Abstract
Repeated compost or inorganic fertilization may increase soil organic C (SOC) but how SOC accumulation relates to changes in soil aggregation, microenvironment and microbial community structure is unclear. Arable soils (Aquic Inceptisol) following a 20-year (1989–2009) application of inorganic fertilizer nitrogen (N), phosphorus (P) and potassium (K) (NPK), fertilizer NP (NP), fertilizer NK (NK), fertilizer PK (PK), compost (CM), half compost N plus half fertilizer N (HCM), and non-fertilization (Control) were collected to evaluate the relationship between SOC accumulation rate, soil aggregation, microenvironment and microbial community composition using phospholipid fatty acid (PLFA) analysis. Compared to the starting year, SOC content after 20 years under CM, HCM and NPK was significantly (P < 0.05) increased by 172 %, 107 % and 56 %, respectively, and by less than 50 % under NP, NK and PK. The mass proportion of macroaggregates was increased by 101–250 % under CM, but was not significantly affected by inorganic fertilizations, except PK. Compost and NPK significantly (P < 0.05) reduced the effective diffusion coefficient of oxygen primarily by increasing the proportion of pores <4 μm, and in contrast, increased the abundance of branched PLFAs and Gram-positive (G+) bacteria, resulting in the reduction of the ratio of monounsaturated/branched PLFAs (M/B) compared with Control. The mass proportion of macroaggregates was significantly (P < 0.01) and negatively correlated with the effective diffusion coefficient of oxygen; the latter was positively associated with M/B ratio. The SOC accumulation rate (z) had a significant interaction with the mass proportion of macroaggregates (x) and M/B ratio (y) (z = 0.514 + 4.345ex-15–0.149ey). Our results suggested that SOC accumulation promoted the macroaggregation and reduced the effective diffusion coefficient of oxygen, causing changes in microhabitats and a shift in microbial community composition to more facultative and/or obligate anaerobes; such microbial community shifts favored accumulation of SOC in turn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concerns about long-term agricultural sustainability and the high environmental costs of conventional cropping practices have made it imperative that reasonable soil and crop management should be developed to enhance C sequestration and improve soil quality, while mitigating global greenhouse gas emissions (Kong et al. 2011). Soil organic C (SOC) content could be more efficiently increased by organic compost application, either alone or in combination with inorganic fertilizers, than inorganic fertilizer application alone (Rudrappa et al. 2006). Fertilization could also promote aggregate formation and stabilization, and enhance the physical inaccessibility of organic C for decomposing microorganisms (Jagadamma et al. 2014). In addition, changes of aggregate formation under different management regimes can influence the soil microenvironment and, consequently, the function of the microbial communities in soil (Kong et al. 2011). In turn, microorganisms strongly influence a wide range of soil processes, and their abundance and composition in soil are being increasingly recognized as crucial for determining C cycle processes such as organic C turnover, stabilization, and accumulation in soil (Bossio et al. 1998; Sun et al. 2004; Denef et al. 2009). For example, soil microbial communities dominated by fungi have greater capability to degrade recalcitrant organic C like lignin and cellulose than soils dominated by bacterial communities because fungi release a broader range of extracellular enzymes (Moore-Kucera and Dick 2008).
Application of organic or inorganic fertilizers could affect the abundance, composition, diversity, and functioning of microorganisms in soils (Marschner et al. 2003; Stark et al. 2007). Organic manure tends to increase the total microbial biomass mainly through increasing the abundance of Gram-positive (G+) and Gram-negative (G−) bacteria (Sun et al. 2004). Bossio et al. (1998) and Cookson et al. (2005) found that fertilization, regardless of the type of fertilizer, stimulated the growth of G+ bacteria. In contrast, a long-term experiment in Tennessee showed that continuous 5-year application of organic manure at the rate of 252 and 504 kg N ha−1 increased the abundance of G− bacteria by 15 and 27 %, respectively (Peacock et al. 2001), and variations in microbial community composition were primarily related to the quantity and quality of organic materials added (Elfstrand et al. 2007). On the other hand, applications of inorganic fertilizers had exerted less effect (positive or negative) on the abundance of bacteria in soils compared with organic fertilizer (Sarathchandra et al. 2001; Plaza et al. 2004).
Fungi play an important role in the breakdown of recalcitrant organic matter in soil (Moore-Kucera and Dick 2008). Fungal biomass is generally low in soils compared with bacteria (Sessitsch et al. 2001) and decreased in agricultural soils treated with organic manure or inorganic fertilizer (Bittman et al. 2005). In contrast, Elfstrand et al. (2007) observed that organic manure increased fungal abundance particularly that of arbuscular mycorrhizal fungi, and de Vries et al. (2007) reported that the fungi/bacteria ratio was higher in organic manure treatment. The abundance of actinobacteria, which are well-adapted to metabolize old soil organic matter, is also decreased by the application of manure but may be stimulated by addition of inorganic N (Peacock et al. 2001). These findings indicate that inorganic and organic fertilizers may exhibit different effects on the abundance and composition of microbial communities, resulting in different levels of organic C accumulation in soil. Understanding the shift of microbial community composition and its relation to changes in soil microenvironment and SOC accumulation rates following long-term fertilization could guide development of sustainable management practices for enhancing SOC sequestration and crop production (Dolfing et al. 2004).
The North China Plain is one of the most important agricultural regions in China. In 1989, a long-term field experiment was established to evaluate the effect of compost and inorganic fertilizer on SOC content and crop productivity. Previous studies from this site have shown that the long-term application of compost or inorganic fertilizer significantly increased the activities of enzymes and SOC content, with higher effects with compost addition (Cai and Qin 2006; Yu et al. 2012a). Crop yields were also significantly improved by compost or inorganic fertilizers application, except inorganic NK fertilizer treatment (Cai and Qin 2006). In addition, soil aggregate structure can be changed by long-term fertilization (Majumder et al. 2010). However, there is a lack of understanding of the effects of long-term fertilization on the abundance and community composition of microorganisms and the relationship of changes in the composition of soil microbial communities with soil microenvironment and the accumulation rate of SOC. We hypothesized that long-term fertilization can influence soil microbial community composition through altering SOC content and soil aggregate structure and consequently oxygen diffusion coefficient (Kong et al. 2005; Hansel et al. 2008), which would in turn affect accumulation of SOC.
In this study, phospholipid fatty acids (PLFAs) were used as biomarkers for various microbial groups to evaluate the variation in the abundance and composition of microbial communities since phospholipids are essential components of cell membranes (Bossio et al. 1998; Peacock et al. 2001). We also measured soil water retention curves and calculated soil pore size and oxygen effective diffusion coefficient under different fertilizations. This study had two objectives: (1) to understand how a 20-year application of compost and inorganic fertilizers affects soil organic C and the abundance and composition of microbial communities, and (2) to evaluate the relationships between organic C accumulation and shifts of the microbial community composition in soils.
Materials and methods
Field experiment and soil sampling, processing, and basic property analyses
A long-term field experiment was established since September 1989 in the Fengqiu Agro-ecological Experimental Station (35°00′N, 114°24′E, ∼70 m above sea level), Chinese Academy of Sciences, Henan, China. The soil with a sandy loam texture is developed from alluvial sediments of the Yellow River and classified as an Aquic Inceptisol (USDA 1994). In the start of the experiment, soil had been cultivated for >50 years in a similar agricultural cropping system, so the heterogeneity of soil fertility was considered to be minimal. In 1989, soil had an average pH of 8.65, 4.48 g organic C kg−1, 0.43 g total N kg−1, 0.50 g total P kg−1, and 18.60 g total K kg−1.
Seven treatments (Table 1) with four replicate plots each were examined in this experiment: (1) compost (CM), (2) half organic compost N plus half inorganic N (HCM), (3) inorganic NPK (NPK), (4) inorganic NP (NP), (5) inorganic NK (NK), (6) inorganic PK (PK), and (7) no-fertilization (Control). The total N, P, and/or K across all treatments were applied in equal amounts (Table 1). The size of each plot was 9.5 m × 5 m. All plots were arranged in a randomized block design. The compost used was composed of wheat straw, oil cake, and cotton cake with a C/N ratio ≈ 8 (Meng et al. 2005), and contained 422 g C kg−1, 54 g N kg−1, 18 g P2O5 kg−1, and 24 g K2O kg−1 (averaged values over 20 years). Since 1989, winter wheat (Triticum aestivum cv. Xinmai 19) has been annually rotated with summer maize (Zea mays cv. Zhengdan 958). Basal fertilizers were broadcasted evenly onto the soil surface before sowing of maize (early June) or wheat (early October) and the surface soil (0–20 cm) was then tilled immediately. Supplemental fertilizer was also broadcast onto the soil surface in late February for wheat and in late July for maize. Field management practices were the same for all plots during the experiment. Further details of the field site, compost preparation, and fertilization treatments have been reported by Yu et al. (2012a).
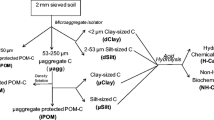
On 7 June 2009, ten soil cores from each replicate plot were collected using a 2.5-cm-diameter auger up to a depth of 20 cm, and then mixed as one composite sample. All samples were stored at 4 °C and transported immediately to the laboratory. Moist soil samples were gently broken apart along natural break points and sieved (<8 mm). Visible plant roots and organic debris in the sieved soil were carefully removed with forceps and these moist samples were used for PLFA analyses and aggregate fractionation. After thorough mixing, a subsample was dried at 105 °C to measure soil moisture. Another soil subsample was air-dried for the analyses of organic C by the wet oxidation-redox titration method (Carter 1993). The carbohydrate content in soil was also measured according to Puget et al. (1999), since the microbial growth is usually limited by available organic C (Hill et al. 2008).
PLFA analyses
PLFAs were extracted following a modified Bligh-Dyer technique (Brant et al. 2006). Briefly, fresh soil samples (3 g dry weight equivalent) were extracted with methanol/chloroform/phosphate buffer (2:1:0.8). Soil extracts were centrifuged (750×g for 10 min) and chloroform phases were collected. Phospholipids were separated from glycolipids and neutral lipids using silicic acid-bonded solid-phase-extraction columns by eluting sequentially with chloroform, acetone, and methanol. Phospholipids were saponified and methylated to fatty acid methyl esters (FAMEs) under N2 at 37 °C, and then dissolved in hexane, which contained a methyl nonadecanoate (19:0) FAME standard.
The resulting FAMEs were analyzed with a Shimadzu Gas Chromatography-Mass Spectrometer (GC-MS) QP 2010 PLUS (Shimadzu, Kyoto, Japan) equipped with a Varian VF23ms column (30 m × 0.25 mm [i. d.] × 0.25 μm film thickness) (Varian Associates Inc., Walnut Creek, CA, USA). The GC used helium as the carrier gas and the oven temperature was programmed to be 50 °C for 2 min, increasing at 3 °C min−1 to 200 °C for 4 min, before increasing further at 5 °C min−1 to 240 °C for 10 min. The interface with the MS was set to maximum oven temperature (240 °C), while the ion source was at 210 °C. The MS was programmed to scan m/z 50–650 at 1.7 scans s−1. Peaks were identified by comparing retention times with known standards, while the abundance of each PLFA was calculated by comparing peak areas with that of the 19:0 FAME standard.
PLFAs have been used as biomarkers for various groups of microorganisms (Bossio et al. 1998): 16:3ω3, 18:1ω9, 20:1ω9, and 18:2ω6c indicate fungi (Frostegard and Bååth 1996); 16:1ω5c is generally attributed to arbuscular mycorrhizal fungi (Olsson 1999); 10Me16:0, 10Me17:0, 10Me18:0, and 10Me20:0 indicate actinobacteria (Sundh et al. 1997); i14:0, a15:0, i15:0, a16:0, i16:0, a17:0, i17:0, and i18:0 indicate G+ bacteria (Sundh et al. 1997); and cy17:0, cy19:0, 16:1ω5t, 16:1ω7c, 16:1ω7t, 16:1ω9, and 18:1ω7c indicate G− bacteria (Sundh et al. 1997). G+ and G− bacteria have different effects on the C cycling process and accumulation, and the higher ratio of G+/G− bacteria is favorable for organic C accumulation in soils (Ponder and Tadros 2002; Zhang et al. 2013). The ratio of fungal/bacterial PLFAs (F/B) decreased with the increase of nutrient availability (Rinnan et al. 2007), and a higher F/B ratio can be seen as an indicator for the dominant supplies of plant nutrients for crop growth by organic matter decomposition and N mineralization (de Vries et al. 2007). In addition, 16:1ω5t, 16:1ω5c, 16:1ω7c, 16:1ω7t, 18:1ω7c, 16:1ω9, 17:1ω5c, 18:1ω9, 19:1ω8, and 20:1ω9 are monounsaturated PLFAs, while i14:0, a15:0, i15:0, a16:0, i16:0, a17:0, i17:0, i18:0, i19:0, 10Me16:0, 10Me17:0, 10Me18:0, and 10Me20:0 are branched PLFAs (Bossio et al. 2006). The ratio of monounsaturated/branched PLFAs (M/B) is suggested to be the relative proportion of aerobic to anaerobic microorganisms, while the ratio of cyclopropyl (cy17:0, cy19:0) to its precursors (16:1ω7c, 18:1ω7c), i.e., the ratio of sum cy/ω7c, indicates the growth stage of G− bacteria (Rajendran et al. 1992; Bossio et al. 2006) and the development of anaerobic conditions in soil (Feng et al. 2003).
Soil aggregate fractionation
Moist soils (<8 mm) were physically fractionated into different aggregates according to the procedure of Elliott (1986). One hundred grams of moist soil samples (on an oven-dried basis) was submerged in deionized water on top of a 250-μm sieve for 5 min. The sieve was manually moved up and down by 3 cm, and this process was repeated 50 times over a 2-min period. Floating organic material was discarded. The fraction remaining on the 250 μm sieve was collected in a pre-weighed aluminum pan and the fraction is termed as macroaggregates. Water plus soil with particle size <250 μm was poured through a 53-μm sieve, and the sieving procedure was repeated. The fraction remaining on the 53-μm sieve was collected in another pre-weighed aluminum pan and the fraction is termed as microaggregates (53–250 μm). All materials through the 53-μm sieve were transferred into 250-ml centrifuge tubes and centrifuged at 3,750×g for 30 min at 4 °C and the supernatant was discarded. The pellets were resuspended in deionized water and the above centrifugation steps were repeated three times to exclude the possible adsorption of dissolved organic C to minerals in the silt + clay fraction. Finally, the pellets were collected to obtain the silt + clay fraction (<53 μm).
Soil water retention curve and oxygen effective diffusion coefficient measurement
Undisturbed core soil samples (cylinder 100 cm3) were taken from each replicate plot at different locations (n = 8) for laboratory measurements. Water retention curves were determined with a ceramic pressure plate assembly at equilibrium matric potentials of −0.1, −0.2, −1, −3.5, −6, −10, −33, −50, −100, −200, −500, and −1,500 kPa in a pressure chamber. The obtained data were used to calculate the soil water retention curves and to derive the van Genuchten parameters by the RETention Curve (RETC) software (van Genuchten 1980) using the following equation.
where θ is the soil water content (cm3 cm−3), θ s and θ r are the saturated and residual water content (cm3 cm−3), respectively, ψ is the matric potential (kPa) as indicated by the pressure head, and the parameters of α, n, and m (m = 1–1/n) are dimensionless.
According to the capillary rise theory, the pore size was calculated as follows when the soil was hydrophilic at 20 °C of water (Kutílek and Nielsen 1994).
where d is the diameter of pore (μm), and |ψ| is the absolute matric potential (kPa).
The effective diffusion coefficient of oxygen (D, m2 s−1) through the pore space of the soil was calculated as follows (Aachib et al. 2004):
where N is soil porosity (%), D a0 is the free diffusion coefficient of oxygen in air (1.8 × 10−5 m2 s−1 at 20 °C), K H is Henry’s equilibrium constant (0.03 at 20 °C), D w0 is the free diffusion coefficient of oxygen in water (2.2 × 10−9 m2 s−1 at 20 °C), Q a and Q w are the proportion of soil porosity occupied by air and water, respectively, i.e., Q a + Q w = 1, and p is a power constant (p = 3.4). The soil porosity (N) was calculated as follows:
where ρ is the soil bulk density (g cm−3), and ρ 0 is the soil particle density (g cm−3). The proportion of soil porosity occupied by water (Q w) was calculated as follows:
where ρ is the soil bulk density (g cm−3), and θ m is the soil moisture content (cm3 g−1).
Statistical analysis
Accumulation rate of organic C in soil was calculated as follows:
Microbial biomass per SOC (mmol g−1 SOC) was calculated as the following:
Statistically significant differences among treatments were evaluated by the analysis of variance (one-way ANOVA) and least significant difference (LSD) calculations at the 5 % level with the SPSS 18 for Windows (SPSS Inc., Chicago, IL, USA). Redundancy analyses (RDA) (ter Braak and Šmilauer 1998) was used to test relationships between the accumulation rate of organic C and the microbial community composition, and regression analyses were used to test relationships between oxygen effective diffusion coefficient and macroaggregate mass proportion, and between microbial community composition and oxygen effective diffusion coefficient.
Results
Soil organic C content and crop yields
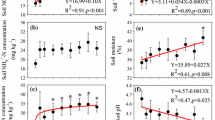
Compared to the experiment starting year of 1989, after 20 years, SOC contents slightly decreased under Control, significantly (P < 0.05) increased by 107–172 % under compost fertilized soils and by 17–56 % under inorganic fertilizer-added soils (Table 2). Compared to NPK, a significant decrease of SOC content was observed under NK but not under NP. The highest accumulation rate of organic C was 0.39 g C kg−1 year−1 under CM, higher under HCM, and the lowest under NK. Compost (CM and HCM) and NPK application also significantly (P < 0.05) enhanced soil carbohydrate content, while unbalance fertilizations, except NP, did not have such an enhancement, relative to the Control. Compared to Control, both wheat and maize yields were significantly (P < 0.05) increased by all fertilizations, except NK, and HCM and NPK treatments more effectively increased crop yields than other fertilizer treatments (Table 2).
Soil aggregation, pore size, and effective diffusion coefficient of oxygen
The microaggregates accounted for 55.1–71.5 % of the total soil mass in all treatments, which were significantly (P < 0.05) higher than the other fractions. The mass proportion of macroaggregates, ranging from 9.9 to 30.8 % (Fig. 1), significantly (P < 0.05) increased by 250 % under CM and 101 % under HCM. In contrast, all inorganic fertilizations, except PK, did not significantly affect the mass proportion of macroaggregates.
The proportion of pores with a neck diameter <4 μm under CM and HCM amounted to 65.1 and 59.9 %, respectively, and was significantly (P < 0.05) higher than in the Control (55.9 %), which had a similar pore proportion with all inorganic fertilizations (53.8–55.4 %) (Fig. 2). Fertilization, regardless of organic or inorganic, significantly (P < 0.05) decreased the proportion of 4–15 μm pores and, in contrast, increased the proportion of 60–300 μm pores compared with the Control. NPK application also increased the proportion of pores greater than 300 μm. Significant (P < 0.05) differences in the proportion of pores with a neck diameter of 15–60 μm could be ranked as Control, PK > NPK, NK, PK > HCM > CM, and the proportion under CM was only 11.3 %.
The estimated effective diffusion coefficient of oxygen under Control amounted to 5.19 × 10−6 m2 s−1, which was significantly (P < 0.05) higher than in the other treatments, with the lowest value of 1.30 × 10−6 m2 s−1 for CM (Fig. 3a). The effective diffusion coefficient of oxygen was ranked in the order of Control > NK > NPK, NP, PK > HCM > CM. The regression analysis showed that the effective diffusion coefficient of oxygen was negatively and exponentially correlated with the mass proportion of the macroaggregates (P < 0.01) and macroaggregates plus microaggregates (P = 0.068) in the soil across the tested treatments (Fig. 3b).
Effects of long-term (20 years) compost and inorganic fertilizations on the effective diffusion coefficient of oxygen in the soil (a) and the relationship between mass proportion of macroaggregates or macroaggregates plus microaggregates and effective diffusion coefficient of oxygen (b). Vertical bars denote the standard deviation of means (n = 4). Different letters indicate significant differences between treatments at P < 0.05
PLFA profiles
A total of 36 PLFAs containing saturated, unsaturated, methyl-branched, and cyclopropane fatty acids were identified and used for the statistical analysis. The abundance of total microbial and bacterial PLFAs was highest under HCM and CM, which was significantly (P < 0.05) higher than in the other treatments, and there was no significant difference between Control and inorganic fertilizations except for the bacterial PLFAs under NPK (Table 3). The microbial biomass per SOC was significantly (P < 0.05) decreased by compost and inorganic fertilizations especially under CM and HCM (Fig. 4).
Fertilization, regardless of organic or inorganic, significantly (P < 0.05) increased G+ bacterial PLFAs, but not G− bacteria, compared with the Control (Table 3). In contrast, the abundance of actinobacterial PLFAs was significantly (P < 0.05) reduced from 14.5 nmol g−1 in the Control soil to 9.4–11.9 nmol g−1 in the soil treated with compost or inorganic fertilizers. The abundance of fungal PLFAs varied from 9.9 to 13.2 nmol g−1 soil across the all treatments, and the highest abundance was under HCM while the lowest under NPK. The abundance of branched (B) PLFAs was significantly (P < 0.05) increased under CM, HCM, and NPK, and in contrast, the abundance of monounsaturated (M) PLFAs significantly (P < 0.05) decreased under CM and NP.
The ratio of monounsaturated/branched PLFAs (M/B), an indicator for the ratio of aerobic to anaerobic microorganisms, was 0.42, 0.68, and 0.87, respectively, under CM, HCM, and NPK, a value that was significantly (P < 0.05) lower than in the other fertilization treatments (Fig. 5a). The ratio of fungal/bacterial PLFAs was significantly (P < 0.05) decreased under compost (CM and HCM) and NPK compared with the control (Fig. 5b). Whereas the ratio of G+/G− bacteria or cy/ω7c significantly (P < 0.05) increased in the fertilization treatments except NK. The G+/G− ratio was ranked in the order of CM > HCM > NPK, PK > NP, NK > Control, while the cy/ω7c ratio was ranked as CM > HCM > NPK, NP, PK > NK, Control (Fig. 5c, d). Correlation analysis showed that the ratio of sum cy/ω7c was significantly (P < 0.01) and negatively, whereas the M/B ratio was significantly (P < 0.05) and positively, correlated to the effective diffusion coefficient of oxygen in the soil across the tested treatments (Fig. 6).
Effects of long-term (20 years) application of compost and inorganic fertilizers on the ratios of monounsaturated/branched PLFAs (M/B, a), fungal/bacterial PLFAs (b), Gram-positive to Gram-negative bacterial PLFAs (G+/G−, c), and sum (cy17:0 + cy19:0)/(16:1ω7c + 18:1ω7c) (sum cy/ω7c, d) in the test soil. Vertical bars denote the standard deviation of means (n = 4). Different letters indicate significant differences between treatments at P < 0.05
Relating microbial PLFAs to accumulation rate of soil organic C
The accumulation rate of SOC across the tested treatments was significantly and positively correlated with the abundance of branched (P < 0.001) and G+ bacterial (P < 0.01) PLFAs, or the ratio of G+/G− (P = 0.003), whereas significantly and negatively correlated with the ratios of M/B (P < 0.001) and fungal/bacterial PLFAs (P < 0.05). The abundance of actinobacterial PLFAs was also marginally and negatively correlated to the accumulation rate of SOC albeit at P < 0.10 (Fig. 7). Further analysis showed that the SOC accumulation rate (z) significantly (P < 0.001) interacted with the mass proportion of macroaggregates (x) and ratio of M/B (y) (z = 0.514 + 4.345ex-15–0.149ey, Fig. 8); the latter two could account for 94 % of variation of SOC accumulation rate.
Discussion
Effect of fertilization on microbial community composition
This study shows that the abundance and composition of soil microbial community were significantly altered by 20 years of organic compost or inorganic fertilization, relative to the Control (Table 3). Our results of a significant (P < 0.05) increase in the abundance of total microbial PLFAs following the 20-year application of compost or compost plus inorganic N, but not due to the long-term application of inorganic fertilizers alone, are consistent with the findings from another long-term fertilization experiment on fluvo-aquic soil in the North China Plain (Ai et al. 2012; Huang et al. 2013). Kandeler et al. (1999) and Peacock et al. (2001) demonstrated that an addition of compost increased soil microbial biomass and enzyme activities. However, there have been contradictory findings regarding the effect of inorganic fertilizer on microbial abundance. For instance, Böhme et al. (2005) and Rinnan et al. (2007) reported that 15- to 100-year NPK fertilization significantly (P < 0.05) increased microbial biomass, whereas Peacock et al. (2001) found a 15 % decrease of total microbial PLFAs in a silt loam soil of northwest Tennessee, USA, after 5 years of ammonium nitrate application compared with the no extra nutrients addition. It has been suggested that the microbial abundance primarily depends on the level of labile organic C rather than total organic C in soils (Hatch et al. 2000; Hill et al. 2008). In the present study, although inorganic fertilizations significantly (P < 0.05) increased organic C in soil by 30–57 %, the carbohydrate content was increased by only 4–22 %, which was significantly (P < 0.05) lower than the 59–104 % increase of carbohydrates in the compost-amended soil (Table 2). Yu et al. (2012c) demonstrated that structural polysaccharides were more efficiently accumulated in the compost-amended soil than in the inorganic fertilizer-added soil. Therefore, it seems that the addition of compost does provide both relatively stable and labile (readily available) substrates for the microbial community, whereas the relatively small increase of labile organic C under inorganic fertilizations may be unable to support the substantial growth of microorganisms (Hatch et al. 2000; Hill et al. 2008).
Both compost and inorganic fertilizers application had no effects on the abundance of G− bacteria; however, fertilization, regardless of fertilizer type, significantly (P < 0.05) increased the abundance of G+ bacterial PLFAs compared to the Control. Thus far, there have been conflicting results concerning the effect of fertilizations on the abundance of G− and G+ bacteria. Bossio et al. (1998) and Cookson et al. (2005) reported that fertilization stimulated the growth of G+ bacteria. A 31-year addition of organic fertilizer increased the abundance of G+ bacteria, but inorganic fertilizer increased G− bacteria (Ai et al. 2012). In contrast, Peacock et al. (2001) documented that a 5-year application of manure decreased the G+ bacterial biomass but increased the G− bacterial biomass. It was hypothesized that the abundance of G− bacteria would increase with available organic substrates because G− bacteria could utilize a variety of organic C sources under well-aerated conditions (Ponder and Tadros 2002) and hence can outcompete G+ bacteria growing on relatively labile organic C (Bossio and Scow 1998; Peacock et al. 2001). However, Marschner et al. (2003) found a shift in the response of G− and G+ bacteria to exogenous organic material addition with time, indicating that G− bacteria were stimulated initially upon the addition of compost, while the abundance of G+ bacteria increased over time. As discussed above, the lack of any increase in the abundance of G− bacteria in the inorganic fertilizer treatments might be due to a relatively low increase in labile organic C such as carbohydrates. However, this could not be the case in the compost-treated plots, as the content of total organic C and labile organic C (carbohydrates) was significantly (P < 0.05) increased (Table 2). We found that in the G− bacteria, the sum cy/ω7c ratio was significantly (P < 0.05) greater in the compost-treated soil than in the soil receiving only inorganic fertilizers (Fig. 5). Furthermore, the sum cy/ω7c ratio was logarithmically and negatively correlated with the effective diffusion coefficient of oxygen (Fig. 6); the latter was significantly (P < 0.01) and negatively correlated to the mass proportion of macroaggregates or marginally (P = 0.068) and negatively correlated to the proportion of macroaggregates plus microaggregates (Fig. 3b). Previous studies demonstrated that the spatial distribution, functioning, and diversity of bacterial populations at the microscale are affected by the size and stability of soil aggregates through changing factors such as predation pressure, water potential, and oxygen availability (Hansel et al. 2008; Kong et al. 2005, 2011). Our findings of increase in the sum cy/ω7c ratio in the compost-treated soil (cf. Control) further indicate the development of anaerobic microsites during the formation of macroaggregates (Bossio et al. 1998; Cookson et al. 2005; Wixon and Balser 2013).
Zhuang et al. (2008) and Yu et al. (2012b) reported that the increase of organic C in microaggregates (free or within macroaggregates) caused an increase in pore-filling organic matter (particulate or amorphous). This may decrease pore connectivity, increase water retention, and as a result, decrease the effective diffusion coefficient of oxygen in soil, as observed in the present study, which in turn could lower oxygen concentration in the soil air and promote the formation of anaerobic microsites (Schjønning et al. 2003; Huang et al. 2004). According to Blagodatsky and Smith (2012), the aeration of intra-aggregate gradually decreased with the formation of aggregates and depending on the kind and intensity of aggregation, intra-aggregate pores could be completely anoxic. Thus, we argue that a short-term compost application, such as in the study of Peacock et al. (2001), would increase total organic C and labile organic C in particular, but may not sufficiently enhance the formation of soil aggregates, and might stimulate the growth of G− bacteria. In contrast, a long-term compost application might not only increase organic C but would also strongly enhance soil aggregation, resulting in the development of anaerobic microhabitats and the suppression of G− bacterial growth.
In the present study, the abundance of fungal PLFAs including arbuscular mycorrhizal and saprotrophic species was low under all treatments (relative to bacterial PLFAs) possibly because tillage significantly (P < 0.05) decreased the abundance of fungi by destroying mycelial networks (He and Nara 2007). Arbuscular mycorrhizal fungi are indeed more susceptible to tillage than saprotrophic fungal species (Douds et al. 1993; de Vries et al. 2007), and consistent with this observation, the abundance of the arbuscular mycorrhizal biomarker PLFA 16:1ω5c in the present study was relatively lower (<2 nmol g−1 soil) compared to forest and grassland soils (Oehl et al. 2010). In addition, bacterial proliferation following the increase of labile organic substrates had antagonistic effects on fungal growth (Lazcano et al. 2013). Hence, the inherently low fungal abundance and inhibiting effect of bacteria might be the reasons for the relatively limited response of the fungal community to the addition of organic and inorganic fertilizers in the test soil (Lazcano et al. 2013).
The observation of decreased abundance of actinobacteria in the long-term compost and inorganic fertilization treatments (Table 3) suggests that the increase in SOC across these treatments (Table 2) exhibited an inhibitory rather than a stimulatory effect on the growth of actinobacteria. Peacock et al. (2001) demonstrated that soils treated with manure were enriched in total bacterial markers but depleted in actinobacterial markers. Clegg et al. (2003) also found that the abundance of PLFA biomarkers for actinobacteria was lower in N-fertilized, compared with unfertilized, grassland soils. A previous study has shown that actinobacteria dominate under stress conditions, for example, where the soil is depleted of soluble C, N, and other nutrients supporting microbial growth (Zhang et al. 2012), because actinobacteria utilize very little newly added or formed organic C but are well-adapted to metabolize old organic matter with a relatively low soil C/N ratio (McCarthy and Williams 1992). It is suggested that the abundance and activity of actinobacteria would be decreased because fertilization may increase new C inputs, which would decrease the opportunity for actinobacteria to access old organic matter and may mainly rely on their storage compounds for maintaining cell wall in fertilized soils (Billings and Ziegler 2008).
Relationship between microbial community composition and organic C accumulation in soil
In the present study, both the accumulation rate of organic C and the abundance of total microbial PLFAs were significantly (P < 0.05) higher under compost than under inorganic fertilizations, whereas the microbial biomass per SOC was lowest under CM, and highest under Control (Fig. 4), which caused the lower specific SOC decomposition rate (decomposition rate of per unit organic C) under CM (0.32 mg C g−1 SOC day−1) than under other fertilizations (0.34–0.52 mg C g−1 SOC day−1, Yu et al. 2012a). Jagadamma et al. (2014) also reported that there was an inverse relationship between the SOC content and the percentage of SOC lost through respiration. This indicated that organic C turnover was not only affected by total microbial abundance in soil. It is known that the decomposition rate of organic C was also dependent on the proportion of large pores (Yoo et al. 2006; Ruamps et al. 2011) and the location in the pore network (Killham et al. 1993; Strong et al. 2004). Organic C turnover was reported to be faster in pores with >4 μm than with <4 μm neck diameters (Killham et al. 1993; Strong et al. 2004). Strong et al. (2004) also found that the accumulation rate of organic C was positively correlated with the proportion of <4 μm pores, but was negatively correlated with the proportion of 15–60 μm pores. In this study, the proportion of <4 μm pores was significantly (P < 0.05) higher and of 15–60 μm pores was significantly (P < 0.05) lower under CM than under other fertilizations (Fig. 2). It is known that soil aeration condition played an important role in the storage and turnover of organic C (Sundh et al. 1997). The accumulation rate of organic C would be higher in soil with oxygen concentration ≤10 % by slowing down the oxidation of labile organic C (Zibilske and Bradford 2007). The soil pore system is closely related to the effective diffusion coefficient of oxygen, and compared with other treatments, the changes of pore systems under CM lowered the effective oxygen diffusion coefficient. This may also be the reason for the lowest specific SOC mineralization in the compost treatment, hence favoring accumulation of SOC and carbohydrate contents in the soil (Killham et al. 1993; Strong et al. 2004; Yoo et al. 2006; Ruamps et al. 2011, Table 2).
Our results demonstrated that the abundance of branched PLFAs was significantly (P < 0.05) higher under CM, HCM, and NPK than under other fertilizations, and the ratio of M/B PLFAs, an indicator for the ratio of aerobic to anaerobic microorganisms (Bossio et al. 2006), was less than 1.0. This suggested that the 20-year application of compost and NPK fertilizer induced a shift in microbial community composition to more facultative and/or obligate anaerobes under CM, HCM, and NPK. Zhong et al. (2010) reported that a 20-year application of compost mainly stimulated the growth of facultative and obligate anaerobes in a paddy soil, which is widely distributed in southeast of China. In this study, the M/B ratio was significantly (P < 0.05) and positively correlated to the effective diffusion coefficient of oxygen in the tested treatments (Fig. 6). It is possible that by promoting soil aggregation, the long-term application of compost also gradually accelerated the development of anaerobic microhabitats (Denef et al. 2009), which in turn suppressed the proliferation of the monounsaturated PLFAs while increasing the branched PLFAs (Rajendran et al. 1992). It has been observed that aerobes decompose organic C more efficiently than anaerobes (Ding and Sun 2005); therefore, we consider that a gradual shift in dominant species of microbial community composition from aerobes to facultative and/or obligate anaerobes could manipulate the accumulation rate of organic C in the tested treatments, due to an interaction between soil microbial community composition, organic C accumulation, and aggregation (Fig. 8).
The accumulation rate of SOC in the tested treatments was significantly (P < 0.01) and positively correlated with the abundance of G+ bacteria or the ratio of G+/G− bacteria. Compared to G− bacteria, G+ bacteria generally possess a greater proportion of peptidoglycan, which contains significant quantities of N-acetylglucosamine that is a precursor of relatively decay-resistant soil organic matter (Simpson et al. 2007). Zhang et al. (2013) verified that 13C from added 13C-glucose was more effectively used by G+ bacteria and preserved in the CM-fertilized soil than in the NPK-fertilized soil and Control soil. Accordingly, a more efficient increase in the abundance of G+ bacteria under NPK, HCM, and CM might result in a more effective accumulation of relatively resistant organic material. This also explains why Murugan and Kumar (2013) observed that microbial residual C stock was higher in soils receiving organic fertilizer than inorganic fertilizers. On the other hand, the abundance of actinobacteria negatively related to the accumulation rate of organic C (Fig. 7). We acknowledge that actinobacteria were efficient decomposers of nutrient-poor organic compounds (Mackenziem and Quideau 2010) and were capable of using chitin as a sole carbon source, which is a major component of fungal cell walls (Zaitlin et al. 2004). The decrease in the abundance of actinobacteria under fertilization would lead to a decrease in the decomposition of old soil organic matter or newly formed relatively recalcitrant organic C (Billings and Ziegler 2008). Thus, we hypothesize that fertilization may favor the accumulation of decay-resistant organic C through suppressing the growth of actinobacteria.
Conclusions
Our study has shown that the increase on SOC and aggregation through the long-term application of compost or balanced NPK fertilization would contribute to altering soil microenvironment, i.e., the size and distribution of pores and effective oxygen diffusion coefficient, and in turn the abundance and composition of soil microbial communities. The shift in microbial community composition to more facultative and/or obligate anaerobes than aerobes would favor organic C accumulation in the soil. Besides, the increase of Gram-positive versus Gram-negative bacteria and the decrease of actinobacteria would favor accumulation of relatively decay-resistant SOC. This study aids in identifying sustainable organic and/or inorganic fertilization practices that increase SOC and soil fertility. Future studies may simultaneously evaluate the environmental sustainability of increasing SOC and altering soil microenvironment through improved fertilization practices in relation to greenhouse gas emissions from soil.
References
Aachib M, Mbonimpa M, Aubertin M (2004) Measurement and prediction of the oxygen diffusion coefficient in unsaturated media, with applications to soil covers. Water Air Soil Poll 156:163–193
Ai C, Liang GQ, Sun JW, Wang XB, Zhou W (2012) Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 173(174):330–338
Billings SA, Ziegler SE (2008) Altered patterns of soil carbon substrate usage and heterotrophic respiration in a pine forest with elevated CO2 and N fertilization. Global Change Biol 14:1025–1036
Bittman S, Forgr TA, Kowalenko CG (2005) Responses of the bacterial and fungal biomass in a grassland soil to multi-year applications of dairy manure slurry and fertilizer. Soil Biol Biochem 37:613–623
Blagodatsky S, Smith P (2012) Soil physics meets soil biology: towards better mechanistic prediction of greenhouse gas emissions from soil. Soil Biol Biochem 47:78–92
Böhme L, Langer U, Böhme F (2005) Microbial biomass, enzyme activities and microbial community structure in two European long-term field experiments. Agri Ecosyst Environ 109:141–152
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Bossio DA, Scow KM, Gunapala N, Graham KJ (1998) Determinants of soil microbial communities: effects of agricultural management, season and soil type on phospholipid fatty acid profiles. Microb Ecol 36:1–12
Bossio DA, Fleck JA, Scow KM, Fujii R (2006) Alteration of soil microbial communities and water quality in restored wetlands. Soil Biol Biochem 38:1223–1233
Brant JB, Sulzman EW, Myrold DD (2006) Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol Biochem 38:2219–2232
Cai ZC, Qin SW (2006) Dynamics of crop yields and soil organic carbon in a long-term fertilization experiment in the Huang-Huai-Hai Plain of China. Geoderma 136:708–715
Carter MR (1993) Soil samples and methods of analysis. Lewis, Boca Raton, pp 190–191
Clegg CD, Lovell RDL, Hobbs PJ (2003) The impact of grassland management regime on the community structure of selected bacterial groups in soils. FEMS Microbiol Ecol 43:263–270
Cookson WR, Abaye DA, Marschner P, Murphy DV, Stockdale EA, Goulding KWT (2005) The contribution of soil organic matter fractions to carbon and nitrogen mineralization and microbial community size and structure. Soil Biol Biochem 37:1726–1737
Denef K, Roobroeck D, Manimel Wadu MCW, Lootens P, Boeckx P (2009) Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol Biochem 41:144–153
de Vries FT, Bloem J, van Eekeren N, Brusaard L, Hoffland E (2007) Fungal biomass in pastures increases with age and reduced N input. Soil Biol Biochem 39:1620–1630
Ding HB, Sun MY (2005) Biochemical degradation of algal fatty acids in oxic and anoxic sediment-seawater interface systems: effects of structural association and relative roles of aerobic and anaerobic bacteria. Marine Chem 93:1–19
Dolfing J, Vos A, Bloem J, Ehlert PAI, Naumova NB, Kuikman PJ (2004) Microbial diversity in archived soils. Science 306:813–813
Douds DDJ, Janke RR, Peters SE (1993) VAM fungus spore populations and colonization of roots of maize and soybean under conventional and low-input sustainable agriculture. Agri Ecosyst Environ 43:325–335
Elfstrand S, Hedlund K, Martensson A (2007) Soil enzyme activities, microbial community composition and function after 47 years of continuous green manuring. Appl Soil Ecol 35:610–621
Elliott ET (1986) Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci Soc Am J 50:627–633
Feng Y, Motta AC, Reeves DW, Burmester CH, van Santen E, Osborne JA (2003) Soil microbial communities under conventional-till and no-till continuous cotton systems. Soil Biol Biochem 35:1693–1703
Frostegard A, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Hansel CM, Fendorf S, Jardine PM, Francis CA (2008) Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl Environ Microbiol 74:1620–1633
Hatch DJ, Lovell RD, Antil RS, Jarvis SC, Owen PM (2000) Nitrogen mineralization and microbial activity in permanent pastures amended with nitrogen fertilizer or dung. Biol Fertil Soils 30:288–293
He XH, Nara K (2007) Element biofortification: can mycorrhizas potentially offer a more effective & sustainable way to curb human malnutrition? Trends Plant Sci 12:331–333
Hill PW, Farrar JF, Jones DL (2008) Decoupling of microbial glucose uptake and mineralization in soil. Soil Biol Biochem 40:616–624
Huang M, Jiang LG, Zou YB, Xu SH, Deng GF (2013) Changes in soil microbial properties with no-tillage in Chinese cropping systems. Biol Fertil Soils 49:373–377
Huang Y, Zou JW, Zheng XH, Wang YS, Xu XK (2004) Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios. Soil Biol Biochem 36:973–981
Jagadamma S, Steinweg JM, Mayes MA, Wang GS, Post WM (2014) Decomposition of added and native organic carbon from physically separated fractions of diverse soils. Biol Fertil Soils 50:613–621
Kandeler E, Stemmer M, Klimanek EM (1999) Response of soil microbial biomass, urease and xylanase within particle-size fractions to long-term soil management. Soil Biol Biochem 31:261–273
Killham K, Amato M, Ladd J (1993) Effect of substrate location in soil and soil porewater regime on carbon turnover. Soil Biol Biochem 25:57–62
Kong AYY, Six J, Bryant DC, Denison RF, van Kessel C (2005) The relationship between carbon input, aggregation, and soil organic carbon stabilization in sustainable cropping systems. Soil Sci Soc Am J 69:1078–1085
Kong AYY, Scow KM, Córdova-Kreylos AL, Holmes WE, Six J (2011) Microbial community composition and carbon cycling within soil microenvironments of conventional, low-input, and organic cropping systems. Soil Biol Biochem 43:20–30
Kutílek M, Nielsen DR (1994) Soil hydrology. Catena, Cremlingen Destedt
Lazcano C, Brandón-Gómez M, Revilla P, Domínguez J (2013) Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol Fertil Soils 49:723–733
Mackenziem MD, Quideau SA (2010) Microbial community structure and nutrient availability in oil sands reclaimed boreal soils. Appl Soil Ecol 44:32–41
Majumder B, Ruehlmann J, Kuzyakov Y (2010) Effects of aggregation processes on distribution of aggregate size fractions and organic C content of a long-term fertilized soil. Eur J Soil Biol 46:365–370
Marschner P, Kandeler E, Marschner B (2003) Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol Biochem 35:453–461
McCarthy AJ, Williams ST (1992) Actinomycetes as agents of biodegradation in the environment—a review. Gene 115:189–192
Meng L, Ding WX, Cai ZC (2005) Long-term application of organic manure and nitrogen fertilizer on N2O emissions, soil quality and crop production in a sandy loam soil. Soil Biol Biochem 37:2037–2045
Moore-Kucera J, Dick RP (2008) Application of 13C-labeled litter and root materials for in situ decomposition studies using phospholipid fatty acids. Soil Biol Biochem 40:2485–2493
Murugan R, Kumar S (2013) Influence of long-term fertilisation and crop rotation on changes in fungal and bacterial residues in a tropical rice-field soil. Biol Fertil Soils 49:847–856
Oehl F, Laczko E, Bogenrieder A, Stahr K, Bösch R, van der Heijden M, Sieverding E (2010) Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem 42:724–738
Olsson PA (1999) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol Ecol 29:303–310
Peacock AD, Mullen MD, Ringelberg DB, Tyler DD, Hedrick DB, Gale PM, White DC (2001) Soil microbial community responses to dairy manure or ammonium nitrate applications. Soil Biol Biochem 33:1011–1019
Plaza C, Hernandez D, Garcia-Gil JC, Polo A (2004) Microbial activity in pig slurry-amended soils under semiarid conditions. Soil Biol Biochem 36:1577–1585
Ponder F, Tadros M (2002) Phospholipid fatty acids in forest soil four years after organic matter removal and soil compaction. Appl Soil Ecol 19:173–182
Puget P, Angers DA, Chenu C (1999) Nature of carbohydrates associated with water-stable aggregates of two cultivated soils. Soil Biol Biochem 31:55–63
Rajendran N, Matsuda O, Imamura N, Urushigawa Y (1992) Variation in microbial biomass and community structure in sediments of Eutrophic Bays as determined by phospholipid ester-linked fatty acids. Appl Environ Microbiol 58:562–571
Rinnan R, Michelsen A, Bååth E, Jonasson S (2007) Fifteen years of climate change manipulations alter soil microbial communities in a subarctic health system. Global Change Biol 13:28–39
Rudrappa L, Purakayastha TJ, Singh D, Bhadraray S (2006) Long-term manuring and fertilization effects on soil organic carbon pools in a Typic Haplustept of semi-arid sub-tropical India. Soil Till Res 88:180–192
Ruamps LM, Nunan N, Chenu C (2011) Microbial biogeography at the soil pore scale. Soil Biol Biochem 43:280–286
Sarathchandra SU, Ghani A, Yeates GW, Burch G, Cox NR (2001) Effect of nitrogen and phosphate fertilizers on microbial and nematode diversity in pasture soils. Soil Biol Biochem 33:953–964
Schjønning P, Thomsen IK, Moldrup P, Christensen BT (2003) Linking soil microbial activity to water- and air-phase contents and diffusivities. Soil Sci Soc Am J 67:156–165
Sessitsch A, Weilharter A, Gerzabek MH, Kirchmann H, Kandeler E (2001) Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl Environ Microbiol 67:4215–4224
Simpson AJ, Song G, Smith E, Lam B, Novotny EH, Hayes MB (2007) Unraveling the structural components of soil humin by use of solution-state nuclear magnetic resonance spectroscopy. Environ Sci Technol 41:876–883
Stark C, Condron LM, Stewart A, Di HJ, O’Callaghan M (2007) Influence of organic and mineral amendments on microbial soil properties and processes. Appl Soil Ecol 35:79–93
Strong DT, Wever HD, Merckx R, Recous S (2004) Spatial location of carbon decomposition in the soil pore system. Eur J Soil Sci 55:739–750
Sun HY, Deng SP, Raun WR (2004) Bacterial community structure and diversity in a century-old manure-treated agroecosystem. Appl Environ Microbiol 70:5868–5874
Sundh I, Nilsson M, Borga P (1997) Variation in microbial community structure in two boreal peatlands as determined by analysis of phospholipid fatty acid profiles. Appl Environ Microbiol 63:1476–1482
ter Braak CJF, Šmilauer P (1998) CANOCO references manual and user’s guide to Canoco for Windows. Microcomputer Power, Ithaca, p 351
USDA (United States Department of Agriculture) (1994) Keys to soil taxonomy. (Soil Conservation Service), 6th Edition. USDA, Washington, DC
van Genuchten MT (1980) A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci Soc Am J 44:892–898
Wixon DL, Balser TC (2013) Toward conceptual clarity: PLFA in warmed soils. Soil Biol Biochem 57:769–774
Yoo G, Spomer LA, Wander MM (2006) Regulation of carbon mineralization rates by soil structure and water in an agricultural field and a prairie-like soil. Geoderma 135:16–25
Yu HY, Ding WX, Luo JF, Geng RL, Ghanni A, Cai ZC (2012a) Effects of long-term compost and fertilizer application on stability of aggregates-associated organic carbon in intensively cultivated sandy loam soil. Biol Fertil Soils 48:325–336
Yu HY, Ding WX, Luo JF, Geng RL, Cai ZC (2012b) Long-term application of compost and mineral fertilizers on aggregation and aggregate-associated carbon in a sandy loam soil. Soil Till Res 124:170–177
Yu HY, Ding WX, Luo JF, Donnison A, Zhang JB (2012c) Long-term effect of compost and inorganic fertilizer on activities of carbon-cycle enzymes in soil aggregates of an intensively cultivated sandy loam. Soil Use Manag 28:347–360
Zaitlin B, Turkington K, Parkinson D, Clayton G (2004) Effects of tillage and inorganic fertilizers on culturable soil actinomycete communities and inhibition of fungi by specific actinomycetes. Appl Soil Ecol 26:53–62
Zhang QC, Shamsi IH, Xu DT, Wang GH, Lin XY, Jilani G, Hussain N, Chaudhry AN (2012) Chemical fertilizer and organic manure inputs in soil exhibit a vice versa pattern of microbial community structure. Appl Soil Ecol 57:1–8
Zhang HJ, Ding WX, Yu HY, He XH (2013) Carbon uptake by a microbial community during 30-day treatment with 13C-glucose of a sandy loam soil fertilized for 20 years with NPK or compost as determined by a GC-C-IRMS analysis of phospholipid fatty acids. Soil Biol Biochem 58:228–236
Zhong WH, Gu T, Wang W, Zhang B, Lin XG, Huang QR, Shen WS (2010) The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 326:511–522
Zhuang J, McCarthy JF, Perfect E, Mayer LM, Jastrow JD (2008) Soil water hysteresis in water-stable microaggregates as affected by organic matter. Soil Sci Soc Am J 72:212–220
Zibilske LM, Bradford JM (2007) Oxygen effects on carbon, polyphenols, and nitrogen mineralization potential in soil. Soil Sci Soc Am J 71:133–139
Acknowledgments
This study was funded by the Chinese Academy of Sciences (XDB15020100), the National Basic Research Program of China (2011CB100503), and National Natural Science Foundation of China (41401282, 41171190).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Ding, W., Yu, H. et al. Linking organic carbon accumulation to microbial community dynamics in a sandy loam soil: result of 20 years compost and inorganic fertilizers repeated application experiment. Biol Fertil Soils 51, 137–150 (2015). https://doi.org/10.1007/s00374-014-0957-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0957-0