Abstract
To effectively improve soil productivity and optimize organic fertilizer management while reducing environmental pollution and resource wasting in farmland system, the present study was conducted in Wuqiao Experiment Station of China Agricultural University, Hebei Province. Taking crop straw treatment as control, four kinds of organic materials including pig manure (PM), biogas residue (BR), biochar (BC) and crop straw (ST) were applied to soil at the same nitrogen (N) level. The soil bacteria community characteristics were explored using Illumina Miseq high-throughput sequencing technologies. The results were as follows: (1) Compared with ST, PM, BR and BC had no significant effect on Chao 1 and Shannon index. The dominant bacterial groups include Proteobacteria, Acidobacteria, Actinobacteria, Bacteroidetes, and Chloroflexi in sandy loam soil after the application of different organic materials. The abundance of Proteobacteria in BC treatment was significantly lower than that of ST (control) treatment (p < 0.05). On the contrary, compared to ST, the abundances of Acidobacteria increased by 65.0, 40.7, and 58.7% in the BC, BR, and PM treatments, respectively. (2) Compared to ST, the BC treatment significantly (p < 0.05) increased in soil organic carbon (SOC) and pH in the arable layer (0–20 cm) in the farmland (p < 0.05), and significantly increased the soil pH with a value of 0.26 level (p < 0.05). (3) Pearson correlation analysis results showed that the PCoA1 scores and soil pH were closely correlated (R 2 = 0.3738, p < 0.05). In addition, pairwise regression between PCoA1 scores and SOC (R 2 = 0.5008, p < 0.05), PCoA2 scores and SOC (R 2 = 0.4053, p < 0.05) were both closely correlated. In general, our results indicated that organic materials amendment shaped the bacterial community in sandy loam soil through changing the soil pH and SOC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A large number of agricultural organic wastes are produced in China annually, which results in resource waste and environmental pollution due to unrecyclable utilization. It is estimated that 7.7 × 108 t/a crops straw, 4.0 × 109 t/a poultry and livestock manure, and over 1.3 × 109 t/a biogas slurry are produced in agricultural systems (Lu et al. 2010; Zhang et al. 2009). Previous studies show that organic wastes, such as crop straw, animal manure, and biogas residue, contain abundant nutrient elements (Bakht et al. 2009; Xun et al. 2016; Zhang et al. 2016; Alburquerque et al. 2012; Li et al. 2016a). Organic materials application on soil is regarded as an effective solution to circularly use the organic materials and avoid environmental pollution (Amusan et al. 2011; Lal 2004). In addition, many researchers utilize animal and plant residues or other biomass to generate highly aromatic carbon-rich solid-state biochar under the high-temperature pyrolysis carbonization with anoxic conditions. At present, about 32.2 million hectare of agricultural land is managed organically by using mainly organic materials globally (Thangarajan et al. 2013). Organic fertilizer amendments have complex effects on the soil physical, chemical, and biological environment. Importantly, microbes are responsible for the circulation of nutrient transformation, organic matter decomposing, humus formation, and system stability in the soil ecological function (Gougoulias et al. 2014; Mader et al. 2002). Consequently, soil microbial characteristics are considered as index for soil quality evaluation.
Bacteria play an important role in the maintenance of soil functions. They are immanent, possess enormous metabolic capabilities, and are indispensable to virtually all biogeochemical processes (Prosser et al. 2007). Agricultural fertilization practices have a great impact on soil bacterial community size and structure. The shifts of soil bacteria community following different agricultural management practices could help us to understand soil nutrient cycling processes. In recent years, some scholars have researched the soil microbial ecological changes under organic wastes application. For example, He et al. (2008) and Shen et al. (2010) have reported that, by using PCR-DGGE combined with cloning and sequencing in upland red clay of southeastern China and loamy brown earth of northeast China, respectively, manure amendment shows a dramatic shift in bacterial community when compared to straw amendment or mineral fertilizers. Li et al. (2016 b) have reported that biochar amendment has positive effects on shifting soil microbial community structure in a paddy field using PLFA. However, previous methods on soil microbial have some limitations which just focus on dominant bacteria in soils (Bossio and Scow 1998; Joseph et al. 2003; Smalla et al. 1998). Traditional methods to study soil microbes are greatly restricted, which mainly examines the cultivable microorganisms and advantage bacterium group in the soil. With the development of molecular biology, high-throughput sequencing truly illustrates the complexity and diversity of the microbial community in the soil environment, which has been considered as a better tool to evaluate soil microbial ecology (Shendure and Ji 2008). Illumina MiSeq sequencing has a power to deeply understand the bacterial community composition and diversity based on 16SrRNA gene libraries (Wang et al. 2015). The present study on bacteria community dynamics of organic material amendment in barren sandy soil is scares. The combination of modern microbiological techniques with the knowledge of soil ecological processes would provide a valuable opportunity to improve agronomical fertilization practices.
The shifts of soil bacteria community following different agricultural management practices could help us to understand soil nutrient cycling processes. The aim of this paper is to examine variation on bacterial communities correspond to organic materials amendment in barren sandy loam soil. Understanding the shift in microbial community composition and its relation to changes in soil microenvironment following fertilization could give a direction on the development of sustainable management practices for farmland ecosystem health. Particularly, the study on barren sandy soil is of profound connotation to fill the knowledge gap in the North China Plain.
Materials and methods
Study site
The experiment was carried out at Wuqiao Experimental Station of China Agricultural University, Hebei Province (37°41′02″N, 116°37′23″E). The region has a temperate monsoon climate with an annual average precipitation of 562 mm mainly occurring between June to August and a mean annual temperature of 12.9 °C. The basic soil properties of the studied sample were as follows: organic matter 2.52 g kg−1, total N 0.21 g kg−1, available P 4.86 g kg−1, available K 55.85 g kg−1, and pH 8.71.
Experiment design
Treatments were arranged in different plots with straw (ST), biogas residue (BR), pig manure (PM), and biochar (BC), with ST treatment as a control. Each treatment was replicated three times using a complete random design and the area of plot is 20.4 m2. The experiment was established in October 2012 in a wheat-maize rotation cropping system. The biochar was generated from wheat straw by a pyrolysis temperature of 500 °C. The chemical properties of the organic wastes are shown in Table 1.
The amount of organic materials was applied by the principles based on the wheat straw and maize straw returning with 7500 kg hm−2 (WST) and 9000 kg hm−2 (MST), respectively. Moreover, the quantity of C added in BC is equivalent to the total amount of C contained in the crop straw of ST treatment. Whereas, the PM and BR are half of ST, considering the low C/N of PM and BR, as well as the situation of the organic fertilizer application of field production. Commonly, nitrogen management affects carbon sequestration in cropland soils (Christopher and Lal 2007). In order to achieve maximum utilization efficiency of materials, the lack of nitrogen in material was supplement with urea when materials returned to the field. As the principle, four treatments were applied for the same amount of nitrogen decided by the highest nitrogen treatment (BR). Because of two season returning of ST treatment, the N of ST was applied in 6:5 ratios in wheat and maize season. To obtain high crop yields, all of the treatments (ST, BR, PM, BC) were incorporated with N 300 kg hm−1, P 150 kg hm−1, and K 150 kg hm−1 in both wheat and maize seasons, except for ST which applied a 6:5 N ratio. The specific experimental design is shown in Table 2.
Sample collection
Soil samples were collected at wheat mature stage in 2014. Five sites were randomly selected from each plot and then uniformly mixed to form a composite sample. Visible roots and residues were removed. A portion of soil was air dried to measure soil pH and soil organic carbon (SOC); the rest was used for microbial analysis.
Microbial analysis
Total DNA was extracted from 0.4 g of soil sample using a Quick Soil Isolation Kit (HuaYueYang, China). The 16S rRNA V3-V4 region was amplified using primers 338F: 5′-barcode-ATG CAG GGA CTA CHV GGG TWT CTA AT-3′ and 806R: 5′-barcode-ACT CCT ACG GGA GGC AGC A-3′ (Wang et al. 2015). Each sample had an eight-base Barcode. PCR reactions were performed in triplicate at a final volume of 20 μL containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of FastPfu Polymerase, and 10 ng of template DNA. Cycling conditions were as follow: 95 °C for 3 min, followed by 27 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s and a final extension at 72 °C for 5 min. Amplified products were extracted from 2% (w/v) agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, U.S.) following manufacturer’s instructions. Subsequently, purified products were quantified with a QuantiFluor™-ST fluorometer (Promega, U.S.). Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300 bp) on an Illumina MiSeq platform according to the standard protocols (Majorbio Bioinformatics Technology Co., China).
Raw fastq files were demultiplexed and quality filtered using QIIME (version 1.17) with the following criteria: (i) Set 10 bp as sliding window, and discard the truncated reads that were shorter than 50 bp if average quality score was < 20. (ii) An exact barcode match for the forward and reverse reads was required. The maximum number of primer mismatches was < 2 nucleotides per sequence. Reads containing ambiguous characters were removed. (iii) Sequences that overlap more than 10 bp were assembled. In addition, sequences which could not be spliced were removed. Operational taxonomic units (OTUs) were clustered at a 97% similarity cutoff using USEARCH (version 7.1). Chimeric sequences were identified and removed. OTUs were classified with RDP classifier (http://rdp.cme.msu.edu/) against the Silva 16SrRNA database using a confidence threshold of 70% (Wang et al. 2007; Quast et al. 2012).
Soil pH and soil organic carbon analyses
The SOC was measured using an exogenous thermal process with potassium dichromate (Walkley and Black 1934). The pH was measured using HANAN-HI-2221 (Mettler, Germany).
Data analysis
The results were initially collated with Excel 2010 (Microsoft, USA). All samples were normalized by rarefying to the minimum number of sequences (McMurdie and Holmes 2014). One-way ANOVA analysis was based on SPSS 20.0 for Windows (SPSS, USA). Multiple comparisons were made with Duncan’s test. The OTU dataset was used to calculate Good’s coverage, Chao 1, and Shannon-Weaver’s diversity indices using Mothur 1.30.1 (Schloss et al. 2011). Weighted UniFrac distances were calculated for the total community dataset using Qiime (Caporaso et al. 2010) and results were visualized by principal coordinate analysis (PCoA) using R 3.2.3. Linear regression was performed with R 3.2.3.
Results
Sequencing statistics
A total of 268,876 reads were obtained from the 12 samples with an average length of 468 bp. At 97% similarity cutoff, Good’s coverage indicated that sequences covered 95–96% of the bacteria in the soil samples. Following rarefaction, 3613 OTUs were obtained from all samples. All subsequent analyses were performed with the normalized data. Of these OTUs, all were classified as bacteria while 38 OTUs were not assigned to phylum level when grouped at the 97% similarity level. The Chao 1 and Shannon indices were estimated from the rarefied OTU dataset using Mothur 1.30.1. The Chao 1 and Shannon indices are used to reflect the microbial community. Compared to ST (control), PM, BR, and BC had no significant effect on OTU number, Chao 1, and Shannon index (Table 3). The results show that there was no significant difference in the community diversity among different treatments.
Bacterial community structure
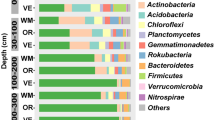
Classified OTUs belonged to 38 phyla among all sites. Bacterial communities in soils were dominated by Proteobacteria, Actinobacteria, Acidobacteria, Bacteroidetes, Chloroflexi, Gemmatimonadetes, Verrucomicrobia, Planctomycetes, and Nitrospirae (Fig. 1). These taxa accounted more than 88% of the bacterial sequences in all the treatments. The percentage of Proteobacteria in the sample was higher than other groups, representing 27.1–36.9% of retrieved sequences (Fig. 1). The abundance of Proteobacteria in BC treatment was significantly lower than that of ST treatment (Fig. 2a, p <0.05). Across all the treatments, there were no differences in Chloroflexi abundance (Fig. 2d, p >0.05), as well as Actinobacteria abundance (Fig. 2b, p >0.05). Remarkably, compared to ST, the abundances of Acidobacteria increased by 65.0, 34.9, and 40.7% in the BC, BR, and PM treatments, respectively (Fig. 2c).
Abundances of OTUs (97% identity, including phylum level classification) that data were mean of three replicates of composite samples. The dominant groups were chosen across treatments. The p value is for One-way ANOVA analysis. Different letters indicate significant differences (p < 0.05) across treatments according to Duncan’s multiple comparison
Beta diversity refers to the magnitude of changes in community composition in a region. Weighted UniFrac distances were used to estimate beta diversity between the different treatments. The PCoA ordination plot (Fig. 3) indicated that 44.0 and 19.0% of the variation in community composition were explained by the first two axes, respectively. As a result, samples within each treatment clustered tightly together, with the exception of PM2, and differentiated between treatments (Fig. 3). This suggests high similarity among the samples from same treatment but variations among samples from different treatments. Clusters between BC and ST treatments were situated further apart indicating that bacterial communities between the two treatments were dissimilar. Conversely, PM and BR treatment clusters were situated closely to each other suggesting more similar bacterial communities as compared with the other treatment clusters (Fig. 3). Thus, it can be seen that the response on soil bacteria community structure is discrepant after organic material amendment when compared to ST treatment.
Soil pH
Compared to ST, the soil pH of BR and PM had no significant change in the sandy loamy soil (Table 3, p < 0.05). However, the soil pH for BC significantly increased to 0.26 compared to ST (Table 3, p < 0.05). The relationship between bacterial communities and soil pH was confirmed using regression analysis. The PCoA1 scores and soil pH closely correlated (R 2 = 0.3738, p < 0.05); however, no significant correlation between PCoA2 scores and soil pH was obtained (Fig. 4b).
SOC
According to the results, the organic materials amendment improved SOC. Compared to straw (ST), biochar (BC) significantly increased SOC of the arable layer (0–20 cm) in the farmland (Table 3, p < 0.05). SOC distribution in the different treatments followed the order of BC > PM > BR > ST. Treatments increased when compared to the initial soil content (1.46 g kg−1). Specifically, the BC, BR, PM, and ST treatments enhanced SOC content to 1.78, 0.48, 0.47, and 0.24 g kg−1, respectively. Correlation analysis showed that changes in the bacterial communities along PCoA axis scores and SOC were closely correlated. Results for pairwise regression between PCoA1 scores and SOC (R 2 = 0.5008, p < 0.05), and PCoA2 scores and SOC (R 2 = 0.4053, p < 0.05) are presented in Fig. 5 (Fig. 5a and b).
Discussion
Soil microbial communities have higher species richness and are genetically more complex compared to other environmental microbial communities (Delmont et al. 2011). Soil microorganisms are important components of soil ecosystems, which are currently widely investigated in soil ecology research. This study attempted to use the V3-V4 region of the 16sRNA gene to assess the impact of organic materials amendment on soil bacterial communities. The V3-V4 region has been widely used to study bacterial diversity in a variety of ecosystems (Fu et al. 2015; Hong et al. 2015; Wang et al. 2015). The Illumina chemistry applied in this study provided a quantitative insight into the main bacterial groups. Furthermore, the technology generated high throughput and reproducibility data to monitor microbial communities and changes therein. The coverage index showed that sequences covered > 95% of the bacteria in the arable topsoil samples. A total of 3613 OTUs were assigned to phylum level, while 38 OTUs could not be classified. It is possible that these unclassified bacteria are members of the “rare biosphere” (Sogin et al. 2006; Staley et al. 2013). Diversity indices suggested that the application of different organic materials had no effect on soil bacterial community structures, which are in line with previous literature (Ahn et al. 2012; Yuan et al. 2013; Sun et al. 2015). These results may be partially explained by the presence of bacterial groups that were less sensitive to environmental changes.
In soil ecosystems, microbial populations perform various ecological functions including (i) soil structure and stability, (ii) recycling of nutrients, (iii) regulation of microclimate, (iv) local hydrological processes, (v) disease resistance, and (vi) carbon storage capacity (Altieri 1999; Li et al. 2016b). Microbial community is sensitive to the changes in soil nutrients, pH, and other external conditions, which respond to changes in soil quality rapidly (Qin et al. 2015). It is an important issue to compare the microbial community and estimate the discrepancy of microbial communities in many different research fields of ecology and microbiology (Lee and Paull 2005; Graham and Fine 2008; Jiang et al. 2013). The results of this study indicated that Proteobacteria, Actinobacteria, Acidobacteria, and Chloroflexi were the main bacterial groups, which correspond to previous studies of agricultural soils (Zhao et al. 2014; Chen et al. 2016). Chloroflexi had a relatively lower abundance, but were still within levels as reported by Poulsen et al. (2013) and Sengupta and Dick (2015). A possible explanation is that soil moisture may significantly impact this facultative anaerobic phylum. Proteobacteria were the most dominant group in the soil samples. It constitutes of gram-negative bacteria, which are sensitive to carbon sources and colonize nutrient-rich environments (Byss et al. 2008). Biochar (BC)-amended soil had a lower abundance of Proteobacteria compared to ST samples. This may be attributed to the impurity of carbon sources in biochar treatment/fertilizer, which in turn influenced the gram-negative bacteria (Lazcano et al. 2013). Also, higher abundance of Proteobacteria in ST samples is presumably related to the higher input of labile organic matter and readily available organic compounds. Increased abundance of soil gram-negative bacteria in relation to the use of organic fertilizers has previously been reported (Lazcano et al. 2013; Zhong et al. 2010).
Actinobacteria is considered to be ecologically important in the turnover of organic matter in soil because it is often associated with the degradation of recalcitrant polymers. Previous studies have shown that the abundance of Actinobacteria in biochar-amended soil increased (Kolton et al. 2011; O’neill et al. 2009; Xu et al. 2016) due to their ability to degrade recalcitrant carbon compounds. On the contrary, Mackie et al. (2015) found that biochar treatment significantly lowered Actinobacteria abundances compared to compost amendments. However, the results of this study showed there were no differences in Actinobacteria abundance with the addition of biochar. It is possible that certain species within the Actinobacteria taxa, which are more easily driven by labile C, became more abundant. Previous studies have demonstrated that Acidobacteria have a negative correlation with pH (Lauber et al. 2009; Rousk et al. 2010). The soil changes to a more alkaline environment with the addition of biochar, which is not favorable for Acidobacteria. However, in our study, the abundance of Acidobacteria increased with biochar addition. It is likely that more anfractuous factors, except for pH, impacted the relative of Acidobacteria. Regression analysis did not find a significant relationship between Acidobacteria and pH, which was corroborated by Poulsen et al. (2013).
Microbial communities are the core driving force of soil metabolism. Many researchers have studied the microbial ecological structure and purification function of organic materials amendment. After applying organic materials into the soil ecosystem, exogenous carbon would promote the strong activity of microbes in soil in the short term, which would cause a change in the quantity, activity, and population structure of microorganisms (Lazcano et al. 2013). UniFrac distances were calculated based on phylogenetic differences of microbial communities and subjected to principal coordinate analysis (PCoA). The PCoA plot indicated that the bacterial community structures of the soil were strongly influenced by organic materials amendment. These results are in agreement with previous studies on different fertilization regimes in agricultural soil (Poulsen et al. 2013; Xun et al. 2016). The effects of amendment on bacterial communities were evident within a short period after application. Similarly, Lazcano et al. (2013) found that fertilizer regions have an effect on soil microbial community structure and function. Bacteria are the most sensitive microbial group to different fertilizers because they have a much shorter turnover time than fungi and react faster to environmental changes in soil.
Many studies have shown that environmental factors determine the soil microbial community, especially soil pH which has been demonstrated to be the strongest factor in shaping microbial community structures in several studies (Fernández-Calviño and Bååth 2010; Lauber et al. 2009; Rousk et al. 2010). For instance, a study by Wakelin et al. (2008) found that soil pH was the dominant driver of microbial community structure in a range of Australian agricultural soils. Wu et al. (2009) have studied the soils from 14 geographic regions in east China through PLFA methods. The authors found that the microbial community structure was mainly influenced by soil pH. This pattern was robust across different spatial scales, soil types, and techniques (Lauber et al. 2009; Liu et al. 2015; Sun et al. 2015). Biochar contains a lot of K+ and Na+ base ions. These ions will exchange Al3+ and H+ from the soil and reduce the concentration of Al3+ and H+ (Wang et al. 2014). Thereby, the application of biochar increases soil pH. Overall, fertilization practices affect soil pH. PCoA1 scores and soil pH correlated significantly. This may be due to the material amendment that affected the soil pH, which in turn charged the soil bacterial community. A similar finding was demonstrated by Donnell et al. (2001) who observed that fertilization could change the soil pH leading to changes in the microbial community structure. It can thus be suggested that similar conditions caused changes in the bacterial communities in this study. It should be noted that the soil type was neutral or acidic in previous studies (He et al. 2008; Shen et al. 2010; Poulsen et al. 2013; Xun et al. 2016); whereas, in this study, soil samples were alkaline. Our results emphasized that soil pH plays an important role in shifting the bacterial community composition in organic material amendment soils.
The effects of fertilizer on soil microorganisms are vital to understand how soil fertility changes and what drives variation between different soil ecosystems. Organic compounds in soil not only provide an active substrate for microbial growth but also improve the soil fertility through microbial metabolism. The SOC is an important index to measure the level of soil fertility. This study showed that organic wastes amendment increase soil organic carbon when compared to the initial value (1.46 g kg−1), especially in biochar amendment. This is in good agreement with previous findings (Calderón et al. 2015; Herath et al. 2015). The increase in SOC may be related to biochar’s thermal chemical stability, its highly condensed aromatic structures, and low solubility that resist biochemical degradation (Singh et al. 2010). Therefore, the lower presence of readily metabolizable C in biochar appears to be responsible for the stronger short-term effects on the structure of the SOC accumulation. The efficiency of manures in increasing the levels of SOC has been well documented (Ding et al. 2012; Gong et al. 2009; Potter et al. 1998). The increase in SOC with the addition of the manure and biogas residue could have resulted due to any of the following mechanisms or combinations: (i) manure and biogas residue augmented products of lignin and lignin-like, which causes them have stronger biological chemical resistance than straw (contains cellulose) (Rovira and Vallejo 2002; Schmidt et al. 2011; Long et al. 2015); (ii) pig manure and biogas residue show high proportions of water-soluble C and easily biodegradable organic compounds (Yanardağ et al. 2015), which may be responsible for microbial growth and rapid transformation of energy and matter; (iii) pig manures that have a better promoting effect on crop growth which result in input of the soil SOC from crop root and stubble (Ding et al. 2012). The correlation analysis showed that bacterial community structures were also influenced by SOC content. This is consistent with previous studies (Guo et al. 2015; Li et al. 2015; Sul et al. 2013). Currently, Zhang et al. (2015) discovered that SOC accumulation is relevant to bacterial community dynamics in sandy loam soil. Similar findings were also reported in hyper saline soils, sediment soils, and black soil (Hollister et al. 2010; Liu et al. 2014). Consequently, the results of the present study show that organic material amendments have a significant short-term impact on the soil microbial community. It is possible that organic materials amendment changed the SOC content, which affected the soil bacterial community structure. In contrast, previous research demonstrated that short-term fertilization did not change the soil microbial community structure. It is likely that the soil nutrient content in this study was extremely low and that available carbon and nitrogen mainly came from foreign material. In addition, previous studies have indicated that soil humidity, soil temperature, and seasons also affect bacterial community structure (Hollister et al. 2010; Sengupta and Dick 2015). Therefore, the influence of organic materials amendment on soil microbial ecology requires future research.
Conclusions
This study focuses on the influence of different organic materials amendment on soil community structures. A large number of sequences, which represented most of the bacteria in the soil, were obtained through high-throughput sequencing method. The results confirmed that there were no significant differences in the levels of bacterial diversity of all treatments. Compared to the traditional straw returning, the bacterial diversity was not affected by the biogas residue and manure and biochar amendment in barren sandy loam soil. However, biochar amendment decreased the relative abundance of Proteobacteria and increased the relative abundance of Acidobacteria. Biochar amendment changed the soil micro-ecological environments, which in turn caused the bacterial community structure to change. The results of the present study showed that biochar amendment have a short-term impact on the soil bacterial community structure. To improve soil quality while reducing environmental impacts, more research is required to understand the association between soil microbial processes and nutrient cycling.
References
Ahn JH, Song J, Kim BY, Kim MS, Joa JH, Weon HY (2012) Characterization of the bacterial and archaeal communities in rice field soils subjected to long-term fertilization practices. J Microbiol 50:754–765
Alburquerque JA, Fuente C, Ferrer-Costa A, Carrasco L, Cegarra J, Abad M, Bernal MP (2012) Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 40:181–189
Altieri MA (1999) The ecological role of biodiversity in agroecosystems. Agric Ecosyst Environ 74:19–31
Amusan AO, Adetunji MT, Azeez JO, Bodunde JG (2011) Effect of the integrated use of legume residue, poultry manure and inorganic fertilizers on maize yield, nutrient uptake and soil properties. Nutr Cycl Agroecosyst 90:321–330
Bakht J, Shafi M, Jan MT, Shah Z (2009) Influence of crop residue management, cropping system and N fertilizer on soil N and C dynamics and sustainable wheat (Triticum aestivum L.) production. Soil Tillage Res 104:233–240
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Byss M, Elhottová D, Tříska J, Baldrian P (2008) Fungal bioremediation of the creosote-contaminated soil: influence of Pleurotus ostreatus and Irpex lacteus on polycyclic aromatic hydrocarbons removal and soil microbial community composition in the laboratory-scale study. Chemosphere 73:1518–1523
Calderón FJ, Benjamin J, Vigil MF (2015) A comparison of corn (Zea mays L.) residue and its biochar on soil C and plant growth. PLoS One 10:e121006
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Huttley GA (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Chen C, Zhang J, Lu M, Qin C, Chen Y, Yang L, Huang Q, Wang J, Shen Z, Shen Q (2016) Microbial communities of an arable soil treated for 8 years with organic and inorganic fertilizers. Biol Fertil Soils 52:455–467
Christopher SF, Lal R (2007) Nitrogen management affects carbon sequestration in North American cropland soils. Crit Rev Plant Sci 26:45–64
Delmont TO, Robe P, Cecillon S, Clark IM, Constancias F, Simonet P, Hirsch PR, Vogel TM (2011) Accessing the soil metagenome for studies of microbial diversity. Appl Environ Microbiol 77:1315–1324
Ding X, Han X, Liang Y, Qiao Y, Li L, Li N (2012) Changes in soil organic carbon pools after 10 years of continuous manuring combined with chemical fertilizer in a Mollisol in China. Soil Tillage Res 122:36–41
Donnell AG, Seasman M, Macrae A, Waite I, Davies JT (2001) Plants and fertilisers as drivers of change in microbial community structure and function in soils. Plant Soil 232:135–145
Fernández-Calviño D, Bååth E (2010) Growth response of the bacterial community to pH in soils differing in pH. FEMS Microbiol Ecol 73:149–156
Fu Y, Li X, Zheng S, Du J, Liang A (2015) Classification and identification of bacteria in the soil treated by AcMNPV using high-throughput sequencing technique. Biotechnol Bioprocess Eng 20:931–936
Gong W, Yan X, Wang J, Hu T, Gong Y (2009) Long-term manuring and fertilization effects on soil organic carbon pools under a wheat-maize cropping system in North China plain. Plant Soil 314:67–76
Gougoulias C, Clark JM, Shaw LJ (2014) The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J Sci Food Agric 94:2362–2371
Graham CH, Fine PV (2008) Phylogenetic beta diversity: linking ecological and evolutionary processes across space in time. Ecol Lett 11:1265–1277
Guo L, Zhang Z, Wang D, Li C, Cao C (2015) Effects of short-term conservation management practices on soil organic carbon fractions and microbial community composition under a rice-wheat rotation system. Biol Fertil Soils 51:65–75
He JZ, Zheng Y, Chen CR, He YQ, Zhang LM (2008) Microbial composition and diversity of an upland red soil under long-term fertilization treatments as revealed by culture-dependent and culture-independent approaches. J Soils Sediments 8:349–358
Herath H, Camps-Arbestain M, Hedley MJ, Kirschbaum M, Wang T, Hale R (2015) Experimental evidence for sequestering C with biochar by avoidance of CO2 emissions from original feedstock and protection of native soil organic matter. GCB Bioenergy 7:512–526
Hollister EB, Engledow AS, Hammett AJ, Provin TL, Wilkinson HH, Gentry TJ (2010) Shifts in microbial community structure along an ecological gradient of hypersaline soils and sediments. ISME J 4:829–838
Hong C, Si Y, Xing Y, Li Y (2015) Illumina MiSeq sequencing investigation on the contrasting soil bacterial community structures in different iron mining areas. Environ Sci Pollut Res 22:10788–10799
Jiang XT, Peng X, Deng GH, Sheng HF, Wang Y, Zhou HW, Tam N (2013) Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microb Ecol 66:96–104
Joseph SJ, Hugenholtz P, Sangwan P, Osborne CA, Janssen PH (2003) Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl Environ Microbiol 69:7210–7215
Kolton M, Harel YM, Pasternak Z, Graber ER, Elad Y, Cytryn E (2011) Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl Environ Microbiol 77(14):4924–4930
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304:1623–1627
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
Lazcano C, Gómez-Brandón M, Revilla P, Domínguez J (2013) Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol Fertil Soils 49:723–733
Lee JH, Paull TT (2005) ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308:551–554
Li J, Li YT, Yang XD, Zhang JJ, Lin ZA, Zhao BQ (2015) Microbial community structure and functional metabolic diversity are associated with organic carbon availability in an agricultural soil. J Integr Agric 14:2500–2511
Li X, Guo J, Dong R, Ahring BK, Zhang W (2016a) Properties of plant nutrient: comparison of two nutrient recovery techniques using liquid fraction of digestate from anaerobic digester treating pig manure. Sci Total Environ 544:774–781
Li M, Liu M, Li ZP, Jiang CY, Wu M (2016b) Soil N transformation and microbial community structure as affected by adding biochar to a paddy soil of subtropical China. J Integr Agric 15:209–219
Liu J, Sui Y, Yu Z, Shi Y, Chu H, Jin J, Liu X, Wang G (2014) High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol Biochem 70:113–122
Liu S, Ren H, Shen L, Lou L, Tian G, Zheng P, Hu B (2015) pH levels drive bacterial community structure in sediments of the Qiantang River as determined by 454 pyrosequencing. Front Microbiol 6:285
Long P, Sui P, Gao WS, Wang BB, Yan LL, Xing Y, Chen YQ (2015) Effects of agriculrural organic wastes incorporation on soil organic carbon and microbial carbon. J China Agric Univ 20:153–160
Lu J, Zhu L, Hu G, Wu J (2010) Integrating animal manure-based bioenergy production with invasive species control: a case study at Tongren Pig Farm in China. Biomass Bioenergy 34:821–827
Mackie KA, Marhan S, Ditterich F (2015) The effects of biochar and compost amendments on copper immobilization and soil microorganisms in a temperate vineyard. Agriculture, Ecosystems & Environment 201:58–69.
Mader P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697
McMurdie PJ, Holmes S (2014) Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol 10(4):e1003531
O’neill B, Grossman J, Tsai MT, Gomes JE, Lehmann J, Peterson J, Thies JE (2009) Bacterial community composition in Brazilian anthrosols and adjacent soils characterized using culturing and molecular identification. Microb Ecol 58(1):23–35
Potter KN, Torbert HA, Jones OR, Matocha JE, Morrison JE, Unger PW (1998) Distribution and amount of soil organic C in long-term management systems in Texas. Soil Tillage Res 47:309–321
Poulsen PH, Al-Soud WA, Bergmark L, Magid J, Hansen LH, Sørensen SJ (2013) Effects of fertilization with urban and agricultural organic wastes in a field trial-Prokaryotic diversity investigated by pyrosequencing. Soil Biol Biochem 57:784–793
Prosser JI, Bohannan BJ, Curtis TP, Ellis RJ, Firestone MK, Freckleton RP, Green JL, Green LE, Killham K, Lennon JJ, Osborn AM (2007) The role of ecological theory in microbial ecology. Nat Rev Microbiol 5:384–392
Qin J, Jiang X, Zhou J, Ma MC, Guan DW, Zhou BK, Zhao BS, Du BH, Li J (2015) Characteristics and driving factors of soil bacterial and archaeal communities under long-term fertilization regimes in black soil. J Plant Nutr Fertil 21:1590–1598 (in Chinese)
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351
Rovira P, Vallejo VR (2002) Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: an acid hydrolysis approach. Geoderma 107:109–141
Schloss PD, Gevers D, Westcott SL (2011) Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One 6:e27310
Schmidt MW, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DA, Nannipieri P (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Sengupta A, Dick WA (2015) Bacterial Community Diversity in Soil Under two Tillage Practices as Determined by Pyrosequencing. Microbial Ecology 70:853–859.
Shen JP, Zhang LM, Guo JF, Ray JL, He JZ (2010) Impact of long-term fertilization practices on the abundance and composition of soil bacterial communities in Northeast China. Appl Soil Ecol 46:119–124
Shendure J, Ji H (2008) Next-generation DNA sequencing. Nat Biotechnol 26:1135–1145
Singh BP, Hatton BJ, Singh B, Cowie AL, Kathuria A (2010) Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J Environ Qual 39:1224–1235
Smalla K, Wachtendorf U, Heuer H, Liu WT, Forney L (1998) Analysis of BIOLOG GN substrate utilization patterns by microbial communities. Appl Environ Microbiol 64:1220–1225
Sogin ML, Morrison HG, Huber JA, Welch DM, Huse SM, Neal PR, Herndl GJ (2006) Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc Natl Acad Sci 103(32):12115–12120
Staley C, Unno T, Gould TJ, Jarvis B, Phillips J, Cotner JB, Sadowsky MJ (2013) Application of Illumina next-generation sequencing to characterize the bacterial community of the Upper Mississippi River. J Appl Microbiol 115(5):1147–1158
Sul WJ, Asuming-Brempong S, Wang Q, Tourlousse DM, Penton CR, Deng Y, Rodrigues J, Adiku S, Jones JW, Zhou J, Cole JR, Tiedje JM (2013) Tropical agricultural land management influences on soil microbial communities through its effect on soil organic carbon. Soil Biol Biochem 65:33–38
Sun R, Zhang X, Guo X, Wang D, Chu H (2015) Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol Biochem 88:9–18
Thangarajan R, Bolan NS, Tian G, Naidu R, Kunhikrishnan A (2013) Role of organic amendment application on greenhouse gas emission from soil. Sci Total Environ 465:72–96
Wakelin SA, Macdonald LM, Rogers SL, Gregg AL, Bolger TP, Baldock JA (2008) Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils. Soil Biol Biochem 40:803–813
Walkley A, Black LA (1934) An examination of the method for determining soil organic matter, and a proposed modification of the chromic acidtitration method. Soil Sci 37:29–38
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Wang L, Butterly CR, Wang Y, Herath H, Xi YG, Xiao XJ (2014) Effect of crop residue biochar on soil acidity amelioration in strongly acidic tea garden soils. Soil Use Manag 30:119–128
Wang ZG, Hu YL, Xu WH, Liu S, Hu Y, Zhang Y (2015) Impacts of dimethyl phthalate on the bacterial community and functions in black soils. Front Microbiol 6
Wu Y, Ma B, Zhou L, Wang H, Xu J, Kemmitt S, Brookes PC (2009) Changes in the soil microbial community structure with latitude in eastern China, based on phospholipid fatty acid analysis. Appl Soil Ecol 43:234–240
Xu N, Tan G, Wang H, Gai X (2016) Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol 74:1–8
Xun W, Xiong W, Huang T, Ran W, Li D, Shen Q, Li Q, Zhang R (2016) Swine manure and quicklime have different impacts on chemical properties and composition of bacterial communities of an acidic soil. Appl Soil Ecol 100:38–44
Yanardağ İH, Zornoza R, Cano AF, Yanardağ AB, Mermut AR (2015) Evaluation of carbon and nitrogen dynamics in different soil types amended with pig slurry, pig manure and its biochar by chemical and thermogravimetric analysis. Biol Fertil Soils 5:183–196
Yuan H, Ge T, Zhou P, Liu S, Roberts P, Zhu H, Zou Z, Tong C, Wu J (2013) Soil microbial biomass and bacterial and fungal community structures responses to long-term fertilization in paddy soils. J Soils Sediments 13:877–886
Zhang PD, Yang Y, Tian YS, Yang XT, Zhang YK, Zheng YH, Wamg LS (2009) Bioenergy industries development in China: dilemma and solution. Renew Sust Energ Rev 13:2571–2579
Zhang H, Ding W, Yu H, He X (2015) Linking organic carbon accumulation to microbial community dynamics in a sandy loam soil: result of 20 years compost and inorganic fertilizers repeated application experiment. Biol Fertil Soils 51:137–150
Zhang P, Chen X, Wei T, Yang Z, Jia Z, Yang B, Han Q, Ren X (2016) Effects of straw incorporation on the soil nutrient contents, enzyme activities, and crop yield in a semiarid region of China. Soil Tillage Res 160:65–72
Zhao J, Ni T, Li Y, Xiong W, Ran W, Shen B, Shen Q, Zhang R (2014) Responses of bacterial communities in arable soils in a rice-wheat cropping system to different fertilizer regimes and sampling times. PLoS One 9:e85301
Zhong W, Gu T, Wang W, Zhang B, Lin X, Huang Q, Shen W (2010) The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 326:511–522
Acknowledgements
This study is supported by the National Science and Technology Research Projects of China (2012BAD14B03) in the 12th 5-year plan period.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Dai, H., Chen, Y., Yang, X. et al. The effect of different organic materials amendment on soil bacteria communities in barren sandy loam soil. Environ Sci Pollut Res 24, 24019–24028 (2017). https://doi.org/10.1007/s11356-017-0031-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0031-1