Abstract
The objective of this study was to investigate the effects of short-term (less than 2 years) conservation managements [no-tillage (NT) and crop residue returning] on top soil (0–5 cm) microbial community composition and soil organic C (SOC) fractions under a rice-wheat rotation at Junchuan town of Hubei Province, China. Treatments were established following a split-plot design of a randomized complete block with tillage practices [conventional tillage (CT) and NT] as the main plot and residue returning level [no residue returning (0) and all residues returned to fields from the preceding crop (S, 2,146 kg C ha−1)] as the subplots. The four treatments were CT with or without residue returning (CT0 and CTS) and NT with or without residue returning (NT0 and NTS). The abundances of microbial groups [total FLFAs, fungal biomass, bacterial biomass, fungal biomass/bacterial biomass (F/B), monounsaturated fatty acids/saturated fatty acids (MUFA/STFA), and microbial stress] were determined by phospholipid fatty acid (PLFA) analysis of soil. The ratio of MUFA/STFA reflects aeration of soil and greater MUFA/STFA means better aeration condition of soil. Moreover, the microbial stress, the ratio of cy19:0 to 18:1ω7, was regarded as an indicator of physiological or nutritional stress of microbial community. PLFA profiles were dominated by the fatty acids iC15:0 (9.8 %), C16:0 (16.5 %), 10Me17:0 (9.9 %), and Cyc19:0 (8.3 %), together accounting for 44.6 % of the total PLFAs. Compared with CT, NT significantly increased microbial biomass C (MBC) by 20.0 % but did not affect concentrations of total organic C (TOC), dissolved organic C (DOC), easily oxidizable C (EOC), and SOC of aggregates. Residue returning significantly increased MBC by 18.3 % and SOC content of 2–1-mm aggregate by 9.4 %. NT significantly increased total PLFAs by 9.8 % and fungal biomass by 40.8 % but decreased MUFA/STFA by 15.5 %. Residue returning significantly enhanced total PLFAs, bacterial biomass, fungal biomass, F/B, and MUFA/STFA by 31.1, 36.0, 95.9, 42.5, and 58.8 %, respectively, but decreased microbial stress by 45.9 %. Multivariate analysis (redundancy analysis and partial correlation analysis) indicated that SOC of 2–1-mm aggregate was related to changes in the composition of soil microbial groups, suggesting that SOC of 2–1-mm aggregate was sensitive to changes in soil microbial community composition affected by short-term conservation management practices in our study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rice-wheat rotation, growing at least one rice and wheat crop on the same field each year, is the major cropping system in South Asia and China, occupying about 13.5 million ha in South Asia and about 4.5 million ha in China (Ladha et al. 2003; Dawe et al. 2004). The rotation is extremely important for the food security of Asia and China (Ladha et al. 2003; Qi et al. 2010), providing stable food grains for more than 20 % of the world’s population (Kumari et al. 2011). Therefore, this cropping system plays an important role in maintaining the balance between food supply and population growth. However, several studies pointed out that the average 2 % per year increases in rice and wheat yields seen from 1970 to 1990 have dropped off caused by a combination of factors including a deteriorating natural resource base, especially soils; inefficient use of inputs (fertilizer, water, and labor); rising cost of cultivation; weather conditions; pest and agronomic management; emerging socioeconomic changes (such as urbanization, labor migration, preference of nonagricultural work); and reduced support for public agricultural research (Ladha et al. 2003, 2009; Gathala et al. 2011a). Therefore, it is important to focus on soil health and system sustainability of rice-wheat rotation system.
Sustainable soil management can be practiced through conservation management practices, including no-tillage (NT), crop residue returning, and crop rotation (Hobbs et al. 2008; Palm et al. 2014). Conventional tillage (CT) can influence physical, chemical, and biological properties of soil, thus affecting soil productivity and sustainability (Palm et al. 2014). It accelerates soil organic matter oxidation, disturb soil aggregate stability, and increase soil erosion due to frequent soil disturbance (Frey et al. 1999; Liu et al. 2010; Gathala et al. 2011b; Mathew et al. 2012). Compared with CT, conservation management practices can reduce soil erosion (Uri 1999), increase soil macro-aggregation, and enhance soil microbial biomass and activity (Hendrix et al. 1998; Schwab et al. 2002; Jacobs et al. 2009; Gathala et al. 2011b; Huang et al. 2013; Murugan et al. 2014).
Soil microorganisms play important roles in ecosystem function and sustainability (Paul and Clark 1989). The abundance and composition of soil microbial communities and their interactions with environment factors are important influencing factors of soil organic C (SOC) dynamic (Wall et al. 2013). Dong et al. (2014) pointed out that any changes in soil microbial community composition is likely to alter SOC availability and subsequently crop productivity. In general, short-term (<5 years) effects of conservation management practices on SOC are difficult to be monitored (West and Post 2002; Liang et al. 2010). In contrast, long-term (>5 years) conservation management practices can increase SOC level (Helgason et al. 2009; Quilty and Cattle 2011; Mathew et al. 2012) and microbial biomass and activity (Gouaerts et al. 2007; Helgason et al. 2009; Mathew et al. 2012). Long-term conservation management practices affect habitats for microorganisms through altering soil physical and chemical conditions, and this can shift soil microbial community compositions (Feng et al. 2003; Helgason et al. 2009). Moreover, effects of short-term conservation management practices on soil microbial community composition have showed inconsistent results (Jackson et al. 2003; Spedding et al. 2004; Minoshima et al. 2007). For example, Minoshima et al. (2007) reported differences in microbial community composition after the first year following conversion from CT to NT, while others did not find a significant effect of short-term tillage disturbance (Carpenter-Boggs et al. 2003; Jackson et al. 2003). The short-term effects of management on SOC fractions and microbial community composition are complex and depend on the climate, soils, crop rotations, returning residue, and the management type (Carpenter-Boggs et al. 2003; Jackson et al. 2003; Spedding et al. 2004; Al-Kaisi et al. 2005; Liang et al. 2010).
Relatively less attention has been paid to the effects of short-term conservation management practices on SOC fractions. Some SOC fractions [i.e., microbial biomass C (MBC), dissolved organic C (DOC), and SOC of aggregates] can be more sensitive to shift in short-term tillage practices than total organic C (TOC) (Carter et al. 1998; Gosling et al. 2013). For example, both MBC and DOC are considered to be early indicators of C sequestration in soil for their quick turnover rate (Blair et al. 1995; Ghani et al. 2003; Haynes 2005). In addition, it has been reported that macroaggregates are sensitive to changes in land use and cultivation practice (Franzluebbers and Arshad 1997; Puget et al. 2000). Therefore, it is possible that SOC of macroaggregates and labile organic C pool may be sensitive to short-term conservation management practices.
In China, farmers generally burn crop residues in their fields to reduce the time and expense of handling them, causing environmental pollution. Therefore, a government policy favors these crop residues to be returned to field, and this has caused a fast development of conservation management practices in paddy field in South China since the 1980s. However, this long-term NT in paddy field can increase soil bulk density, decrease the plow layer of paddy soil, and promote weed growth, and for this reason, long-term NT should be avoided promoting conversion of 3-year NT to CT in paddy field. It is important to investigate the effects of short-term conservation management practices on SOC fractions and compositions of microbial communities in paddy field so as to evaluate if the research and the application of conservation management practices are suitable in China.
In this study, we have hypothesized that SOC fractions and compositions of microbial communities can respond quickly to short-term conservation management practices. We have monitored changes in the composition of main microbial groups by phospholipid fatty acids (PLFAs).
Materials and methods
Experimental site
Field experimental site is situated at Junchuan, Suizhou City, Hubei Province (31°69′ N latitude, 115°33′ E longitude, 84 m above sea level), China. The study site has a subtropical monsoon climate with an average annual temperature of 15.2 °C and an annual precipitation of 970 mm. The hydromorphic paddy soil is a silty clay loam derived from Quaternary yellow sediment. The main soil properties (0–20-cm depth) were pH, 5.38; organic C, 23.82 g kg−1; total N, 1.42 g kg−1; NH4 +-N, 0.49 mg kg−1; NO3 −-N, 12.87 mg kg−1; available P, 50.28 mg kg−1; and available K, 44.38 mg kg−1.
The experimental site was a rice-wheat rotation, where rice was direct seeded and grown from May to October each year and wheat was direct seeded and grown from October to May. The rice variety was Huanghuazhan (Oryza sativa L.) and the wheat variety, Zhengmai 9023 (Triticum aestivum L.).
Experimental design and agronomic management
The experiment was initiated in May 2011 with a split-plot design of a randomized complete block and with tillage practices (NT and CT) as the main plot and wheat residue returning (0- and 2,146-kg C ha−1 residue returning) as the subplots. Each treatment was replicated three times, and each plot had an area of 90 m2. The four treatments were CT0, CTS, NT0, and NTS. In the CT0 and NT0 treatment, wheat residues were removed and not returned to the field. In the CTS and NTS treatments, crop residues collected from preceding crops were air-dried, chopped to approximately 5–7 cm in length, and plowed into soil in the CT practice and mulched on the soil in the NT practice. In the case of the CT treatment, soil was cultivated to an 8–10-cm depth by hoe and subsequently mold-board plowed twice to a 20-cm depth 2–3 days before rice or wheat sowing. The C and N contents of the wheat residue used were 408.7 and 14.80 g kg−1, respectively.
During rice growing seasons, the weeds were controlled by spraying 36 % glyphosate at 3 L ha−1 in June 2011 and 2012. Rice was direct seeded manually at a rate of 22.5 kg ha−1 in June after herbicide application. The rice was then harvested in October. According to local conventional irrigation-drainage practices, the plots were irrigated immediately upon the germination of rice seeds. Thereafter, the plots were reirrigated to a depth of 10 cm whenever the water depth decreased to 1–2 cm above the soil surface during the growing season. After the rice was harvested, air-dried residues of rice were returned to the soil 3 weeks before wheat was seeded. Wheat was direct seeded at a rate of 150 kg ha−1 in November and harvested manually in June. During wheat growing seasons, no irrigation was conducted.
During rice growing season, commercial inorganic NPK fertilizer (N:P2O5:K2O = 15 %:15 %:15 %), urea (N 46 %), single superphosphate (P2O5 16 %), and potassium chloride (K2O 60 %) were applied at a rate of 210 kg N ha−1, 105 kg P2O5 ha−1, and 180 kg K2O ha−1. Commercial inorganic NPK fertilizers were broadcasted at the rate of 84 kg N ha−1 as basal fertilizers immediately after sowing and 126 kg N ha−1 of N-urea were equally divided into three doses applied at the mid-tillering, jointing, and panicle initiation stages. Both P and K fertilizers were only applied immediately after seeding.
Soil sampling and analytical methods
Soil samples were collected from the top layer (0–5-cm depth) in October 2012 (just after rice harvest) with a soil core sampler (inner diameter 7 cm) at five random points in each plot.
TOC was determined by oxidation with potassium dichromate and titration with ferrous ammonium sulfate (Jones 2001).
Aggregates were separated following the dry-sieving method described by Gartzia-Bengoetxea et al. (2009). Visible plant residues and stones were removed and then soil particles were sieved (<5 mm) by gently pressing large aggregates by hand. Aggregates were separated by placing 100 g of air-dried soil fragments (<5 mm) in a nest of sieves mounted on Retsch AS200 control (Retsch Technology, Düsseldorf, Germany). Sieves were mechanically shaken (amplitude 1.5 mm) for 2 min to separate soil into the following aggregate-size classes: 2–1 mm (macroaggregate), 1–0.25 mm, 0.25–0.053 mm, and <0.053 mm. Concentration of SOC of aggregate fractions was determined by a FlashEA 1112 elemental analyzer (Thermo Finnigan, Italy).
MBC was estimated by the fumigation-extraction method (Vance et al. 1987). Fumigated and nonfumigated soils were extracted with 0.5 M K2SO4 for 30 min (soil/extractant ratio = 1:4), and organic C in the soil extracts was measured by oxidation with potassium dichromate and titration with ferrous ammonium sulfate. MBC was calculated as EC/KEC, where EC is the organic C extracted from fumigated soil minus organic C extracted from nonfumigated soil and KEC is 0.38. The total C of soil was determined by a FlashEA 1112 elemental analyzer (Thermo Finnigan, Italy). Because the soil was free of carbonate, the soil organic C was assumed to be equal to the total C.
DOC was extracted as described by Jiang et al. (2006). Moist soil samples (equivalent to a 10-g oven-dry weight, 1:2.5 ratio) were shaken with water for 1 h at 250 r min−1, and then centrifuged for 10 min at 4,500 × g. The supernatant was filtered with a 0.45-μm membrane filter. The DOC of the filtrate was measured by oxidation with potassium dichromate and titration with ferrous ammonium sulfate (Jones 2001).
Easily oxidizable C (EOC) was determined as described by Blair et al. (1995). Air-dried soil samples (10 g) were shaken with 20 ml 333 M KMnO4 for 1 h at 60 r min−1. The suspension was then centrifuged for 5 min at 2,000 × g. The supernatant was diluted and the optical density was measured at 565 nm. Analyses were replicated three times.
PLFA analysis of soil
Composition of soil microbial communities was determined by PLFA analysis as described by Bossio et al. (1998) and Blair et al. (1995). Lipids were extracted from 3 g of freeze-dried soil by a single-phase chloroform-methanol-citrate buffer (1:2:0.8). The soil extracts were filtered and the chloroform phases were collected. The polar lipids were separated from neutral lipids and glycolipids on solid phase extraction columns (Supelco Inc, USA) by eluting with CHCl3, acetone, and methanol. Phospholipids were saponified and methylated to fatty acid methyl esters (FAME). An internal standard 19:0 FAME was used. Concentrations of PLFAs were determined by an Agilent (6890-5973N series) Gas Chromatograph equipped with a flame ionization detector and an HP-5 capillary column (30 m × 0.25 mm × 0.25 μm) with ultra super purified He as a carrier gas. Identification of individual compounds was based on the comparison of retention time and mass spectral data obtained from standard compounds isolated from microbial monoculture and environmental samples. FAME was analyzed by a MIDI Sherlock Microbial Identification System (MIDI, Newark, DE, USA) according to the manufacturer’s instructions.
Standard fatty acid/PLFA nomenclature (A:BwC) was used as follows: the number before the colon refers to the total number of C atoms and the number(s) following the colon refers to the number of double bonds and their location (after the “w”) in the fatty acid molecule. Abbreviations “Me,” “cy,” “I,” and “a” refer to methyl groups, cyclopropane groups, and iso- and anteiso-branched fatty acids, respectively. “Cis” and “trans” geometries are indicated by the suffixes “c” and “t,” respectively (Frostegård and Bååth 1996; Angela and Six 2012). The following biomarkers were used: microbial biomass, total PLFAs, the sum of all identified PLFAs, from C14 to C20; bacterial biomass, the sum of i14:0, 14:0, i15:0, a15:0, C15:0, i16:0, i17:0, a17:0, 16:1ω7c, cy17:0, 17:1ω8c, 18:1ω7c, C18:0, cy19:0, 18:1ω9t, and 18:1ω9c (Bach et al. 2010); fungal biomass, 18:2ω6c (Bach et al. 2010); monounsaturated fatty acids (MUFA), the sum of 16:1ω7c, 17:1ω8c, 18:1ω9c, and 18:1ω9t (Bausenwein et al. 2008); and saturated fatty acids (STFA), the sum of 14:0, 15:0, 16:0, and 18:0 (Bausenwein et al. 2008). We have also calculated the fungal to bacterial ratio (Kaur et al. 2005). Microbial stress was expressed by the cy19:0 to 18:1ω7 ratio (Jackson et al. 2003; Moore-Kucera and Dick 2008). PLFAs that contributed less than 0.5 % of the total amount extracted from each sample, and that were only observed in one sample, were eliminated from the data set; thus 21 PLFAs were used for statistical analysis in the present study.
Statistical analysis
To test the main effects and interactions of tillage and residue returning, general linear model analysis of variance designed for split plot was performed with tillage and residue returning as fixed factors and replicates as random factors. The least significant difference (LSD) test was used when tillage, residue returning, and/or their interactions were significant. Difference at P < 0.05 level was considered to be significant. All statistical analyses were performed by SPSS 16.0 (SPSS Inc, Chicago, IL). Differences in composition of microbial communities were evaluated by principal component analysis based on the relative abundances of PLFAs. Partial correlation analysis and redundancy analysis were performed to gain insights into the relationships between soil microbial community composition and organic C fractions under different treatments by SPSS 16.0 (SPSS Inc, Chicago, IL) and CANOCO software, respectively.
Results
SOC fractions
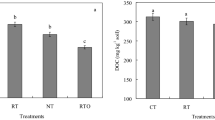
No significant differences in concentrations of TOC, DOC, and EOC were found between CT and NT treatments (Table 1). Compared with CT treatments, NT treatments only significantly increased MBC by 20.0 % (P < 0.05). Both MBC and DOC concentrations were 18 and 24 % higher (P < 0.05) under residue returning treatments than under no residue treatments, respectively. There were no interactive effects of tillage and residue returning on MBC and contents of TOC, DOC, and EOC.
Table 2 shows no significant differences in concentrations of SOC of aggregate fractions between CT and NT treatments. Residue returning only significantly increased concentration of SOC of 2–1-mm aggregate by 10 % (P < 0.05). The interaction between tillage and residue returning significantly affected concentration of SOC in 2–1-mm aggregate (P < 0.05).
Composition of soil microbial communities
No significant effects of tillage on bacterial biomass, F/B, and microbial stress are shown in Table 3. However, total PLFAs and fungal biomasses were 9.8 and 40.8 % higher under NT treatments than under CT treatments (P < 0.05), respectively, while MUFA/STFA were 15.5 % lower (P < 0.05). Significant differences between residue returning treatments were found in total PLFAs, bacterial biomass, fungal biomass, F/B, MUFA/STFA, and microbial stress. Residue returning significantly increased total PLFAs, bacterial biomass, fungal biomass, F/B, and MUFA/STFA by 31.1, 36.0, 95.9, 42.5, and 58.8 % (P < 0.05), respectively, but significantly reduced microbial stress by 45.9 % (P < 0.05). The interaction between tillage and residue returning significantly affected total PLFAs, bacterial biomass, fungal biomass, and MUFA/STFA (P < 0.05).
A total of 21 PLFAs were identified under different treatments (Fig. 1). PLFA profiles were dominated by the iC15:0 (9.8 %), C16:0 (16.5 %), 10Me17:0 (9.9 %), and Cyc19:0 (8.3 %) fatty acids, which together accounted for 43.0–47.3 % of the total PLFAs. Principal component analysis extracted two principal components, PC1 and PC2, accounting for 55.9 and 16.5 % of the overall variances, respectively. The dominant PLFAs of PC1 were C14:0, iC16:0, C16:0, C18:2w6c, and Cyc19:0 (loading value >0.9). Microbial community composition was strongly affected by treatments. The PLFA profiles showed a significant separation when comparing soil samples to CTS and NTS to CT0 and NT0 along PC1. A clear separation was also found among CT0, CTS, NT0, and NTS when comparing the PLFA patterns of soil samples along PC2. The most PLFAs were located to the right side along PC1 for NTS.
a Loading values for individual PLFAs shown by principal component analysis. b Principal component analysis of the PLFA pattern from soils (0–5 cm) under different treatments. Two components (PC1 and PC2) were extracted by principal component analysis, and PC1 accounted for 55.9 % and PC2 for 16.5 % of the original variance. CT0, conventional tillage; CTS, conventional tillage plus residue returning; NT0, no-tillage; NTS, no-tillage plus residue returning
Redundancy analysis of soil microbial communities shows that the coordinate from the first two ordination axes explained 99.5 % (the first axis 88.5 % and the second 11.0 %) of the variances (Fig. 2). Microbial community composition under different treatments was distinctly separated by the first two principal components. The SOC contents of 2–1- and 0.25–0.053-mm aggregates along the first axis were the most affecting microbial community composition. The SOC content of 2–1-mm aggregate was negatively correlated with the first axis, and the SOC content of 0.25–0.053-mm aggregate was positively related to the first axis. In addition, among TOC and labile organic C fractions, the SOC content of 2–1-mm aggregate had the highest degree of correlation with microbial community composition.
Redundancy analysis of soil microbial communities and SOC fractions under different treatments. The amount of variability explained by all the canonical axes was 99.5 %. TOC, total organic C; MBC, microbial biomass C; DOC, dissolved organic C; EOC, easily oxidizable C; MUFA/STFA, monounsaturated fatty acids/saturated fatty acids; F/B, fungal biomass/bacterial biomass; microbial stress, cy19:0/18:1ω7; CT0, conventional tillage; CTS, conventional tillage plus residue returning; NT0, no-tillage; NTS, no-tillage plus residue returning
Partial correlation analysis indicated that MBC was significantly correlated with total PLFAs and bacterial and fungal biomasses (Table 4). The SOC of 2–1-mm aggregate was significantly and positively correlated with total PLFAs, bacterial biomass, fungal biomass, and MUFA/STFA, and the partial correlation coefficient of SOC within 2–1-mm aggregate (2–1 mm) were higher than the coefficients of other organic C fractions. In general, there were no significant relationships between TOC, DOC, EOC, and SOC contents of other aggregate fractions with microbial parameters.
Discussion
Effect of tillage and residue returning on SOC fractions
Long-term NT can increase TOC concentrations at top soil layer (Helgason et al. 2009; Mathew et al. 2012), while the effects of short-term NT on TOC concentration are contradictory (Carpenter-Boggs et al. 2003; Spedding et al. 2004; Helgason et al. 2010). In our short-term study, no significant differences between tillage systems were found in TOC contents of different aggregate fractions (Tables 1 and 2), probably because the duration (less 2 years) of NT was not long enough to cause a significant change in the soil organic matter content (Kay and VandenBygaart 2002; Mathew et al. 2012). Kay and VandenBygaart (2002) reported that assessments of tillage-induced changes in organic matter content are most consistent after a minimum of 15 years following the conversion of CT to NT.
Crop residue plays important roles in increasing organic C sequestration, reducing soil temperature fluctuation, and conserving soil moisture (Schwab et al. 2002; Zhu et al. 2014). It is well established that TOC needs a long period (>5 years) to respond to residue amendments for its slow turnover rate and the great soil organic C pool (West and Post 2002; Wang et al. 2012), while labile organic C fractions (i.e., MBC and DOC contents) can respond to soil management more quickly than TOC content (Blair et al. 1995; Ghani et al. 2003; Haynes 2005). In our study, MBC and DOC contents of the 0–5-cm soil layer were higher under residue returning treatments than under no residue treatments (Table 1). Similarly, Zhu et al. (2014) found that straw returning significantly increased MBC and DOC contents of the 0–7-cm soil layer. This is due to favorable water content and soil temperature of top soil layer during crop growing season, causing a fast residue decomposition with microbial growth and microbial necromass production (Miltner et al. 2012).
In the present study, residue returning significantly increased concentration of SOC of 2–1-mm aggregate, but not that of SOC contents of other aggregate fractions (P < 0.05, Table 2), possibly because macroaggregates are more sensitive than micro-aggregates to changes in land use and cultivation practice (Franzluebbers and Arshad 1997; Puget et al. 2000). Jastrow (1996) reported that SOC of macroaggregates has a faster turnover than that of micro-aggregates. Furthermore, residue returning increased soil C content and enhanced microbial activity (Chen et al. 2009; Zhu et al. 2014), which have contributed to the binding of residue and soil particles into macroaggregates (Jastrow 1996; Six et al. 1999), thus increasing not only aggregates stability but also SOC content and C sequestration (Mathew et al. 2012; Zhu et al. 2014).
Effect of tillage and residue returning on soil microbial community
No-tillage compared to CT reduces soil physical disturbance with enrichment of organic matter in soil surface, providing a more beneficial environmental condition for soil biota (Sundermeier et al. 2011). In this study, as expected, total PLFAs and fungal biomasses were higher under NT than under CT (Table 3). However, we did not observe a shift toward fungal dominance as indicated by F/B under NT. Similar results were also reported by Spedding et al. (2004). Probably not only fungal activity but also bacterial activity was favored by NT (Helgason et al. 2009). MUFA/STFA, which is a sensitive indicator of soil microorganisms in response to agricultural management (Bossio et al. 1998), was significantly lower in NT than in CT soils (Table 3), possibly due to more anaerobic conditions under NT as a result of lower physical disturbance (Gouaerts et al. 2007; Spedding et al. 2004); this may have also lead to bacteria stimulation under NT.
Principal component analysis shows that residue returning was the main factor influencing the composition of microbial communities (Fig. 1). Total PLFAs, bacterial biomass, fungal biomass, and F/B were higher under residue returning than under no residue returning treatments (Table 3). Given the dependence of microbial community composition on resource quality and availability (Helgason et al. 2010), it is likely that crop residues provide a C source for microbial activity (Zhu et al. 2014) improving environmental conditions for biota (Helgason et al. 2010). Moreover, lower microbial stress under residue returning treatments (Table 3) may indirectly explain the increases in microbial biomasses. Additionally, higher MUFA/STFA under residue returning than under no residue returning treatments (Table 3) suggests that residue returning might improve aeration of top soil layer (Bossio et al. 1998). Increased F/B under residue returning treatments might be attributed to increased C/N in soil. Fierer et al. (2009) reported that the F/B ratio generally increases with increasing C/N in soil because fungi have a lower N demand and use C more efficiently than bacteria.
The relationships between microbial communities and SOC fractions
Redundancy analysis of soil microbial communities showed that SOC content of 2–1-mm aggregate was related to soil microbial diversity (Fig. 2). Moreover, partial correlation analysis also showed that the SOC content of macroaggregate (2–1 mm) was significantly and positively related to microbial parameters, and the partial correlation coefficient of SOC of macroaggregate (2–1 mm) was higher than coefficients of other organic C fractions (Table 4). Changes in SOC content of macroaggregate (2–1 mm) induced by short-term conservation management practices may cause changes in microbial community composition. Similarly, it was reported that SOC content of macroaggregates was related to changes in management practices (Franzluebbers and Arshad 1997; Carter et al. 1998; Freixo et al. 2002). Crop residue returning can contribute to the binding of residue and soil particles into macroaggregates (Jastrow 1996; Six et al. 1999), thus improving aggregate formation and stabilization (Golchin et al. 1997; Six et al. 2002). Moreover, the quantity and quality of organic substrates can modulate activity, function, and composition of microbial community (Bending et al. 2002; Bausenwein et al. 2008). Griffiths et al. (1999) pointed out that microbial community composition changed by increasing C substrate supply. Further, soil aggregates regulate both the composition and activity of microbial community (Gupta and Germida 1988; Miller et al. 2009; Plaza-Bonilla et al. 2014) by affecting microbial access to oxygen, substrates, and water (Young and Ritz 2000). In this way, SOC can affect microbial community composition through influencing aggregate formation and stabilization.
Conclusions
No significant differences between CT and NT treatments were found in TOC, labile organic C fractions (except for MBC), and SOC of aggregate fractions, while residue returning significantly increased MBC and SOC content of macroaggregate (2–1 mm). No-tillage compared to CT significantly increased total PLFAs, fungal biomass, and MUFA/STFA. Total PLFAs, bacterial biomass, fungal biomass, F/B, and MUFA/STFA were higher under residue returning than under no residue returning treatments, but microbial stress was lower. Changes in SOC of 2–1-mm aggregate induced by short-term conservation management practices might be important in influencing soil microbial community composition. In addition, we only conducted this study in a site, and thus a further study about microbial community composition and contents of SOC under conservation management practices from different typical paddy fields is needed to verify the relationships between compositions of soil microbial communities and SOC fractions under a rice-wheat cropping system.
References
Al-Kaisi MM, Yin XH, Licht MA (2005) Soil carbon and nitrogen changes as influenced by tillage and cropping systems in some Iowa soils. Agric Ecosyst Environ 105:635–647
Angela YY, Six KJ (2012) Microbial community assimilation of cover crop rhizodeposition within soil microenvironments in alternative and conventional cropping systems. Plant Soil 356:315–330
Bach EM, Baer SG, Meyer CK, Six J (2010) Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol Biochem 42:2182–2191
Bausenwein U, Gattinger A, Langer U, Embacher A, Hartmann HP, Sommer M, Munch JC, Schloter M (2008) Exploring soil microbial communities and soil organic matter: variability and interactions in arable soils under minimum tillage practice. Appl Soil Ecol 40:67–77
Bending GD, Turner MK, Jones JE (2002) Interactions between crop residue and soil organic matter quality and the functional diversity of soil microbial communities. Soil Biol Biochem 34:1073–1082
Blair GJ, Lefory RDB, Lise L (1995) Soil carbon fractions based on their degree of oxidation and the development of a carbon management index for agricultural system. Aust J Agric Res 46:1459–1466
Bossio DA, Scow KM, Gunapala N, Graham KJ (1998) Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microb Ecol 36:1–12
Carpenter-Boggs L, Stahl PD, Lindstrom MJ, Schumacher TE (2003) Soil microbial properties under permanent grass, conventional tillage, and no-till management in South Dakota. Soil Till Res 71:15–23
Carter MR, Gregorich EG, Angers DA, Donald RG, Bolinder MA (1998) Organic C and N storage, and organic C fractions, in adjacent cultivated and forested soils of eastern Canada. Soil Till Res 47:253–261
Chen HQ, Hou RX, Gong YS, Li HW, Fan MS, Kuzyakov Y (2009) Effects of 11 years of conservation tillage on soil organic matter fractions in wheat monoculture in Loess Plateau of China. Soil Till Res 106:85–94
Dawe D, Frolking S, Li C (2004) Trends in rice-wheat area in China. Field Crops Res 87:89–95
Dong HY, Kong CH, Wang P, Huang QL (2014) Temporal variation of soil friedelin and microbial community under different land uses in a long-term agroecosystem. Soil Biol Biochem 69:275–281
Feng Y, Motta AC, Reeves DW, Burmester CH, Santen EV, Osborne JA (2003) Soil microbial communities under conventional-till and no-till continuous cotton systems. Soil Biol Biochem 35:1693–1703
Fierer N, Schimel JP, Holden PA (2009) Variations in microbial community composition through two soil depth profiles. Soil Biol Biolchem 35:167–176
Franzluebbers AJ, Arshad MA (1997) Soil microbial biomass and mineralizable carbon of water-stable aggregates. Soil Sci Soc Am J 61:1090–1097
Freixo AA, Machado PL, Santos HP (2002) Soil organic carbon and fractions of a rhodic ferralsol under the influence of tillage and crop rotation systems in southern Brazil. Soil Till Res 64:221–230
Frey SD, Elliott ET, Paustian K (1999) Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol Biolchem 31:573–585
Frostegård A, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Gartzia-Bengoetxea N, González-Arias A, Merinob A, Aranoa IM (2009) Soil organic matter in soil physical fractions in adjacent semi-natural and cultivated stands in temperate Atlantic forests. Soil Biol Biochem 41:1674–1683
Gathala MK, Ladha JK, Kumar V, Saharawat YS, Kumar V, Sharma PK, Sharma S, Pathak H (2011a) Tillage and crop establishment affects sustainability of South Asian rice-wheat system. Agron J 103:961–971
Gathala MK, Ladha JK, Saharawat YS, Kumar V, Sharma PK (2011b) Effect of tillage and crop establishment methods on physical properties of a medium-textured soil under a seven-year rice-wheat rotation. Soil Sci Soc Am J 75:1851–1862
Ghani A, Dexter M, Perrott WK (2003) Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilization, grazing and cultivation. Soil Biol Biochem 35:1231–1243
Golchin A, Baldock JA, Oades JM (1997) A model linking organic matter decomposition, chemistry, and aggregate dynamics. In: Lal R, Kimble JM, Follett RF, Stewart BA (eds) Soil processes and carbon cycle. CRC, Boca Raton, pp 245–266
Gosling P, Parsons N, Bending GD (2013) What are the primary factors controlling the light fraction and particulate soil organic matter content of agricultural soils? Biol Fertil Soils 49:1001–1014
Gouaerts B, Mezzalama M, Unno Y, Sayre KD, Marco LG, Vanherck K, Dendooven L, Deckers J (2007) Influence of tillage, residue management, and crop rotation on soil microbial biomass and catabolic diversity. Appl Soil Ecol 37:18–30
Griffiths BS, Ritz K, Ebblewhite N, Dobson G (1999) Soil microbial community structure: effects of substrate loading rates. Soil Biol Biochem 31:145–153
Gupta VVSR, Germida JJ (1988) Distribution of microbial biomass and its activity in different soil aggregate size classes as affected by cultivation. Soil Biol Biochem 20:777–786
Haynes RJ (2005) Labile organic matter fractions as central components of the quality of agricultural soils: an overview. Adv Agron 85:221–268
Helgason BL, Walley FL, Germida JJ (2009) Fungal and bacterial abundance in long-term no-till and intensive-till soils of the Northern Great Plains. Soil Sci Soc Am J 73:120–127
Helgason BL, Walley FL, Germida JJ (2010) Long-term no-till management affects microbial biomass but not community composition in Canadian prairie agroecosytems. Soil Biol Biochem 42:2192–2202
Hendrix PF, Franzluebbersm AJ, McCracken DV (1998) Management effects on carbon accumulation and loss in soils on the southern Appalachian Piedmont of Georgia, USA. Soil Till Res 47:245–251
Hobbs PR, Sayre K, Gupta R (2008) The role of conservation agriculture in sustainable agriculture. Phil Trans R Soc B 363:543–555
Huang M, Jiang L, Zou Y, Xu SH, Deng GF (2013) Changes in soil microbial properties with no-tillage in Chinese cropping systems. Biol Fertil Soils 49:373–377
Jackson LE, Calderon FJ, Steenwerth KL, Scow KM, Rolston DE (2003) Responses of soil microbial processes and community structure to tillage events and implications for soil quality. Geoderma 114:305–317
Jacobs A, Rauber R, Ludwing B (2009) Impact of reduced tillage on carbon and nitrogen storage of two Haplic Liuvsols after 40 years. Soil Till Res 102:158–164
Jastrow JD (1996) Soil aggregate formation and the accrual of particulate and mineral associated organic matter. Soil Biol Biochem 28:656–676
Jiang PK, Xu QF, Xu ZH, Cao ZH (2006) Seasonal changes in soil labile organic pools within a Phyllostachys praecox stand under high rate fertilization and winter mulch in subtropical China. For Ecol Manage 236:30–36
Jones JJB (2001) Laboratory guide for conducting soil tests and plant analysis. CTC, Boca Raton London, pp 140–142
Kaur A, Chaudhary A, Kaur A, Choudhary R, Kaushik R (2005) Phospholipid fatty acid—a bioindicator of environment monitoring and assessment in soil ecosystem. Curr Sci 89:1103–1112
Kay BD, VandenBygaart AJ (2002) Conservation tillage and depth stratification of porosity and soil organic matter. Soil Till Res 66:107–118
Kumari M, Chakraborty D, Gathala MK, Pathak H, Dwivedi BS, Tomar RK, Garg RN (2011) Soil aggregation and associated organic carbon fractions as affected by tillage in a rice-wheat rotation in North India. Soil Biol Biochem 75:560–567
Ladha JK, Pathak H, Padre AT, Dawe D, Gupta RK (2003) Productivity trends in intensive rice-wheat cropping systems in Asia. In: Ladha JK, Hill JE, Duxbury JM, Gupta RK, Buresh RJ (eds) Improving the productivity and sustainability of rice-wheat systems: issues and impacts. American Society of Agronomy. Series. ASA Special Publication, Madison, pp 45–76
Ladha JK, Kumar V, Alam MM, Sharma S, Gathala MK, Chandna P, Saharawat YS, Balasubramanian V (2009) Integrating crop and resource management technologies for enhanced productivity, profitability and sustainability of the rice-wheat system in South Asia. IRRI, Los Baños, Philippines, pp 69–108
Liang AZ, Yang XM, Zhang XP, Shen Y, Shi XH, Fan RQ, Fang HJ (2010) Short-term impacts of no tillage on aggregate-associated C in black soil of Northeast China. Agric Sci China 9:93–100
Liu XB, Herbert SJ, Zhang XY (2010) Feeding China’s growing needs for grain. Nature 465:420–420
Mathew RP, Yu CF, Githinji L, Githinji L, Ankumah R, Balkcom KS (2012) Impact of no-tillage and conventional tillage systems on soil microbial communities. Appl Environ Soil Sci 2012:1–10
Miller MN, Zebarth BJ, Dandie CE, Burton DL, Goyer C, Trevors JT (2009) Denitrifier community dynamics in soil aggregates under permanent grassland and arable cropping systems. Soil Sci Soc Am J 73:1843–1851
Miltner A, Bomback P, Schmidt-Brucken B, Kastner M (2012) SOM genesis: microbial biomass as a significant source. Biogeochemistry 111:41–55
Minoshima H, Jackson LE, Cavagnaro TR, Ferris SSM, Temple SR, Mitchell JP (2007) Soil food webs and carbon dynamics in response to conservation tillage in California. Soil Sci Soc Am J 71:952–963
Moore-Kucera J, Dick RP (2008) PLFA profiling of microbial community structure and seasonal shifts in soils of a Douglas-fir chronosequence. Microbial Ecol 55:500–511
Murugan R, Koch HJ, Joergensen RG (2014) Long-term influence of different tillage intensities on soil microbial biomass, residues and community structure at different depths. Biol Fertil Soils 50:487–498
Palm C, Blanco-Canqui H, DeClerck F, Gatere L, Grace P (2014) Conservation agriculture and ecosystem services: an overview. Agric Ecosyst Environ 187:87–105
Paul EA, Clark FE (1989) Soil microbiology and biochemistry. Academic, San Diego
Plaza-Bonilla D, Cantero-Martínez C, Álvaro-Fuentes J (2014) Soil management effects on greenhouse gases production at the macroaggregate scale. Soil Biol Biochem 68:471–481
Puget P, Chenu C, Balesdent J (2000) Dynamics of soil organic matter associated with particle-size fractions of water-stable aggregates. Eur J Soil Sci 51:595–605
Qi J, van Keulen H, Hengsdijk H (2010) Modeling biomass, nitrogen and water dynamics in rice-wheat rotations. Agric Systems 103:433–443
Quilty JR, Cattle SR (2011) Use and understanding of organic amendments in Australian agriculture: a review. Soil Res 49:1–26
Schwab EB, Reeves DW, Burmester CH, Rapper RL (2002) Conservation tillage systems for cotton in the Tennessee Valley. Soil Sci Soc Am J 66:569–577
Six J, Elliott ET, Paustian K (1999) Aggregate and soil organic matter dynamics under conventional and no-tillage systems. Soil Sci Soc Am J 63:1350–1358
Six J, Conant RT, Paul EA, Paustian K (2002) Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241:155–176
Spedding TA, Hamel C, Mehuys GR, Madramootoo CA (2004) Soil microbial dynamics in maize-growing soil under different tillage and residue management systems. Soil Biol Biochem 36:499–512
Sundermeier AP, Islam KR, Raut Y, Reeder RC, Dick WA (2011) Continuous no-till impacts on soil biophysical carbon sequestration. Soil Sci Soc Am J 75:1779–1788
Uri ND (1999) Factors affecting the use of conservation tillage in the United States. Water, Air, Soil Pollut 116:621–638
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wall DH, Bardgett R, Behan-Pelletier V, Herrick JE, Jones TH, Strong DR (2013) Soil ecology and ecosystem services. Oxford University Press, UK
Wang JJ, Li XY, Zhu AN, Zhang XK, Zhang HW, Liang WJ (2012) Effects of tillage and residue management on soil microbial communities in North China. Plant Soil Environ 58:28–33
West TO, Post M (2002) Soil organic carbon sequestration rates for crops with reduced tillage and enhanced rotation. Soil Sci Soc Am J 66:1930–1946
Young IM, Ritz K (2000) Tillage, habitat space and function of soil microbes. Soil Till Res 53:201–213
Zhu LQ, Hu NJ, Yang MF, Zhang XH, Zhang ZW (2014) Effects of different tillage and straw return on soil organic carbon in a rice-wheat rotation system. Plos One 9:e88900
Acknowledgments
This work was funded by the National Technology Project for High Food Yield, China (2011BAD16B02), National Natural Science Foundation of China (31100319), Fundamental Research Funds for the Central Universities (2013PY106), and Program for Changjiang Scholars and Innovative Research Team in University of China (IRT1247). We are grateful to Paolo Nannipieri, editor-in-chief of Biology and Fertility of Soils, and four anonymous referees for their critical comments on the original manuscript.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Guo, LJ., Zhang, ZS., Wang, DD. et al. Effects of short-term conservation management practices on soil organic carbon fractions and microbial community composition under a rice-wheat rotation system. Biol Fertil Soils 51, 65–75 (2015). https://doi.org/10.1007/s00374-014-0951-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0951-6