Abstract
The objective of this study was to determine concentration and bioaccumulation of organochlorine pesticides and heavy metals in edible fish from Wuhan, China, in order to assess health risk to the human via fish consumption. Two edible fish species (Aristichthys nobilis and Hypophthalmichthys molitrix) were collected and analyzed for 11 organochlorine pesticides (OCPs) and eight heavy metals (HMs). Concentrations of ∑HCHs, ∑DDTs, and ∑OCPs in fish samples were in the range of 0.37–111.20, not detected (nd)–123.61, and 2.04–189.04 ng g−1 (wet weight), respectively. Bioaccumulation factors (BAFs) of OCPs in bighead carp (A. nobilis) were higher than those in silver carp (H. molitrix). Concentrations of ∑HMs in bighead carp and silver carp were 352.48 and 345.20 mg kg−1 (dw), respectively. Daily exposure of OCPs and HMs for consumers was estimated by comparing estimated daily intake (EDI) with different criteria. The results revealed that the EDIs in our study were all lower than those criteria. Target hazard quotient (THQ) and risk ratio (R) were used to evaluate non-carcinogenic and carcinogenic risks, respectively. As regard to non-carcinogenic effects of the contaminants, hazard quotients (THQ) of OCPs and HMs were both lower than 1.0, implying negligible non-carcinogenic risk via fish consumption in study area. Nevertheless, in view of carcinogenic effects of the contaminants, the total value of risk ratio (R) of OCPs was lower than the threshold of tolerable risk while the total value of risk ratio (R) of HMs was higher than the threshold of tolerable risk due to the high carcinogenic risk ratios of As and Cr, indicating high carcinogenic risks via fish consumption. The results demonstrated that HMs in edible fish from Wuhan, China, especially As and Cr required more attention than OCPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organochlorine pesticides (OCPs) and heavy metals (HMs) are of global concern in recent decades because of their persistence in environment, bioaccumulation in organisms, and toxicity to human and wildlife. Bioaccumulation of OCPs in biota can lead to some adverse effects on reproduction, nervous systems, and immunity (King et al. 2003; Longcore and Stendell 1977; Misumi et al. 2005). HMs include potentially toxic elements (arsenic, cadmium, lead and mercury, etc.), probably essential elements (nickel, vanadium, cobalt), and essential elements (copper, zinc, iron, manganese) for fish. The potentially toxic elements can be very hazardous at low concentration such as mercury which caused a serious epidemic called Minamata disease in Japan 1950s (Uluozlu et al. 2007), and the essential elements can cause nephritis, anuria, and extensive lesions in kidney if they were excessively took in (Çelik and Oehlenschläger 2007; Luckey and Venugopal 1978; Rahman and Islam 2009).
Among all kinds of aquatic organisms, fish is a suitable indicator for environmental pollution monitoring (Fausch et al. 1984; Roset et al. 2007; Van Ael et al. 2014). The uptake of the pollutants in fish is directly from water via gill and skin and indirectly from diet. Data of pollutants accumulated in fish are indicative not only for the environment but also for the transfer of pollutants through the trophic web (Gobas et al. 1999). The edible fish is an important source of protein and omega-3 polyunsaturated fatty acids which can well guard against cardiovascular disease for human (Hu et al. 2003). The consumption of contaminated edible fish can cause accumulation of pollutants in human body (Li et al. 2008).
Wuhan is the largest city in central China with another name of “city of hundreds of lakes,” where the residents are habituated to eating fish and fish consumption is huge every year. In recent years, with the rapid economic growth of Wuhan, many lakes have been subject to serious pollution (Liu et al. 2014; Tang et al. 2007; Yang et al. 2014; Yang et al. 2009; Yun et al. 2014). In the present study, we choose the East Lake as sampling site which not only used to be the largest urban lake in China but also an important producing area of fish in Wuhan. It is a typical shallow lake in middle and lower drainage basin of the Yangtze River and has an average depth of 2.2 m, a mean temperature of 16.7 °C and a maximum temperature of 40 °C all through the year in the lake area. Due to more and more intensive anthropogenic activities in the surroundings for the past few years, the East Lake suffered considerable OCP and HM pollution and the water quality was graded at IV according to 2013 Wuhan Environmental Aspect Bulletin (WEPB 2014a). Reports analyzing concentrations of OCPs in surface water of the East Lake and revealing carcinogenic risk for human have aroused great concern of the government (Yang et al. 2014). Yun et al. and Liu et al. also revealed that OCPs and HMs in sediments of the East Lake might cause potential ecological risk to aquatic organisms (Liu et al. 2014; Yun et al. 2014). What is more, the East Lake was close to a heavy industrial area where several enterprises such as iron and steel company, foundries plants, and machinery factory included into list of the key enterprises of heavy metal emission published by Wuhan Environmental Protection Bureau were located (WEPB 2014b). There are also hundreds of thousands of residents, several hospitals, and farmlands around the lake which might be the source of OCP and HM pollution in the lake. Although OCP and HM pollution in this area was so serious, almost no information of OCPs and HMs in fish was reported either in East Lake or in other lakes of Wuhan.
Therefore, the objectives of this study are to clarify the concentrations and accumulation levels of OCPs and HMs in edible fish in Wuhan, assess the daily exposure and health risk of human via fish consumption in this area, and provide references for pollution research in other urban lakes of the world. This study gives a comprehensive overview of OCP and HMs’ status in edible fish of Wuhan and provides a basis for government to perform environmental contamination management and take effective measures to reduce potential health risks.
Materials and methods
Study area and sample collection
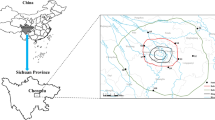
Details of sampling sites can be found in Fig. 1. Twenty fish, 42 water, and 42 sediment samples were collected from the East Lake in June 2013. The fish samples included 10 bighead carp (Aristichthys nobilis) and 10 silver carp (Hypophthalmichthys molitrix) whose dietary habits were all filter feeder, belonging to cyprinid which were also important edible fishes in Wuhan even in China (Xie et al. 2004) (Table 1). The water and fish samples were analyzed for 11 OCPs including α-HCH, β-HCH, γ-HCH, δ-HCH, p,p’-DDE, p,p’-DDD, p,p’-DDT, heptachlor, heptachlor epoxide, aldrin, and methoxychlor. The content of eight heavy metal elements including Cr, Ni, Cu, Zn, As, Cd, Hg, and Pb in water, sediment, and fish samples was also determined simultaneously in this study. Yun et al. had already tested concentrations of OCPs in sediments in June 2013, so the experiment was not repeated in this study (Yun et al. 2014). All of the samples were stored in an ice-chest and transported immediately to laboratory. Throughout the sampling process, the positions of sampling sites were fixed by a global positioning system (GPS).
In the laboratory, fish were dissected and the bladder, intestine, heart, brain, liver, and muscle tissues of the dorsal of fish were taken for analysis. Fish samples were freeze-dried, pulverized, and then stored at −20 °C before extraction. The particulate in the water samples was removed by filtering with 0.45-μm hydrophilic filters. Sediment samples were lyophilized, pulverized, and sifted through 60-mesh sieve. All the sample preparation was finished in 7 days.
Sample extraction and cleanup
Organochlorine pesticides
The concentrations of 11 OCPs were determined in water and fish including the muscle and liver.
OCPs in water samples were tested as described in Muir and Sverko (2006). A recovery surrogate 2,4,5,6-tetrachloro-m-xylene (TCmX) was added into 1 L of filtered water sample. The sample was performed liquid-liquid extraction by dichloromethane (35 mL). Water in the organic phase was removed by Na2SO4 column, and then, solvent of the extract was replaced into n-hexane. After that, a column packed with neutral silica (4.0 g, 3 % deactivated), neutral alumina (2.0 g, 6 % deactivated), and anhydrous sodium sulfate (1 cm) from the bottom to the top was used to remove impurities in the extract. Finally, the purified extract was blown to dryness by purity nitrogen and the residue was dissolved with 20 μL n-hexane.
OCPs in the fish livers and muscles were extracted according to matrix solid-phase dispersion method with some appropriate alternations (Villaverde-de-Sáa et al. 2013). The fish sample (0.5 g) was blended thoroughly with a known amount of 2,4,5,6-tetrachloro-m-xylene (TCmX) as a surrogate and 0.5 g octadecylsilane-bonded silica (C18) (SiliCycle Inc., Québec, Canada) as dispersion sorbent in a glass mortar using a glass pestle for 5 min to obtain a homogeneous mixture. The mixture was transferred into a polyethylene syringe barrel column (10 mL) which contained a membrane filter (0.22 μm). From bottom to top, the column was packed with neutral silica (0.5 g, 100–200 mesh, 180 °C 4 h activated), acidic silica (2.5, 30 % fume sulfuric acid, w/w), florisil (1.75 g, 60–100 mesh, 150 °C 10 h activated), acidic alumina (1.0 g, 150 °C 8 h and 5 % deactivated), and sample mixture. The other membrane filter was placed on the top. The packed column was eluted by 15 mL of dichloromethane flowing through at a rate of 2 mL min−1. Then, the eluent was dried with a gentle stream of high purity nitrogen followed by the redissolution of dry residue with 200 μL of n-hexane and addition of internal standard pentachloronitrobenzene (PCNB). The analytes were analyzed qualitatively with gas chromatography-mass spectrometer (GC-MS) and quantified with gas chromatography-electron capture detector (GC-ECD).
Heavy metals
The concentrations of eight heavy metals were determined in water, sediment, and fish including the muscle, liver, heart, brain, intestine, and bladder.
About 10 mL water sample was filtered under vacuum with 0.45-μm hydrophilic filters to get rid of particulates. Lyophilized sediment sample (0.1–0.2 g) and fish tissue samples (0.1–0.2 g) including the bladder, intestine, heart, brain, liver, and muscle were weighed directly into acid-washed Teflon digestion vessels. Eight milliliters of pure concentrated HNO3 and 2 mL of hydrogen peroxide (30 %) were added before microwave digestion. Each batch included a reagent blank and certified reference materials included NRCDOLT-3 (Dogfish liver) and GBW08573 (Yellow-fin tuna) (both from National Standard Material Center, Beijing, People’s Republic of China). Samples were digested for 30–40 min at 180 °C. After cooling for at least 1 h, the vessels were placed in a solvent evaporation system at a temperature of 140 °C for 1.5 h to remove most of the extractant. Following cooling for at least 1 h, the digested samples were transferred to 50-mL volumetric flasks, and the volume was made up to 50 mL with deionized water.
Lipid
The lipid content of the fish muscle was determined gravimetrically as described in a previous study with some modifications (Smedes 1999). Twenty milliliters water, cyclohexane, and 16 mL isopropyl alcohol were added to 2 g freeze-dried muscle powder, followed by ultrasonic extraction. The organic phase was collected after the mixture reached the statically separated equilibrium. The extraction was repeated by the addition of 3 mL isopropyl alcohol and 18 mL cyclohexane, and then, organic phase was combined and dried under a gentle nitrogen stream. The residue was weighed. The extraction steps of lipid content in the fish liver were similar to those in the fish muscle, but 0.5 g sample and half dosage of the solvent were used.
Experimental protocol and quality assurance and quality control (QA/QC)
For OCPs, the samples were analyzed on an Agilent 7890A gas chromatography equipped with an electron capture detector (GC-ECD) (Agilent Technologies, Santa Clara, CA, USA) and HP-5 capillary column (30 m × 0.25 mm i.d. × 0.25-μm film thickness, Agilent). Nitrogen was the carrier gas (1 mL min−1). Temperatures of the injector and detector were kept at 250 and 300 °C, respectively. Starting from 80 °C and holding for 1 min, the oven temperature was risen to 150 °C at the rate of 20 °C min−1 and finally to 300 °C (5 min hold) at the rate of 5 °C min−1. The splitless injection volume was 1 μL.
During analysis, the instruments were calibrated with calibration standards every day. A solvent blank and matrix blank were tested every 10 samples throughout whole procedure. Each sample was analyzed in duplicate. High, medium, and low levels of recoveries were analyzed during the experiment. The average recoveries of the surrogate standard TCmX were 80 ± 5 % in water samples and 73 ± 8 % in fish samples. The recoveries of OCPs ranged from 70 to 105 % in water samples and from 62 to 87 % in fish samples. The method detection limits (MDLs) were confirmed by the concentrations of analytes whose signal-to-noise (S/N) ratio was three and ranged from 0.15 to 0.30 ng L−1 in water samples and from 0.07 to 0.33 ng g−1 in fish samples. Those samples with concentrations detected less than MDLs were treated as not detected (nd).
For HMs, samples were analyzed for Cr, Ni, Cu, Zn, As, Cd, Hg, and Pb using an Inductively Coupled Plasma Mass Spectrometer (ICP-MS) (Thermo Electron Co. Ltd, USA). Results were quantified using an empirical calibration curve generated from the responses obtained from multiple dilutions of a mixed calibration standard prepared from individual element standards (GBW07401, obtained from Chinese Academy of Measurement Science). Analytical quality control was performed with 2 % ultrapure nitric acid blank and a procedural blank. The detection values of all elements were in good agreement with the certified values, with recoveries ranging from 83 to 105 %.
Data analysis
Spearman correlation test was performed by SPSS 16.0 for windows (IBM Corporation, USA). Biota-water accumulation factor (BAF) is calculated as shown in the following equation:
where C l is the pollutant concentration in fish (ng g−1) normalized by lipid content and c is pollutant concentration in water (ng L−1).
Risk assessment
In order to evaluate potential risk to human health of tested fishes, the estimate daily intake (EDI), the target hazard quotients (THQs), and carcinogenic risk ratio (R) were used in the risk assessment. THQ < 1 denotes that the daily exposure seems to cause no deleterious effects to human health and the acceptable level of R was in the range of 1 × 10−6–1 × 10−4 (Chien et al. 2002; Yu et al. 2014). The determination of THQ and R was referred to method described by Chien et al. and Yu et al., respectively (Chien et al. 2002; Yu et al. 2014). The calculation formulas of EDI, THQ, and R were listed as follows:
where
C = pollutant concentration in food (μg g−1), W F = daily average consumption of fish in this area (according to the population size and annual consumption of aquatic products in Wuhan, assuming 75 g day−1 person−1), and W AB = average body weight (70 kg for adults).
where
E F = exposure frequency (350 days year−1); E D = exposure duration (70 years); F IR = food ingestion rate (g person−1 day−1), R FD = oral reference dose (mg kg−1 day−1 (BW)), T A = average exposure time (365 days year−1 × lifetime, assuming 70 years), and SF = oral cancer slope factor (mg kg−1 day−1)−1. Concentrations used in risk calculations in the present study are all on a wet weight basis. Risk-based concentration table was used in the study to search for R FD and SF values (US EPA 2009).
Sometimes, the exposure is not only to one signal toxicant. When the toxicants are two or more, some additive and/or interactive effects may come up and the risk addition hypothesis is applicable to this situation. The hazard index (HI) is summation of the non-carcinogenic risks, and R T is the total carcinogenic risks. The calculation formulas of HI and R T cited from Yu et al. (2014) were listed as follows:
In addition, it has been assumed that the cooking process does not alter the available contaminant concentration into human bodies and the ingested pollutants are absorbed completely by the consumer.
Results and discussion
Concentrations of OCPs and HMs in water and sediment samples
All of the 11 OCPs were detected in water samples. The mean concentrations of ΣOCPs, ΣDDTs, and ΣHCHs were 16.75, 2.00, and 8.51 ng L−1, respectively (Table 2). Yang et al. also sampled water in the same positions in winter 2012 and tested OCPs (Yang et al. 2014). Research showed that water of the East Lake suffered more serious pollution compared with other lakes in the world. In comparison with their results (the total concentration of 12.38 ng L−1 of nine OCPs including α-HCH, β-HCH, γ-HCH, δ-HCH, p,p’-DDE, p,p’-DDD, p,p’-DDT, heptachlor, and heptachlor epoxide), the concentration of 13.79 ng L−1 of the nine OCPs in our research performed in summer 2013 was marginally higher. No obviously seasonal change trends of concentrations of individual OCP compound were found in this study. Pollution was mainly from the anthropogenic activities such as fishing in winter, recreational activities in summer, and lake tunnel construction recently.
Only four heavy metals were detectable in water samples with the mean concentration of 0.0076 mg L−1 for As, 0.00012 mg L−1 for Hg, 0.0012 mg L−1 for Ni, and 0.0078 mg L−1 for Zn, respectively (Table 3). The concentrations of heavy metals in Bozova of Atatürk Dam Lake (Turkey) which were 0.0154 mg L−1 for Ni, 0.025 mg L−1 for Cu, and 0.064 mg L−1 for Pb were higher than those in the present study (Karadede and Ünlü 2000); the heavy metals in Biétri Bay in Ebrié Lagoon, Ivory Coast, at rainy season were found at the levels of 0.2, 0.1, 3.53, 9.43, and 17.04 mg L−1 for Hg, Cd, Pb, Cu, and Zn, respectively, which were much more higher than those in the East Lake (Coulibaly et al. 2012); the mean concentrations of Cu, Cd, Cr, Ni, and Zn in the Taihu Lake (China) were 0.002876, 0.000047, 0.00129, 0.002443, and 0.008778 mg L−1, respectively, which were comparable with those in the present study (Tao et al. 2012). It was obvious that water in the East Lake suffered a slight contamination of heavy metals.
In sediment samples, the mean concentrations of As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn were 17.72, 0.43, 49.02, 49.89, 0.31, 41.6, 19.59, and 401.76 mg kg−1, respectively (Table 3). Compared with the results of Liu et al. in winter 2012, the concentrations of As, Cd, Cr, and Cu in this study were lower while the concentrations of Hg, Ni, Pb, and Zn were higher (Liu et al. 2014).
Concentrations and tissue distribution of OCPs and HMs in fish samples
As is shown in Table 2, 10 organochlorine pesticides were detected in fish samples except p,p’-DDT. ΣOCPs concentrations (wet weight, ww) ranged from 2.04 to 139.17 ng g−1 in the muscle samples and from 5.22 to 189.04 ng g−1 in the liver samples. The mean concentration of ΣDDTs detected in the present study was 3.43 ng g−1 in the bighead carp muscle which was lower than those reported before (Li et al. 2008; Wang et al. 2012) and 9.38 ng g−1 in the silver carp muscle which was higher than those in the Taihu Lake, Poyang Lake, and Lake Ziway in Ethiopia (Wang et al. 2012; Yohannes et al. 2014; Zhao et al. 2014) (Table S1). With respect to HCHs, the general concentration ranges were from 0.37 to 45.81 ng g−1 in the muscle samples and from 1.89 to 111.20 ng g−1 in the liver samples. Compared with those from other studies, the mean concentrations (5.38 ng g−1) in the bighead carp muscle and (7.17 ng g−1) in the silver carp muscle were higher than those from the Taihu Lake and Lake Ziway, but lower than those found in the Poyang Lake and Hemiculture leuciscultures from the Gaobeidian Lake (Table S1). Compared with fish collected from other water bodies, fish in the East Lake were moderately contaminated by OCPs.
A preferential accumulation in the muscle compared with the liver was observed for OCPs except β-HCH in bighead carp as well as p’p-DDE and β-HCH in silver carp (Table 2). Sun et al. reported 16 OCPs in different organs of silver carp, and the results revealed that the concentrations of OCPs in the muscle and liver were similar. HCHs in the liver were slightly more than those in the muscle, and DDTs in the muscle were marginally more abundant than those in the liver (Sun et al. 2005). A report from Miranda et al. was different: OCPs in the liver of Hoplias malabaricus were more than those in the muscle (Miranda et al. 2008). On the contrary, Svobodovà et al. observed more DDTs in the muscle of wels in contrast with the liver (Svobodova et al. 1995). Biotransformation of compounds was always conducted in the liver, and the main function of muscle was mobility (van der Oost et al. 2003). Different accumulation processes might occur in the two tissues which resulted in the difference of chemical accumulation.
As is shown in Table 3, eight elements were detectable in both bighead carp and silver carp except Hg in the intestine and brain of the two fishes. Average concentrations (dry weight, dw) of ΣHMs were 352.48 and 345.20 ng g−1 in bighead carp and silver carp, respectively (Table 1). Cu, Zn, and Cr were the predominant elements among eight elements. The general order of HMs in the muscle was Cd < Hg < Pb < Ni < As < Cu < Cr < Zn, in the liver was Hg < Ni < Pb ≈ Cd < As < Cr < Cu < Zn, in the bladder was Cd < Pb < As ≈ Hg < Ni < Cu < Zn < Cr, in the intestine was Hg < Cd < Pb < Ni < As < Cu < Cr < Zn, in the heart was Hg < Cd < Pb < As < Ni < Cu < Cr < Zn, and in the brain was Hg < Cd < Pb ≈ Ni < As < Cu < Cr < Zn. The results of the present study were compared with previous studies of HMs in freshwater fishes of different places and listed in Table S2. For instance, As was found at an average level of 0.39 mg kg−1 in the muscle in the present study which was comparable to that in the Danube River, Serbia (0.35 mg kg−1), almost three times of that in the Taihu Lake, Jiangsu, China (0.15 mg kg−1), but much lower than that in the Yangtze River, Nanjing, China (3.45 mg kg−1) (Fu et al. 2013; Hao et al. 2013). Hg levels found in the present study were one order of magnitude lower than those in most of other studies listed in Table S2. The contamination of HMs in fish in this area was at a low level.
Fish tissues, i.e., the muscle, liver, brain, heart, bladder, and intestine, were investigated for the tissue distribution of HMs. As could be seen from Fig. 2, Cu, Zn, and Cr were more than other elements in both two fishes. Numerous studies also reported Cu and Zn were the most abundant elements in fish (Ahmad et al. 2014; Djedjibegovic et al. 2012; Guerin et al. 2011; Turkmen et al. 2013). They were all essential elements for fish, particularly Cu participating in synthesis of several enzymes and hemoglobin (Sivaperumal et al. 2007). Accumulation of Zn trended to occur in more fatty tissue of fish, which could be verified by the concentrations of more fatty silver carp which were higher than those of bighead carp (Rahman et al. 2012). Except Hg, the other seven elements were more easily concentrated in the liver, intestine, and heart which were more metabolically active organs than the muscle and bladder. Many researchers considered that the liver and intestine had relative high potential for accumulation of HMs (Demirak et al. 2006; Karadede et al. 2004; Uysal et al. 2009; Yang et al. 2007; Yılmaz et al. 2007). This point was generally in accordance with our studies. The highest content of Hg was found in the bladder of both fishes. Bladder is an organ related to adjustment of specific gravity of fish, and both of the two fishes we studied are physostomous which means adjustment of specific gravity of fish is conducted by gas exchange with atmosphere. Some study has already reported that one important approach for Hg into the East Lake was by borne particulate in atmosphere, which indicated that the exposure of fish bladder to atmosphere containing Hg particulate might be the reason why bladder accumulated more Hg than other tissues (Su et al. 2004).
Potential sources of OCPs and HMs in water, sediment, and fish samples
Organochlorine pesticides
p,p’-DDE and p,p’-DDD were detectable in all of the samples, and p,p’-DDT was detected only in 12 of the 42 water samples. It is well known that DDT/(DDE + DDD) > 1 indicates new pollution sources which might be existed while DDT/(DDE + DDD) < 1 suggests historical use of DDT. Namely, the historical use of DDT was the main source of DDTs in water. However, the ratio of DDT/(DDE + DDD) exceeding 1 in 11 of the 42 water samples meant new input of DDT, which was consistent with the conclusions from Yang et al. (2014). Although the use of DDT has been prohibited since 1983 in China, some reports showed that the usage of DDT for malaria control was still present in our country (Qiu et al. 2005). p,p’-DDE was the most abundant in fish samples, where the similar results have also been documented in other studies (Eqani et al. 2013; Hu et al. 2010; Sharma et al. 2009). However, in the present study, the non-detectable levels of p,p’-DDT in both studied fish species might be due to low exposure level since bighead and silver carps are both at low trophic position and DDT was not detected in most of the water and sediment samples.
Gamma-HCH accounted for the most percentage of HCHs in muscle samples. However, in the liver samples, β-HCH accounted for the most percentage of HCHs. Technical HCH usually contains α-HCH (55–90 %), β-HCH (5–14 %), γ-HCH (8–15 %), and δ-HCH (2–16 %), while lindane contains more than 98 % of γ-HCH (Ge et al. 2013). Relative abundances of HCH congeners in fish samples are shown in Fig. 3. HCHs in surface water consisted of 15 % α-HCH, 39 % β-HCH, 11 % γ-HCH, and 35 % δ-HCH. The ratios of α-/γ-HCH varied from 0.74 to 1.87 indicating historical use of technical HCHs and lindane in this area, which was consistent with those from previous studies (Yang et al. 2014; Yun et al. 2014). The ratios of α/γ-HCH in all fish samples ranged from 0.58 to 1.89 indicating the same source of HCHs as that in water and sediments, which also confirmed that these two fishes were good bio-indicators for HCH pollution monitoring in the East Lake.
Regarding rest of the OCPs analyzed, heptachlor, heptachlor epoxide, aldrin, and methoxychlor contributed about one third of the total OCPs in fish. The ranges of concentration of heptachlor were 1.11–11.59, 1.9–27, and 0.14–14.54 ng L−1 in water, sediments, and fish samples, respectively, while concentrations of heptachlor epoxide (degradation product of heptachlor) were in the range of nd–0.30, nd–7.6, and nd–7.79 ng g−1 in water, sediments, and fish samples, respectively (Yun et al. 2014). Recent usage of heptachlor in the East Lake was revealed by the ratio of heptachlor to its degradation product. Aldrin accounted for about 7 % of total OCPs in fish in present study has never been produced and used on a large scale in China. The most probable source of aldrin in the East Lake might be the global migration (MEPC 2011).
Heavy metals
It has been reported that no point source pollution of HMs was found in the proximity of the East Lake (Liu et al. 2014). The most probable origins of HMs in the East Lake were natural and anthropogenic activities such as weathering; erosion of bed rocks; ore deposits; and wastewater from local residents, hospitals, and industries. Table 4 reports correlations between the eight elements in both fish and sediment samples listing Spearman’s rho correlation coefficients. Moderate correlations were found in three of the eight elements (Cd-Ni, Cd-Pb, Pb-Ni, p < 0.01) in fish. However, four of the eight elements (Cu-Cr, Cu-Ni, Cu-As, Cr-Ni, p < 0.01) were correlated significantly in sediment, which was different from that in fish. Therefore, the conclusion that two elements which present a correlated relationship had the same origin like many studies reported should not be made arbitrarily (Joksimovic et al. 2011; Rahman et al. 2012; Yang et al. 2007). There might be totally different patterns in sediment. Only four elements were detectable in water, so the data were not analyzed for Spearman’s correlation.
Bioaccumulation factors of OCPs in fish samples
The bioaccumulation factor (BAF) of fish is the ratio of concentration of a chemical inside the fish to the concentration of surrounding water. Due to the lipophilic characteristic of OCPs, the concentrations in organism needed to be normalized by lipid content. In all investigated samples, BAFs of methoxychlor, heptachlor epoxide, and p,p’-DDE were higher than those of the other seven chemicals, especially those BAFs of methoxychlor and heptachlor epoxide which were remarkably high. BAFs of the rest of the seven chemicals were fairly low or none (Fig. 4). Higher BAFs of the three chemicals might be ascribed to the relative high log K ow of them (Table 2). By comparing BAFs of the two fishes, a transparent trend could be obtained: BAFs in bighead carp were higher than those in silver carp. It is known that bioaccumulation of pollutants in fish was potentially controlled by many physiological and environmental elements such as trophic levels, age, lipid content of fish, environmental concentration of pollutants, and so on (Nichols et al. 1998; Russell et al. 1999). Two fishes (bighead carp and silver carp) had similar trophic level and size, so the possible effects induced by the two environmental elements could be eliminated (Table 1). In general, lipid content always correlated with concentration of hydrophobic chemicals positively. However, in our study, the lipid content of silver carp was almost two times of that in bighead carp while the BAFs of silver carp were lower than those of bighead carp. There were also some studies pointing out no relationships between lipid content and concentration of hydrophobic chemicals (Crimmins et al. 2002; Jackson et al. 2001). BAF is always assessed based on the hypothesis that is the sampled organisms are at steady state with surrounding water; however, the steady state is nearly impossible in natural environment. In addition, silver carp and bighead carp were all pelagic fishes which meant they could be more easily affected by dynamic water. Therefore, lipid content was not the main factor resulting in differences of BAFs of the two fishes and the difficulty for obtaining the actual concentration of pollutants in ambient water enlarged the error of BAFs.
Risk assessment of OCPs and HMs in the fish collected in the East Lake
One of major ways for contaminants into human body is fish consumption. EDIs were used to carry out the assessment of potential human exposure to OCPs and HMs by calculating with the mean concentration of target contaminants in fish muscle. Table 5 shows the comparison of our results with other prevailing criteria issued by different regulatory agencies. All of the values from the present study were several orders of magnitude lower than the listed standards except for Cr. For bighead carp, the EDI of Cr was 0.41, and for silver carp, was 0.31, which were almost half of 0.8 given by reference. The results implied that potential health risks related to daily intake of OCPs and HMs through consumption of bighead carp and silver carp were insignificant.
Table 6 lists the results of health risk assessment of the two fishes. The THQs were all less than 1.0. The THQs of As, Cr, Hg, aldrin, and heptachlor epoxide were one or two orders of magnitude higher than those of the other contaminants in which the THQ of As was the highest. The HI of OCPs which meant the non-carcinogenic risks were 0.19 for silver carp and 0.14 for bighead carp, lower than 1.0. All of the HIs of HMs were also lower than 1.0, which were 0.56 for silver carp and 0.43 for bighead carp, respectively. The HI of OCPs and HMs indicated lower health risk to human via fish consumption in study area. From another aspect, the R and R T of OCPs associated with fish consumption whose values were several orders of magnitude lower than 1 × 10−4 indicated that the cancer risk of exposure to these contaminants by fish consumption was negligible. Nevertheless, the Rs of As and Cr were all higher than 1 × 10−4 in both two fishes, which suggested that the cancer risk of As and Cr caused by fish consumption cannot be ignored.
Conclusions
Two commonly consumed fishes from Wuhan, China, were collected and analyzed for 11 organochlorine pesticides (OCPs) and eight heavy metals (HMs). The concentrations of OCPs and HMs in fish were all lower or slightly higher than those in other water bodies in the world. Bighead carp trended to accumulate more OCPs than silver carp, and a preferential accumulation in the muscle compared with the liver was observed for most OCPs. Zn as an essential element was most abundant in two fishes. The liver and intestine of the two fishes were proved to be target organs of HMs. The EDIs in our study were all lower than criteria issued by different regulatory agencies. The HI of OCPs and HMs were both lower than 1.0, which indicated lower non-carcinogenic risk to human via fish consumption in study area. Carcinogenic risks of exposure to OCPs by fish consumption were negligible, but carcinogenic risks of exposure to As and Cr were high. In addition, we assessed the consumption rate of fish at the upper band in the present study which meant the actual risks might be lower.
References
Ahmad K, Azizullah A, Shama S, Khattak MN (2014) Determination of heavy metal contents in water, sediments, and fish tissues of Shizothorax plagiostomus in river Panjkora at Lower Dir, Khyber Pakhtunkhwa, Pakistan. Environ Monit Assess 186:7357–7366
CE (2011) The Guidelines on metals and alloys used as food contact materials are part of the Council of Europe’s Policy Statements concerning materials and articles intended to come into contact with foodstuffs. In: Europe Co (Hrsg.), Strasbourg
Çelik U, Oehlenschläger J (2007) High contents of cadmium, lead, zinc and copper in popular fishery products sold in Turkish supermarkets. Food Control 18:258–261
Chien LC, Hung TC, Choang KY, Yeh CY, Meng PJ, Shieh MJ, Han BC (2002) Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci Total Environ 285:177–185
Coulibaly S, Atse BC, Koffi KM, Sylla S, Konan KJ (2012) Seasonal accumulations of some heavy metal in water, sediment and tissues of black-chinned tilapia Sarotherodon melanotheron from Biétri Bay in Ebrié Lagoon, Ivory Coast. B Environ Contam Toxicol 88:571–576
Crimmins BS, Brown PD, Kelso DP, Foster GD (2002) Bioaccumulation of PCBs in aquatic biota from a tidal freshwater marsh ecosystem. Arch Environ Contam Toxicol 42:396–404
Demirak A, Yilmaz F, Tuna AL, Ozdemir N (2006) Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere 63:1451–1458
Djedjibegovic J, Larssen T, Skrbo A, Marjanović A, Sober M (2012) Contents of cadmium, copper, mercury and lead in fish from the Neretva river (Bosnia and Herzegovina) determined by inductively coupled plasma mass spectrometry (ICP-MS). Food Chem 131:469–476
EFSA (2005) Opinion of the scientific panel in contaminants in the food chain on a request from the commission related to Gamma-HCH and other hexachlorocyclohexanes as undesirable substances in animal feed. EFSA J 250:1–39
Eqani SAMAS, Malik RN, Cincinelli A, Zhang G, Mohammad A, Qadir A, Rashid A, Bokhari H, Jones KC, Katsoyiannis A (2013) Uptake of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) by river water fish: the case of River Chenab. Sci Total Environ 450–451:83–91
Faroon O, Harris MO (2002) Toxicological profile for DDT, DDE, and DDD. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry
Fausch KD, Karr JR, Yant PR (1984) Regional application of an index of biotic integrity based on stream fish communities. Trans Am Fish Soc 113:39–55
Fu J, Hu X, Tao X, Yu H, Zhang X (2013) Risk and toxicity assessments of heavy metals in sediments and fishes from the Yangtze River and Taihu Lake, China. Chemosphere 93:1887–1895
Ge J, Woodward LA, Li QX, Wang J (2013) Composition, distribution and risk assessment of organochlorine pesticides in soils from the Midway Atoll, North Pacific Ocean. Sci Total Environ 452–453:421–426
Gobas FA, Wilcockson JB, Russell RW, Haffner GD (1999) Mechanism of biomagnification in fish under laboratory and field conditions. Environ Sci Technol 33:133–141
Guerin T, Chekri R, Vastel C, Sirot V, Volatier JL, Leblanc JC, Noel L (2011) Determination of 20 trace elements in fish and other seafood from the French market. Food Chem 127:934–942
Hale SE, Martin TJ, Goss K-U, Arp HPH, Werner D (2010) Partitioning of organochlorine pesticides from water to polyethylene passive samplers. Environ Pollut 158:2511–2517
Hao Y, Chen L, Zhang X, Zhang D, Zhang X, Yu Y, Fu J (2013) Trace elements in fish from Taihu Lake, China: levels, associated risks, and trophic transfer. Ecotox Environ Safe 90:89–97
Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE (2003) Fish and long-chain ω-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation 107:1852–1857
Hu G, Dai J, Mai B, Luo X, Cao H, Wang J, Li F, Xu M (2010) Concentrations and accumulation features of organochlorine pesticides in the Baiyangdian Lake freshwater food web of North China. Arch Environ Contam Toxicol 58:700–710
Jackson LJ, Carpenter SR, Manchester-Neesvig J, Stow CA (2001) PCB congeners in Lake Michigan coho (Oncorhynchus kisutch) and Chinook (Oncorhynchus tshawytscha) salmon. Environ Sci Technol 35:856–862
JECFA 2010 Seventy-second meeting, Rome, 16-25 February 2010: summary and conclusions. Rome: Joint FAO/WHO Expert Committee on Food Additives, JECFA/72/SC
Joksimovic D, Tomic I, Stankovic AR, Jovic M, Stankovic S (2011) Trace metal concentrations in Mediterranean blue mussel and surface sediments and evaluation of the mussels quality and possible risks of high human consumption. Food Chem 127:632–637
Karadede H, Ünlü E (2000) Concentrations of some heavy metals in water, sediment and fish species from the Atatürk Dam Lake (Euphrates), Turkey. Chemosphere 41:1371–1376
Karadede H, Oymak SA, Ünlü E (2004) Heavy metals in mullet, Liza abu, and catfish, Silurus triostegus, from the Atatürk Dam Lake (Euphrates), Turkey. Environ Int 30:183–188
King KA, Zaun BJ, Schotborgh HM, Hurt C, Jones CA (2003) DDE-induced eggshell thinning in white-faced ibis: a continuing problem in the western United States. Southwest Nat 48:356–364
Li X, Gan Y, Yang X, Zhou J, Dai J, Xu M (2008) Human health risk of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in edible fish from Huairou Reservoir and Gaobeidian Lake in Beijing, China. Food Chem 109:348–354
Liu M, Yang Y, Yun X, Zhang M, Li QX, Wang J (2014) Distribution and ecological assessment of heavy metals in surface sediments of the East Lake, China. Ecotoxicology 23:92–101
Longcore JR, Stendell RC (1977) Shell thinning and reproductive impairment in black ducks after cessation of DDE dosage. Arch Environ Contam Toxicol 6:293–304
Luckey TD, Venugopal B (1978) Metal toxicity in mammals. Plenum Press, New York
Ministry of Environment Protection of the People’s Republic of China (MEPC) (2011) The national directory of environmental pollutants health risks. China Environmental Science Press, Beijing, p 10 (in Chinese)
Miranda AL, Roche H, Randi MAF, Menezes ML, Ribeiro CAO (2008) Bioaccumulation of chlorinated pesticides and PCBs in the tropical freshwater fish Hoplias malabaricus: histopathological, physiological, and immunological findings. Environ Int 34:939–949
Misumi I, Vella AT, Leong J-AC, Nakanishi T, Schreck CB (2005) p, p’-DDE depresses the immune competence of chinook salmon (Oncorhynchus tshawytscha) leukocytes. Fish Shellfish Immun 19:97–114
Muir D, Sverko E (2006) Analytical methods for PCBs and organochlorine pesticides in environmental monitoring and surveillance: a critical appraisal. Anal Bioanal Chem 386:769–789
Nichols JW, Jensen KM, Tietge JE, Johnson RD (1998) Physiologically based toxicokinetic model for maternal transfer of 2,3,7,8-tetrachlorodibenzo-p-dioxin in brook trout (Salvelinus fontinalis). Environ Toxicol Chem 17:2422–2434
NRC (1989) Recommended dietary allowances. National Academy Press, Washington, pp 241–243
Qiu X, Zhu T, Yao B, Hu J, Hu S (2005) Contribution of dicofol to the current DDT pollution in China. Environ Sci Technol 39:4385–4390
Rahman MS, Islam MR (2009) Effects of pH on isotherms modeling for Cu (II) ions adsorption using maple wood sawdust. Chem Eng J 149:273–280
Rahman MS, Molla AH, Saha N, Rahman A (2012) Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem 134:1847–1854
Roset N, Grenouillet G, Goffaux D, Pont D, Kestemont P (2007) A review of existing fish assemblage indicators and methodologies. Fish Manag Ecol 14:393–405
Russell RW, Gobas FA, Haffner GD (1999) Maternal transfer and in ovo exposure of organochlorines in oviparous organisms: a model and field verification. Environ Sci Technol 33:416–420
Sharma CM, Rosseland BO, Almvik M, Eklo OM (2009) Bioaccumulation of organochlorine pollutants in the fish community in Lake Arungen, Norway. Environ Pollut 157:2452–2458
Shen L, Wania F (2005) Compilation, evaluation, and selection of physical-chemical property data for organochlorine pesticides. J Chem Eng Data 50:742–768
Sivaperumal P, Sankar TV, Nair PGV (2007) Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food Chem 102:612–620
Smedes F (1999) Determination of total lipid using non-chlorinated solvents. Analyst 124:1711–1718
Su Q, Jiang J, Ma Z (2004) Status of mercury contaminant of Wuhan urban lake. Geol Sci Technol Inf 23:83–88 (in Chinese)
Sun YZ, Wang XT, Li XH, Xu XB (2005) Distribution of persistent organochlorine pesticides in tissue/organ of silver carp (Hypophthalmichthys molitrix) from Guanting Reservoir, China. J Environ Sci-China 17:722–726
Svobodova Z, Piacka V, Vykusova B, Machova J, Hejtmanek M, Hrbkova M, Bastl J (1995) Residues of pollutants in siluriformes from various localities of the Czech-Republic. Acta Vet Brno 64:195–208
Tang Z, Yang Z, Shen Z, Niu J, Liao R (2007) Distribution and sources of organochlorine pesticides in sediments from typical catchment of the Yangtze River, China. Arch Environ Contam Toxicol 53:303–312
Tao Y, Yuan Z, Wei M, Xiaona H (2012) Characterization of heavy metals in water and sediments in Taihu Lake, China. Environ Monit Assess 184:4367–4382
Turkmen M, Tepe Y, Turkmen A, Kemal Sangun M, Ates A, Genc E (2013) Assessment of heavy metal contamination in various tissues of six ray species from Iskenderun Bay, northeastern Mediterranean Sea. B Environ Contam Toxicol 90:702–707
Uluozlu OD, Tuzen M, Mendil D, Soylak M (2007) Trace metal content in nine species of fish from the Black and Aegean Seas, Turkey. Food Chem 104:835–840
US EPA (2009) Risk-based concentration table. Philadelphia PA: United States Environmental Protection Agency, Washington
Uysal K, Kose E, Bulbul M, Donmez M, Erdogan Y, Koyun M, Omeroglu C, Ozmal F (2009) The comparison of heavy metal accumulation ratios of some fish species in Enne Dame Lake (Kutahya/Turkey). Environ Monit Assess 157:355–362
Van Ael E, Belpaire C, Breine J, Geeraerts C, Van Thuyne G, Eulaers I, Blust R, Bervoets L (2014) Are persistent organic pollutants and metals in eel muscle predictive for the ecological water quality? Environ Pollut 186:165–171
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Phar 13:57–149
Villaverde-de-Sáa E, Valls-Cantenys C, Quintana JB, Rodil R, Cela R (2013) Matrix solid-phase dispersion combined with gas chromatography–mass spectrometry for the determination of fifteen halogenated flame retardants in mollusks. J Chromatogr A 1300:85–94
Wang DQ, Yu YX, Zhang XY, Zhang SH, Pang YP, Zhang XL, Yu ZQ, Wu MH, Fu JM (2012) Polycyclic aromatic hydrocarbons and organochlorine pesticides in fish from Taihu Lake: their levels, sources, and biomagnification. Ecotox Environ Safe 82:63–70
WEPB (2014a) 2013 Wuhan Environmental Aspect Bulletin. Wuhan Environmental Protection Bureau
WEPB (2014b) List of the key enterprises of heavy metal emission in Wuhan in 2014. Wuhan Environmental Protection Bureau. http://www.whepb.gov.cn/zjSwrfkzdqymd/104912.jhtml
Xie L, Xie P, Ozawa K, Honma T, Yokoyama A, Park H-D (2004) Dynamics of microcystins-LR and-RR in the phytoplanktivorous silver carp in a sub-chronic toxicity experiment. Environ Pollut 127:431–439
Yang R, Yao T, Xu B, Jiang G, Xin X (2007) Accumulation features of organochlorine pesticides and heavy metals in fish from high mountain lakes and Lhasa River in the Tibetan Plateau. Environ Int 33:151–156
Yang Z, Shen Z, Gao F, Tang Z, Niu J (2009) Occurrence and possible sources of polychlorinated biphenyls in surface sediments from the Wuhan reach of the Yangtze River, China. Chemosphere 74:1522–1530
Yang Y, Yun X, Liu M, Jiang Y, Li QX, Wang J (2014) Concentrations, distributions, sources, and risk assessment of organochlorine pesticides in surface water of the East Lake, China. Environ Sci Pollut Res 21:3041–3050
Yılmaz F, Özdemir N, Demirak A, Tuna AL (2007) Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chem 100:830–835
Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SM, Ishizuka M (2014) Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a Rift Valley lake-Lake Ziway, Ethiopia. Ecotox Environ Safe 106:95–101
Yu YX, Zhang SH, Huang NB, Li JL, Pang YP, Zhang XY, Yu ZQ, Xu ZG (2012) Polybrominated diphenyl ethers and polychlorinated biphenyls in freshwater fish from Taihu Lake, China: their levels and the factors that influence biomagnification. Environ Toxicol Chem 31:542–549
Yu B, Wang Y, Zhou Q (2014) Human health risk assessment based on toxicity characteristic leaching procedure and simple bioaccessibility extraction test of toxic metals in urban street dust of Tianjin, china. PLoS One 9, e92459
Yun X, Yang Y, Liu M, Wang J (2014) Distribution and ecological risk assessment of organochlorine pesticides in surface sediments from the East Lake, China. Environ Sci Pollut Res 21:10368–10376
Zhao Z, Wang Y, Zhang L, Cai Y, Chen Y (2014) Bioaccumulation and tissue distribution of organochlorine pesticides (OCPs) in freshwater fishes: a case study performed in Poyang Lake, China’s largest lake. Environ Sci Pollut Res 21:8740–8749
Acknowledgments
This project was supported by Natural Science Key Foundation of Hubei Province of China (NO. 2014CFA114) and the Hundred Talents Program of the Chinese Academy of Sciences (Y329671K01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Cui, L., Ge, J., Zhu, Y. et al. Concentrations, bioaccumulation, and human health risk assessment of organochlorine pesticides and heavy metals in edible fish from Wuhan, China. Environ Sci Pollut Res 22, 15866–15879 (2015). https://doi.org/10.1007/s11356-015-4752-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4752-8