners a mass balance approach 3. An update. Sci Total Environ 377:296–307
Brown JF, Lawton RW (2001) Factors controlling the distribution and levels of PCBs after occupational exposure. In: Robertson LW, Hansen LG (eds) PCBs recent advances in environmental toxicology and health effects. The University Press of Kentucky
Buser HR, Muller MD (1995) Isomer and enantioselective degradation of HCH isomers in sewage sludge under anaerobic conditions. Environ Sci Technol 29:664–672
CCME (Canadian Council of Ministers of the Environment) (2002) Canadian sediment quality guidelines for the protection of aquatic life. Canadian Environmental Quality Guidelines. Canadian Council of Ministers of the Environment, Winnipeg, MB.
Commission regulation (E.C.) No 1259/2011 of 2 December 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for dioxins, dioxin-like PCBs and non dioxin-like PCBs in foodstuffs. Official J. Eur. Union. 3.12.2011 L320 p. 18.
Dearth MA, Hites RA (1991) Complete analysis of technical chlordane using negative ionization mass spectrometry. Environ Sci Technol 25:245–254
Doong RA, Sun YC, Liao PL, Peng CK, Wu SC (2002) Distribution and fate of organochlorine pesticide residues in sediments from the selected rivers in Taiwan. Chemosphere 48:237–246
Dunnivant FM, Elzerman AW, Jurs PC, Hasan MN (2002) Quantitative structure–property relationships for aqueous solubilities and Henry’s law constants of polychlorinated biphenyls. Environ Sci Technol 26:1567–1573
El Nemr A, El-Sadaawy MM (2016) Polychlorinated biphenyl and organochlorine pesticide residues in surface sediments from the Mediterranean Sea (Egypt). Int J Sed Res 31:44–52
ElKady AA, Wade TL, Klein AG, Sweet ST (2015) Distribution and assessment of heavy metals in the aquatic environment of Lake Manzala, Egypt. Ecol Indic 58:445–457
El-Kady AA, Abdel-Wahhab MA, Henkelmann B, Belal MH, Morsi MKS, Galal SM, Karl-Werner Schramm K-W (2007) Polychlorinated biphenyl, polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofuran residues in sediments and fish of the River Nile in the Cairo region. Chemosphere 68:1660–1668
El-Kholy R, El-Saadi A, Abdin A (2012) Pollution and measures towards de-pollution of the Mediterranean Sea. In: Integrated water resources management in the Mediterranean Region: dialogue towards new strategy, Redouane Choukr-Allah, Ragab Ragab, Rafael Rodríguez-Clemente (Eds.), Springer, P, 175.
El-Marsafy AM (2004) Risk assessment of dietary exposure to chlorpyrifos residues on some fruits, vegetables and herbs in Egypt during 2002. J Adv Agric Res:775–787
Elmenofi GAG, El Bilali H, Berjan S (2014) Governance of rural development in Egypt. Ann Agric Sci 59:285–296
El-Sebae AH, Abou-Zeid M, Saleh MA (1993) Status and environmental impact of toxaphene in the Third World—a case study of African agriculture. Chemosphere 27:2063–2072
FAO (2013) FAO Fisheries and Aquaculture Department has published the Global Aquaculture Production Statistics for the year 2011. 2013 ed.: ftp://ftp.fao.org/FI/news/GlobalAquacultureProductionStatistics2011.pdf.
Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske JR, Lasarev MR, Rohlman DS, Anger WK, Lein PJ, Olson RA (2011) Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ Health Persp 119:801–806
Fleming LE, Broad K, Clement A, Dewailly E, Elmir S, Knap A, Pomponi SA, Smith S, Gabriele HS, Walsh P (2006) Oceans and human health: emerging public health risk in the marine environment. Mar Pollut Bull 53:545–560
Gevao B, Semple KT, Jones KC (2000) Bound pesticide residues in soils: a review. Environ Pollut 108:3–14
Gilpin A (1995) Environmental impact assessment: cutting edge for the twenty-first century. Cambridge University Press, Cambridge
Guo Y, Yu H, Zeng E (2009) Occurrence, source diagnosis, and biological effect assessment of DDT and its metabolites in various environmental compartments of the Pearl River Delta, South China: a review. Environ Pollut 157:1753–1763
Hilmy AM, Badawi HK, Shabana MB (1983) Organochlorine pesticide residues in 12 freshwater Egyptian fish species with special emphasis on Aguilla vulgaris and Mugil cephalus. Comp Biochem Physiol Part C: Comp Pharmacol 76:163–171
Hong H, Chen W, Xu L, Wang X, Zhang L (1999) Distribution and fate of organochlorine pollutants in the Pearl River estuary. Mar Pollut Bull 12:376–382
Khaled A, El Nemr A, Said TO, El-Sikaily A, Abd-Alla AM (2004) Polychlorinated biphenyls and chlorinated pesticides in mussels from the Egyptian Red Sea coast. Chemosphere 54:1407–1412
Lee KT, Tanabe S, Koh CH (2001) Distribution of organochlorine pesticides in sediments from Kyeonggi Bay and nearby areas, Korea. Environ Pollut 114:207–213
Li YF (1999) Global technical hexachlorocylohexane usage and its contamination consequences in environment: from 1948 to 1997. Sci Total Environ 232:123–160
Li Q, Zhang H, Luo Y, Song J, Wu L, Ma J (2008) Residues of DDTs and their spatial distribution characteristics in soils from the Yangtze River Delta, China. Environ Toxicol Chem 27:24–30
Liu G, Zheng M, Ba T, Liu W, Guo L (2009) A preliminary investigation on emission of polychlorinated dibenzo-p-dioxins/dibenzofurans and dioxin-like polychlorinated biphenyls from coke plants in China. Chemosphere 75:692–695
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Macfadyen G, Nasr-Alla AM, Al-Kenawy D, Fathi M, Hebicha H, Diab AM, Hussein SM, Abou-Zeid RM, El-Naggar G (2012) Value-chain analysis-an assessment methodology to estimate Egyptian aquaculture sector performance. Aquaculture 362-363:18–27
Mansour SA (2004) Pesticide exposure—Egyptian scene. Toxicology 198:91–115
Megson D, O’Sullivan G, Comber S, Worsfold PJ, Lohan MC, Edwards MR, Shields WJ, Sandau CD, Patterson DG Jr (2013) Elucidating the structural properties that influence the persistence of PCBs in humans using the National Health and Nutrition Examination Survey (NHANES) dataset. Sci Total Environ 461-462:99–107
Montgomery JH (2000) Groundwater chemicals desk reference, 3rd ed. Lewis Publishers and CRC Press, Boca Raton, p 1384
Osfor MM, El-Dessouky SA, El-Said A, Higazy RA (1998) Relationship between environmental pollution in Manzala Lake and health profile of fishermen. Nahrung 42:42–45
Park J-S, Wade TL, Sweet S (2001) Atmospheric deposition of organochlorine contaminants to Galveston Bay, Texas. Atmospheric Environ 35:3315–3324
Park J-S, Wade TL, Sweet S (2002) Atmospheric deposition of PAHs, PCBs, and organochlorine pesticides to Corpus Christi Bay, Texas: role of air-water gas exchange. Atmospheric Environ 36:1707–1720
Rasmussen EK, Petersen OS, Thompson JR, Flower RJ, Ahmed MH (2009) Hydrodynamic-ecological model analyses of the water quality of Lake Manzala (Nile Delta, Northern Egypt). Hydrobiologia 622:195–220
Sheng J, Wang X, Gong P, Joswiak DR, Tian L, Yao T, Jones KC (2013) Monsoondriven transport of organochlorine pesticides and polychlorinated biphenyls to the Tibetan plateau: three year atmospheric monitoring study. Environ Sci Technol 47:3199–3208 http://dx.doi.org/10.1021/es305201s
Shereif MM, Mancy KH (1995) Organochlorine pesticides and heavy metals in fish reared in treated sewage effluents and fish grown in farms using polluted surface waters in Egypt. Wat Sci Tech:32:153–32:161
Strandberg B, Bavel BV, Bergqvist PA, Broman D, Ishaq R, Naf C, Pettersen H, Rappe C (1998) Occurrence, sedimentation, and spatial variations of organochlorine contaminants in settling particulate matter and sediments in the northern part of the Baltic Sea. Environ Sci Technol 32:1754–1759
Szlinder-Richert J, Barska I, Mazerski J, Usydus Z (2008) Organochlorine pesticides in fish from the southern Baltic Sea: levels, bioaccumulation features and temporal trends during the 1995–2006 period. Mar Pollut Bull 56:927–940
Thomas JE, Ou LT, All-Agely A (2008) DDE remediation and degradation. Rev Environ Contam Toxicol 194:55–69
Thompson JR, Flower RJ, Ramdani M, Ayache F, Ahmed MH, Rasmussen EK, Petersen OS (2009) Hydrological characteristics of three North African coastal lagoons: insights from the MELMARINA project. Hydrobiologia 622:45–84
Toan VD, Thao VD, Walder J, Ha CT (2009) Residue, temporal trend and half-life time of selected organochlorine pesticides (OCPs) in surface soils from Bacninh, Vietnam. Bull Environ Contam Toxicol 82:516–521
Tomlin C (1994) The pesticide manual: incorporating the agrochemicals handbook, tenth ed. British Crop Protection Council Publications and the Royal Society of Chemistry. 407–408.
US EPA (U.S. Environmental Protection Agency) (2000) Guidance for assessing chemical contaminant data for use in fish advisories, vol. 2: risk assessment and fish consumption limits, third ed. Available at: https://www.epa.gov/sites/production/files/201506/documents/volume2.pdf (accessed June, 22, 2016).
Wade TL, Brooks JM, Kennicutt MC II, McDonald TJ, Sericano JL, Jackson TJ (1993) GERG trace organics contaminant analytical techniques. National Status and Trends Program for Marine Environmental Quality, NOAA Tech. Memo. NOS ORCA 71, Sampling and analytical methods of the National Status and Trends Program National Benthic Surveillance and Mussel Watch Projects 1984-1992, Vol IV. Comprehensive Descriptions of Trace Organic Analytical Methods, Silver Spring, MD 1993:121–139
Wade TL, Soliman YS, Sweet ST, Wolff GA, Presley BJ (2008) DGoMB trace elements and polycyclic aromatic hydrocarbons (PAH). Deep Sea Research II 55:2585–2593
Wang X, Li J, Xing H, Xu S (2011) Review of toxicology of atrazine and chlorpyrifos on fish. J Northeast Agric Univ (English edition) 18:88–927
Wang D, Yu Y, Zhang X, Zhang D, Zhang S, Wu M (2013) Organochlorine pesticides in fish from Taihu Lake, China, and associated human health risk assessment. Ecotox Environ Safe 98:383–389
Willett KL, Ulrich EM, Hites A (1998) Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environ Sci Technol 32:2197–2207
Xue N, Zhang D, Xa X (2006) Organochlorine pesticide multiresidues in surface sediments from Beijing Guanting reservoir. Water Res 40:183–194
Yahia D, Elsharkawy EE (2014) Multi pesticide and PCB residues in Nile tilapia and catfish in Assiut city, Egypt. Sci Total Environ 466-467:306–314
Yang RQ, Lv AH, Shi JB, Jiang GB (2005) The levels and distribution of organochlorine pesticides (OCPs) in sediments from the Haihe River, China. Chemosphere 61:347–354
Acknowledgements
This work was carried out at Texas A&M University by the Geochemical and Environmental Research Group (GERG) through scholarship programs provided by the Fulbright organization at Cairo, Egypt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Responsible editor: Hongwen Sun
Rights and permissions
About this article
Cite this article
El-Kady, A.A., Wade, T.L., Sweet, S.T. et al. Distribution and residue profile of organochlorine pesticides and polychlorinated biphenyls in sediment and fish of Lake Manzala, Egypt. Environ Sci Pollut Res 24, 10301–10312 (2017). https://doi.org/10.1007/s11356-017-8714-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8714-1