Abstract
The present study aimed to assess the effects of anthropogenic activities on the heavy metal levels in the Langat River by transplantation of Corbicula javanica. In addition, potential ecological risk indexes (PERI) of heavy metals in the surface sediments of the river were also investigated. The correlation analysis revealed that eight metals (As, Co, Cr, Fe, Mn, Ni, Pb and Zn) in total soft tissue (TST) while five metals (As, Cd, Cr, Fe and Mn) in shell have positively and significantly correlation with respective metal concentration in sediment, indicating the clams is a good biomonitor of the metal levels. Based on clustering patterns, the discharge of dam impoundment, agricultural activities and urban domestic waste were identified as three major contributors of the metals in Pangsun, Semenyih and Dusun Tua, and Kajang, respectively. Various geochemical indexes for a single metal pollutant (geoaccumulation index (I geo), enrichment factors (EF), contamination factor (C f) and ecological risk (Er)) all agreed that Cd, Co, Cr, Cu, Fe, Mn, Ni and Zn are not likely to cause adverse effect to the river ecosystem, but As and Pb could pose a potential ecological risk to the river ecosystem. All indexes (degree of contamination (C d), combined pollution index (CPI) and PERI) showed that overall metal concentrations in the tropical river are still within safe limit. River metal pollution was investigated. Anthropogenic activities were contributors of the metal pollution. Geochemical indexes showed that metals are within the safe limit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The potential role of heavy metals to humans are vastly different; some metals such as Cu ,Zn and Fe only become toxic when excess, while other non-essential elements, such as Pb, Cd and Ni, are toxic even in low concentration (Schwartz et al. 2010). Through bioaccumulation and biomagnification effect, the bioconcentration of heavy metals may increase significantly through the food chains.
The impact of agricultural activities on the elevations of metal concentrations in biological samples had been reported in the literature. For example, the vineyard farms had been found to be associated to the usage of pesticides in viticulture (Rocha et al. 2015) while large-scale usage of fertilisers and pesticides at a paddy field had contributed to the enriched metal concentrations in bivalve Vollorita cyprinoides (George et al. 2013). Atafar et al. (2010) found that the elevated levels of Cd, Pb and As in the soil used for cultivation of durum wheat were related to over-application of fertilisers and pesticides. Zn was found to be the main contributor to total risk in all organic wastes, which acted as agricultural fertiliser (Lopes et al. 2011). Aoun et al. (2015) reported that Pb, Cu and Zn were highly accumulated in edible marine fish including Diplodus sargus, Oblada melanura, Pagellus erythrinus, Siganus rivulatus and Redcoat squirrelfish (Nylon). These metals could be originated from the wastes of a phosphate fertiliser plant.
The effects of domestic discharges on heavy metal levels in molluscs had been widely reported in the literature (Walsh et al. 1994). For example, Maanan (2008) reported that elevated Pb levels in marine mussels that received large domestic wastewater source while George et al. (2013) reported that the urban domestic wastes and agricultural inputs have contributed to increased metal concentrations in Villorita cyprinoides. Sewage discharge, industrial estates and mining activity had also been found to correlate to the higher levels of metals in marine molluscs (Usero et al. 2005).
The concern on the impact of river dam on the dynamic of biogeochemical cycles of metallic pollutants had also been reported in the literature. Zhao et al. (2013) reported that the longitudinal distributions of As, Cd, Cr, Cu, Ni, Pb and Zn in sediments collected from the vicinity of Manwan Dam (China) were consistently higher than those from upstream sampling points. Besides, enriched Hg concentrations in the bottom sediment of the reservoir bed had been previously reported (Hall et al. 2005; Teisserenc et al. 2014). Therefore, its potential impact on the downstream of the dam should be assessed.
The developmental activities in Malaysia have resulted in deterioration of river quality, depletion of river resources, public health risks and loss of river biodiversity (Lim et al. 2013). Rapid development and urbanisation contributed to the increased input of heavy metals into the ecosystem (Aris and Looi 2015). Various industrial processes such as mining, smelters, combustion of fossil fuel and gasoline, usage of automobile, waste incinerators, and productions of jewellery, paint pigments, pottery glazes, inks, dyes, rubbers, plastics and medicines are the main contributors for these anthropogenic heavy metals (Kochubovski 2011; Landis and Yu 2003; Ozaki et al. 2004).
The establishment of biomonitor for freshwater region is necessary, and the freshwater clam Corbicula javanica was chosen based on (1) its availability at the study site and (2) its potential as a good biomonitor similar to its genus sibling C. fluminea, which is well reported as a good biomonitor of heavy metals (Marie et al. 2006; Tran et al. 2001). However, there is limited information in the literature on the use of C. javanica as a biomonitor of heavy metal pollution. In the present study, transplantation of the C. javanica from sites where this species can be abundantly found to other sites of the same river was conducted to overcome the uneven distribution of this clam species.
Besides comparing the heavy metal concentrations between river surface sediment and the shell and total soft tissues (TST) of C. javanica, ecological risk assessment (ERA) was done to assess the potential risk of the heavy metals in the environment. It was achieved by calculating some established risk assessment indexes based on heavy metal concentrations in surface sediment. Among all the ERAs, enrichment factors (EF) (Buat-Menard and Chesselet 1979), geoaccumulation index (I geo) (Muller 1969) and potential ecological risk index (PERI) (Hakanson 1980) were calculated in this study.
The objectives of the present study were to assess the heavy metal pollution in the Langat River by transplantation of C. javanica from upstream pristine site to downstream polluted sites of the river, and to determine the potential ecological risk of heavy metals in the surface sediments of the Langat River.
Material and methods
Study sites
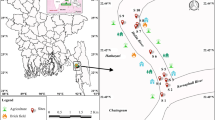
Several surveys, samplings and trial-and-error transplantation studies in the Langat River were conducted between June 2012 and June 2013. The sampling sites in this study were located along upstream and midstream of Langat River and its branch, Semenyih River (Fig. 1). Overall, a total of eight, seven, two and six times of trial-and-error transplantations had been conducted in Pangsun (PS), Semenyih (SM), Dusun Tua (DT) and Kajang (KJ), respectively.
Three-day transplantation and field measurements
Cages for transplantation were prepared by using folded 13 × 13-cm plastic net tied with cable tie. Owing to the limitation of the distribution of C. javanica in the downstream of Langat River, transplantation from the upstream to the downstream sites was carried out for this biomonitoring study. A total of 12 cages (30 clams for each cage) was captured in the upstream PS and transplanted to downstream sites at DT, SM and KJ. During the transplantation, the cages were fixed to the riverbank by rope tied to some trees or other stationary object with the cage at the other end of the rope. The cages were then gently inserted into river stream. Before transplantation, 30 clams were also collected at PS for background study. The cages were collected after 3 days of exposure. The present study used 3-day exposure because of massive death of the exposed clams at longer period of exposure and the short period is sufficient for bioaccumulation of heavy metals in bivalves based on several previous studies. For example, Cardoso et al. (2015) showed that 3 days of exposure was enough for the bivalve to react to the environment while Won et al. (2016) artificially exposed Manila clam Ruditapes philippinarum to Cu and Pb for 24 h and found that the two metal levels increased in the clams after the short period of exposure. The collected clams and surface sediments (0–10 cm) near the riverbank were placed in plastic bags and put into an ice compartment until transported to laboratory. In the laboratory, they were stored at −20°C until further analysis.
Sample preparation
Sediments were dried in the oven at 60°C for at least 16 h until a constant dry weight. Later, dried sediments were grinded by using pestle and mortar and sieved through a 63-μm stainless steel aperture. While sieving, the sieve was shaken vigorously to produce homogeneity (Yap et al. 2002). The sieving is important to standardise the effects of particle grain size (Dhanakumar et al. 2015; Yuan et al. 2014).
The clams were thawed at room temperature prior to the dissection. Before the dissection of the clam, the shell length, width and heights of each individual were measured by using digital calliper (Mitutoyo ABSOLUTE Digimatic Calliper 0–200 mm) with an accuracy up to 0.01 cm. The shell lengths, widths and heights for each population of C. javanica ranged from 10.32 to 24.95, 8.53 to 19.52 and 5.65 to 13.33 mm, respectively (Table 5). The TST and shell were carefully separated by using forceps and scalpels washed with distilled water. The dissected samples in a single cage were pooled together to get enough samples for analysis. Three replicative metal analyses were done in order to get accurate heavy metal concentrations for each individual cage/homogenised pool. The dissected pooled tissues were then dried in 60°C for at 72 h minimum until a constant dry weight (dw) was achieved (Yap et al. 2002).
Sample treatment
Sample digestion
For sediments, 0.50 g of sample (<63-μm diameter) was digested according to Milestone Microwave Laboratory System method for sea sediment (Milestone 2014). The dried sediment sample was mixed with analytical grade 6 ml of HNO3 (65%), 1 ml of H2O2 (35%) and 1 ml of HClO4 in an acid-washed closed Teflon vessels. The vessels were sealed and placed in a microwave oven at 200°C for 30 min. For samples of TST and shells of clams, 0.50 g of sample was digested according to Milestone Microwave Laboratory System method for oyster tissue (Milestone 2014). The dried sample (TST or shell) was mixed with 7 ml of HNO3 and 1 ml of H2O2 in an acid-washed closed Teflon vessels. The vessels were sealed and placed in a microwave oven at 200°C for 25 min.
The digests were transferred to 100-ml volumetric flask and diluted to 100 ml with ultrapure water. During dilution, the Teflon vessel was rinsed at least three times with ultrapure water, and the rinsing water was poured into volumetric flask to ensure all digests were washed into the 100-ml volumetric flask. The sample was then filtered through Whatman no. 1 filter paper, and the filtrate was stored in an acid-washed pill box until further metal analysis (Cheng and Yap 2015).
Sequential extraction technique
A sequential extraction technique (SET) was performed using the four-stage procedure originally recommended by Badri and Aston (1983), and has been extensively used for the speciation of heavy metals in the sediments into four geochemical fractions namely, the easily, freely, leachable or exchangeable (EFLE); acid-reducible (AR); oxidisable-organic (OO); and the resistant (RES). The SET gives a better view of the available fractions of the total metal concentration that may undergo changes due to alterations in the physico-chemical and biological characteristics of the environment (Cristina et al. 1986). It is also believed that the distribution of heavy metals in various geochemical fractions is important to assess the readily available forms of metals that are associated with sediments (Giordano et al. 1992).
Metal determination
The filtered samples were analysed for Cu, Fe, Ni, Pb and Zn by using an air-acetylene flame atomic absorption spectrometry (FAAS; Perkin-Elmer Model AAnalyst 800) while As, Cd, Co, Cr and Mn by using an inductively coupled plasma-mass spectrometry (ICP-MS; Perkin-Elmer Model Elan 600).
Quality control and quality assurance
All glassware and equipment used were soaked in acid (5% HNO3) for 72 h. Procedural blanks and quality control samples were analysed after every 5–10 samples in order to check for the sample accuracy. For each fraction of the SET procedure, a blank was employed using the same procedure to ensure that the samples were free of contaminants. For data validation, present procedural analytical methods were checked with certified reference material (CRM) from dogfish liver (DOLT-3, National Research Council Canada), mussel tissue (Standard Reference Material® 2976; National Institute of Standards Technology), soil (NSC) and marine sediments (MESS-3, National Research Council Canada, Beaufort Sea). The recoveries of CRMs are being satisfactory and are presented in S1.
Statistical analyses
Statistical analyses were done by using a statistical software, Statsoft STATISTICA version 10 for Windows. The present study included Pearson’s correlation analysis (CA), cluster analysis, factor analysis and multiple linear stepwise regression analysis (MLSRA). Prior to all the analyses, log10 (mean + 1)-transformed data were performed because the transformed ones gave better results as compared to the untransformed raw data (Yap et al. 2010; Zar 1996). This is because the data transformation can improve the normality of variables (Osborne 2002). Cluster analysis was performed based on Ward’s method Euclidean distances. The Student-Newman-Keuls (SNK) post hoc analysis was done by using the SPSS software version 21 for Windows to determine the differences between the mean values (Zar 1996).
Data treatment
Geoaccumulation index and enrichment factor
The values of I geo were calculated according to Muller’s (1969) formula:
where C n: heavy metal concentrations measured in the sediments; B n: geochemical background according to the preindustrial reference values (μg/g dw) namely 15, 1.0, 90, 50, 70 and 175 for As, Cd, Cr, Cu, Pb and Zn (Hakanson, 1980), respectively, 17.3, 50,400 and 47 for Co, Fe and Ni (Rudnick and Gao, 2003), respectively, and 1000 for Mn (Emsley, 2001). The factor 1.5 was introduced to minimise the effect of possible variation in the background values, which might be attributed to lithologic variations in the sediments (Binta Hasan et al. 2013) and allows content fluctuations of a given substances in the environment as well as very small anthropogenic influences (Loska et al. 1997). Six classes of geoaccumulation have been distinguished by Muller (1969) to classify extend of heavy metal pollution based on values of I geo (S2).
The EF in this study was determined using a formula defined by Buat-Menard and Chesselet (1979), with Fe as a normaliser:
where (C n/C Fe) sample: metal to Fe ratio in the sediments; (C n/C Fe) crust: metal to Fe ratio in the earth crust, which considered as preindustrial unpolluted metal value of a sediment.
The normalisation using Fe is necessary to correct for differences in sediment grain size and mineralogy (Schi and Weisberg 1999). Fe is a major sorbent phase for trace metals and is a quasiconservative tracer of the natural metal-bearing phases in fluvial and coastal sediments (Schi and Weisberg 1999). According to Binta Hasan et al. (2013), natural resources (98%) vastly dominated the input of Fe. In this expression, the normaliser (reference element) is assumed to have little variability of occurrence and is present in trace concentration in the examined environment (Loska et al. 1997). The degrees of EF are categorised by Taylor (1964) and Birth (2003), as shown in S2.
Ecological risk assessment by potential risk of individual metal and potential ecological risk index
Contamination factor (\( {C}_{\mathrm{f}}^{\mathrm{i}} \)) was proposed by Hakanson (1980) to describe the contamination of toxic substances in an aquatic environment. The \( {C}_{\mathrm{f}}^{\mathrm{i}} \) was calculated using a formula below:
where \( {C}_{\mathrm{f}}^{\mathrm{i}} \): contamination factor; \( {C}_{\mathrm{D}}^{\mathrm{i}} \): mean metal concentration in the sediment; \( {C}_{\mathrm{R}}^{\mathrm{i}} \): preindustrial reference values (PRV) in the sediments. Hakanson (1980) has classified the \( {C}_{\mathrm{f}}^{\mathrm{i}} \) values into four categories (S2).
Degree of contamination (C d) by Hakanson (1980) is mathematically calculated as a formula as following:
where \( {C}_{\mathrm{f}}^{\mathrm{i}} \): contamination factors for individual metal; \( {C}_{\mathrm{D}}^{\mathrm{i}} \): mean metal concentration; \( {C}_{\mathrm{R}}^{\mathrm{i}} \): preindustrial reference values in the sediments as mentioned above. The descriptions of C d proposed by Hakanson (1980) are presented in S2.
According to Hakanson (1980), the potential risk for individual metal can be calculated using formula as follows:
where \( {T}_{\mathrm{r}}^{\mathrm{i}} \): toxic-response factor namely 10, 30, 2, 5, 5 and 1 for As, Cd, Cr, Cu, Pb and Zn (Hakanson 1980), respectively, and 1 and 5 for Mn and Ni (Xu et al. 2008), respectively. Since the \( {T}_{\mathrm{r}}^{\mathrm{i}} \) value for Co and Fe are not available, Er for both metals are not calculated. \( {C}_{\mathrm{f}}^{\mathrm{i}} \) is the contamination factor for the same substance. The extend of potential risk for individual metal can be described using Hakanson’s (1980) standard (S2)
The PERI can be defined as the sum of the potential risk of individual metal:
where Eri: potential risk of individual metal, \( {T}_{\mathrm{r}}^{\mathrm{i}} \): toxic-response factor of the individual metal and \( {C}_{\mathrm{f}}^{\mathrm{i}} \): contamination factor of the individual metal. The PERI can be described according to categories suggested by Hakanson (1980) (S2).
Combined pollution index
Combined pollution index (CPI) was calculated by dividing the summation of contamination factors with total numbers of metal analysed (Zhou et al. 2007; Zhu et al. 2012).
where \( {C}_{\mathrm{f}}^{\mathrm{i}} \) is contamination factor is for an individual metal and m is the total number of metals included in this study. The extent of contamination by CPI can be determined by using the gradation standard as proposed by Abrahim and Parker (2008) in S2.
Results and discussion
Heavy metal concentrations in the surface sediments and clam
Figure 2 showed comparisons of the heavy metal concentrations in the TST and shells of clams, and habitat sediments at PS and the three transplanted sites at SM, DT and KJ, while the overall statistics are presented in S3. The ranges of metal concentrations (μg/g dw) in the sediments were 35.4–112 for As, 0.12–0.34 for Cd, 2.33–9.68 for Co, 28.1–54.5 for Cr, 10.5–17.4 for Cu, 12,784–34,603 for Fe, 106.7–306.8 for Mn, 12.4–20.3 for Ni, 48.1–64.3 for Pb and 55.6–84.7 for Zn. The ranges of metal concentrations (μg/g dw) in the TST of C. javanica were 1.29–19.6 for As, 0.13–0.38 for Cd, 0.39–1.38 for Co, 2.03–6.96 for Cr, 12.2–26.1 for Cu, 675–4641 for Fe, 1.81–9.19 for Mn, 6.70–22.1 for Ni, 7.53–55.7 for Pb and 87.5–305 for Zn. The ranges of metal concentrations (μg/g dw) in the shell of C. javanica were 0.14–3.22 for As, 0–0.12 for Cd, 0.16–0.88 for Co, 0.63–6.24 for Cr, 8.85–12.7 for Cu, 226–4010 for Fe, 0.55–2.46 for Mn, 30.8–37.8 for Ni, 45.7–73.0 for Pb and 8.12–38.3 for Zn.
The first pattern from Fig. 2 was that PS had higher concentrations of Co, Cr, Fe, Mn, Ni and Zn the surface sediments, TST and shells of clams after 3-day transplantation. After transplantation, the Zn concentration (μg/g dw) in the clam TST decreased from PS (180) to SM (149), DT (142) and KJ (114). Similar pattern occurred for Zn concentrations in the clam shells in the order of PS > SM > KJ > DT. The Ni concentrations in the clam TST increased slightly from PS (13.3) to SM (13.7) but decreased at DT (10.3) and KJ (11.6). After 3 days of transplantation, the concentrations of Ni in the clam shells decreased from PS (34.3) to SM (34.3) and KJ (34.2) but increased at DT (36.4). The Ni concentrations of the shell were found in the order of DT > SM > KJ > PS.
The Mn concentration (μg/g dw) in the clam TST decreased from PS (4.08) to SM (3.79), DT (2.45) and KJ (2.96). However, the Mn concentrations in the shell increased from PS (0.76) to SM (1.00), DT (0.93) and KJ (1.36). The Fe concentrations (μg/g dw) decreased from PS (2335) to SM (1523), DT (1748) and KJ (1018). Increment of Fe concentrations in the shells was found in SM (1768) after 3 days of transplantation from PS (892) but still decreased at DT (679) and KJ (523) when compared to that at PS. The Cr concentrations (μg/gdw) in the TST decreased from PS (4.29) to SM (3.31), DT (2.72) and KJ (3.44) while those in the clam shells followed the same order (PS > SM > KJ > DT), after the transplantation period. PS had the highest Co concentrations when compared to the three sites.
However, geochemical study revealed that these higher levels of metals at PS were mainly contributed by natural origins (>80%) than the anthropogenic one (Table 1). Therefore, the dam effect had caused higher metal levels but might not have an environmental impact on the river ecosystem health. But still, higher accumulations of Cr, Fe, Mn, Ni and Zn were found in the clams (whether TST or shell) at PS, which indicated higher bioavailabilities of these metals.
The second pattern from Fig. 2 was that KJ had higher concentrations of Cu and Pb in the sediments. The geochemical study revealed that almost half (49–54%) of the total Cu and Pb were contributed by anthropogenic sources (Table 1). Therefore, the higher levels of these metals could pose a health risk to the living organisms. However, such clear pattern is difficult to be seen based on clam TST and shells since the highest Cu concentrations are not found at KJ after transplantation. After 3-day transplantation, Pb level increased significantly (p < 0.05) from PS to KJ in the clam TST but not in the shells.
The third pattern from Fig. 2 was that SM had higher concentrations of As and Cd in the sediments. Based on geochemical study, for Cd, more than 99% were contributed by anthropogenic inputs but only 12.7% were originated from anthropogenic sources for As (Table 1). This indicated that Cd could have ecological impact on the river ecosystem. These sediment results were also supported by those in both clam TST and shells for As in which SM accumulated significantly (p < 0.05) higher levels of As at SM after 3 days of exposure. The clam TST showed the highest Cd levels at SM. These results indicated higher bioavailabilities of As and Cd at SM.
Comparison with reported sediment data
When compared to previous studies on Langat River (Table 2), the present Cd levels were comparable to and lower than those reported by Shafie et al. (2014) and Sarmani (1989), respectively. However, levels of As, Cr, Cu, Ni, Pb and Zn in this study were found to be higher than those reported by Lim et al. (2013). This may be due to the difference in season when the sampling was done and the possible differences in the specific sites chosen (Ma et al. 2015; Sundaray et al. 2014).
Levels of Zn, Co and Cd found in this study were lower when compared to those previously reported by Sarmani (1989). Sarmani (1989) reported higher metal levels when compared to those in the present findings, and this could be due to increased effluent discharges into river system during 1970s to 1980s, when Malaysia had undergone rapid industrialisation (Lim et al. 2013). Compared to other studies, the Zn concentrations in the surface sediment of Langat River were found to be lower than those from Mamut River (Muhammad Ali et al. 2015) and Rawal Lake (Iqbal et al. 2013). The present Cu level was lower than those reported for Buloh River, Malaysia (Nemati et al. 2011), Mamut River (Muhammad Ali et al. 2015), and Lake Chapala of Mexico (Rosales-Hoz et al. 2000), but higher than that for Rawal Lake (Iqbal et al. 2013).
Pb concentrations were found to be lower than most reported values except for those in Buloh River. Present Ni levels were comparable to those in Buloh River while Fe levels were also similar with those for Cauvery River, India (Venkatesha et al. 2012) and Rawal Lake. Similarly, the concentrations of Mn were also found to be lower than most reported values, except for those in Cauvery River while Co levels of this study were similar to those in Buloh River and Rawal Lake, but lower than those in Mamut River and Lake Chapala. Present Cr levels were found to be lower than those in Mianyan River, Southwest China (Shi et al. 2015), Middle reach of Yangtze River (Song et al. 2011), Central China and Lake Chapala while were comparable to those in Sungai Buloh, Cauvery River and Mangla Lake, Pakistan (Saleem et al. 2015).
Multivariate analysis
Correlation analysis
The correlation coefficients (CEs) of metals between the clam TST and the sediments are presented in Table 3. All metals concentrations, except for those of Cd and Cu, were positively and significantly correlated with respective heavy metals in surface sediments. The correlations of As, Co, Cr, Fe, Mn, Ni, Pb and Zn concentrations between TST and their habitat sediments indicated the ability of clam TST to reflect their respective metal levels in their environment. The clam shells also showed significant correlations with the surface sediments for As, Cd, Cr, Fe, Mn and Ni. This again suggested the potentials of C. javanica shell to be a biomonitor for the six metals.
Factor loadings by factor analysis
Table 4a showed factor loadings, eigenvalues and variance explained by each factor of the ten metals in the surface sediments collected from the four sites along Langat River. According to Table 4a, three factors were obtained, which explained 85.2% of the total variance, with the first factor accounting for 44.7%, the second factor for 24.5% and the third factor for 15.9%. The first factor was characterised by high loading (≥0.70) of Co, Cr, Ni and Zn. The second factor was heavily loaded (≥0.70) with Cu and Pb. Lastly, the third factor was heavily loaded with As. Therefore, of all the ten metals investigated, all the metals except for Fe and Mn were selected on the basis of their high PCA loadings (≥0.70). From Table 4a, it was shown that none of Fe and Mn was loaded into any of the three factors, suggesting that other metals were not come from lithogenic sources.
Table 4b showed factor loadings, eigenvalues and variance explained by each factor of the ten metals in the clam TST. Four factors were obtained, which explained 83.7% of the total variance, with the first factor accounting for 37.3%, the second factor for 21.4%, the third factor for 13.3% and the forth factor for 11.7%. The first factor was characterised by high loading (≥0.70) of Cr, Mn and Ni. The second factor was heavily loaded (≥0.70) with Fe, Pb and Zn. The third factor was heavily loaded with As and Co. Lastly, the fourth factor was heavily loaded with Cu. Therefore, of all the ten metals investigated, all the metals except for Cd were selected on the basis of their high PCA loadings (≥0.70).
Table 4c showed factor loadings, eigenvalues and variance explained by each factor of the ten metals in the shell of C. javanica. According to Table 4c, two factors were obtained, which explained 69.5% of the total variance, with the first factor accounting for 53.6% and the second factor for 15.9%. The first factor was characterised by high loading (≥0.70) of As, Co, Cr, Cu, Fe, Mn and Zn. The second factor was heavily loaded (≥0.70) with Cd and Pb. Therefore, of all the ten metals investigated, all the metals except for Ni were selected on the basis of their high PCA loadings (≥0.70).
Cluster analysis
Clustering patterns based on the heavy metals in the surface sediments, TST and shells of the four sites are shown in Fig. 3a–c, respectively. The clustering pattern based on heavy metals in sediments (Fig. 3a) and TST (Fig. 3b) clearly indicated the possible source of the heavy metals at the four sites. PS was being clustered as a single entity, while SM formed a relatively independent subcluster when compared to DT and KJ, which were clustered closely (Fig. 3a). The only subcluster with PS indicated that the enriched heavy metal levels in the sediment were resulted from discharge of dam impoundment. Another subcluster with SM suggested a different source of metal levels in the sediment. The vicinity of a large area of oil palm plantation at SM could be a potential contributor for the elevated levels of As and Cd in SM. The high levels of As and Cd could be due to the usage of pesticides and phosphate fertilisers (Osman 2014) during agricultural activities. Figure 3a, b also showed that DT and KJ are closely clustered, and this could be attributable to potential domestic inputs. The cluster analysis based on the heavy metal concentration in the TST indicated the bioavailabilities of heavy metals of the sampling site (Cheng and Yap 2015).
The clustering pattern for the four sites based on the heavy metal concentrations in the shells of the clams (Fig. 3c) showed two major clusters with PS and SM as a single entity, while DT and KJ formed another entity. The clustering pattern was in agreement with the geographical pattern like TST. This could be due to different accumulation sites between the shell and TST of the clams (Foster and Cravo 2003). Knowledge of differences of metal levels between shells and TST of bivalves had been widely reported in the literature (Yap et al. 2003, 2008, 2009).
Multiple linear stepwise regression analysis
To investigate the dependence of the heavy metal concentrations of C. javanica (shells and TST) upon the heavy metals concentrations in sediments, MLSRA was performed (Table 5). The independent variables included were the metal concentrations of the four geochemical fractions and the total summation (SUM) of all the four fractions. The result of the MLSRA showed that the SUM of the sediment has great influence on the metal concentrations in shell and TST of the clams since it was the predictive parameter for most of the models. The EFLE was predictive toward the clam TST for all the metals except for Cr in shells and As, Cd, Cr, Fe and Zn in TST. The AR was able to predict the metals except for Fe and Mn in TST, and Fe and Zn in shells. The OO displayed a relative influence toward the clam TST for all the metals except for Ni and Zn in TST, and Zn in shell. The R influences on the metal concentration in the clam TST except for Cd. All the above models showed that the accumulations of metals in TST and shells (dependent variables) of clams were significantly influenced by those in the selected metals in the geochemical fractions of the surface sediments (Yap et al. 2011). Thus, these results further supported the use of TST and shells to reflect the selected metals in their habitat sediments.
Ecological risk assessments
Comparison with established sediment quality guidelines
Heavy metal concentrations in the surface sediments of Langat River were compared with some existing sediment quality guidelines (SQG). According to Canadian Council of Ministers of Environment (CCME 1999) and USEPA (2002), there are three ranges of chemical pollutants can be consistently defined according to threshold effect level (TEL) and probable effect level (PEL):
-
1.
The minimal effect range within which adverse effects rarely occur. Fewer than 25% adverse effects occur below the TEL;
-
2.
The possible effect range within which adverse effects occasionally occur. The range between the TEL and PEL; and
-
3.
The probable effect range within which adverse biological effects frequently occur. More than 50% adverse effects occur above the PEL.
Concentrations of Zn, Cu and Cd were found to be lower than any guidelines including preindustrial reference values (PRV) by Hakanson (1980), and interim SQG and PEL by CCME (1999). The concentrations of Pb were found higher than interim SQG by CCME (1999). With Pb level below PEL, the Pb contamination in the studied area might cause occasion adverse impact on biota within this area.
All Ni concentrations in surface sediments were lower than PRV by Hakanson (1980), the Ni levels in SM, DT and KJ were lower than these reference guidelines except for PS. The Ni levels in the surface sediment from PS were found higher than TEL and PEL. This showed that the Ni contamination in PS might cause occasion adverse biological effect on the aquatic life around PS.
The Fe, Mn and Co concentrations in the surface sediments from all the sites were lower than the PRV by Hakanson (1980). This indicated that there was no pollution of Fe, Mn and Co within the studied area. The concentrations of Cr in sites were lower than PRV by Hakanson (1980) and PEL by CCME (1999). However, the Cr levels from PS and KJ were higher than interim SQG. The Cr level from DT was lower than TEL. This suggested that the Cr contamination at PS and KJ might cause occasional adverse biological effect to the biota around these two sites.
Geoaccumulation index and enrichment factor
The values I geo and EF of heavy metals in the surface sediment of all the sites are presented in Table 6. The values of I geo ranged from −2.24 to −1.63 for Zn, −2.84 to −2.11 for Cu, −1.13 to −0.71 for Pb, −2.51 to −1.80 for Ni, −2.56 to −1.13 for Fe, −3.82 to −3.25 for Mn, −3.48 to −1.83 for Co, −2.55 to −1.68 for Cr, −0.22 to 2.09 for As and −3.77 to −2.55 for Cd.
The classifications of heavy metal pollution based on I geo proposed by Muller (1969) are presented in S2A. All I geo values for Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn are found to be less than 0, and therefore, they can be categorised as ‘unpolluted’ or I geo class 0. In the case of As, DT was scored as 0.66, placing it at ‘unpolluted to moderately polluted’ category. KJ scored 1.50, placing it at ‘moderately polluted’ category. While the I geo of SM scored 2.32, placing it at ‘moderately to strongly polluted’ category.
The EF values ranged from 0.63 to 1.49 for Zn, 0.32 to 1.37 for Cu, 1.00 to 3.62 for Pb, 0.63 to 1.15 for Ni, 0.15 to 0.48 for Mn, 0.30 to 0.97 for Co, 0.51 to 1.72 for Cr, 1.87 to 16.76 for As and lastly 0.19 to 0.69 for Cd. The EF is a convenient measurement of geochemical trends and is applied for speculating a lithogenic or anthropogenic origin of heavy metal (Sutherland 2000). The degree of enrichment was categorised according to the value of EF from ‘no enrichment’ to ‘extremely severe enrichment’ at S2B (Birth 2003; Binta Hasan et al. 2013; Lim et al. 2013 Taylor 1964).
For PS, the EF value showed that the sediment at PS is minor enriched with Pb and As (EF = 1.00 and 1.87, respectively). At SM, based on the EF value, the sediment at SM was minor enriched with Pb (EF = 1.73) and severely enriched with As (EF = 12.8). At DT, it was found that the sediment collected at SM was minor enriched with Zn, Pb, Ni and Cr (EF = 1.43, 2.65, 1.01 and 1.20, respectively). The sediment of SM was also moderately severe enriched with As (EF = 9.05). The KJ sediments were found to be metal-enriched except for Mn, Co and Cd. The sediment at KJ was found to be minor enriched with Zn, Cu, Ni and Cr (EF = 1.49, 1.37, 1.15 and 1.72, respectively). Pb and As in the KJ sediments were found to be moderate (EF = 3.62) and severe (EF = 16.8) enrichments, respectively.
The I geo values suggested that there was no pollution to the sediment at the four sites studied, but EF showed that there was some enrichment of metal at all the sites. This showed the difference of these two indices. For I geo, it showed that there was no pollution (I geo < 0) of Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn. While for EF, all the sites were shown to have some form of metal enrichment (EF ≥ 1). According to Haris and Aris (2013), I geo was more objective than EF in determining the enrichment of metal pollution in sediments. This could be due to the usage of average crust concentration for the calculation of EF may not reflect the true enrichment level in the study area, and this can result in over or underestimation of EF (Haris and Aris 2013).
The EF value of Pb indicated that there was minor to moderate enrichment of the said metal at the four sites studied, with the enrichment increasing along with the flow of river water from upstream sites to downstream. For Zn, some minor enrichment at DT and KJ was found, which could be related to vehicular activities. Yang et al. (2011) reported that Zn in an urban area was contributed by the vehicular activities in the vicinity. The increased amount of vehicle running at DT and KJ may also contribute to the increased enrichment of Zn, Cu, Pb, Ni, Cr and As. Fuge (2013) stated that oil and coal combustion in Europe contributed significantly to atmospheric deposition of As, Cd, Cr, Cu, Ni and V.
Both of the I geo and EF were in agreement that there was enrichment of As at SM, DT and KJ. SM and KJ showed severe enrichment of As. For SM, this may be due to the agricultural activity (oil palm plantation) around the site. Aris et al. (2015) reported that the elevated As level may be explained by the possibility to the use of arsenic pesticide/herbicide at that area. Han et al. (2003) stated that the burning of coal as well as petroleum is a major anthropogenic source of As in the environment. Therefore, it is logical to associate the enrichment of As in urban area with the burning of fuel.
Cheng and Yap (2015) utilised EF as one of the indices in their investigation of the heavy metal pollution at mangrove sediments of peninsular Malaysia. Martínez-Martínez et al. (2013) used EF as one of the indices in their investigation of the Pb and Zn levels in natural soils and tailing ponds. They found that the EF values of all of the sediment of the ponds studied were categorised as ‘severe enrichment’ according to Taylor’s (1964) standard.
Ecological risk assessments
The values of C f, Cd, CPI, Er and PERI of the sediments of the four sites along the Langat River are also presented in Table 6. The C f values ranged from 2.36 to 7.47 for As, 0.21 to 0.34 for Cd, 0.13 to 0.56 for Co, 0.31 to 0.61 for Cr, 0.21 to 0.35 for Cu, 0.25 to 0.69 for Fe, 0.11 to 0.31 for Mn, 0.26 to 0.43 for Ni, 0.69 to 0.92 for Pb and 0.32 to 0.48 for Zn. The values of C f for all the metals, except for As, were lower than 1; therefore, according to the classifications by Hakanson (1980) (S2C), they can be classified as ‘low contamination’. For As, PS and DT were classified as ‘moderate contamination’ because their C f values were 1.28 and 2.36, respectively. The urban site of KJ was categorised as ‘considerable contamination’ while SM as ‘very high contamination’.
The increasing order of C d of the sampling sites along the Langat River was PS < DT < KJ < SM. According to the classifications established by Hakanson (1980), as presented in S2D, the PS, DT and KJ were categorised as ‘low degree of contamination’, while SM showed ‘moderate degree of contamination’. The higher C d value at SM was due to the ‘very high contamination factor’ of As, while other metals as ‘low contamination factor’. Therefore, it is confirmed that the elevated concentration in As was the main factor for the high C d in SM. Abrahim and Parker (2008) found that the C d of their sediment cores ranged from ‘moderate degree of contamination’ to ‘considerable degree of contamination’ according to Hakanson’s (1980) standard.
The CPI values found in this study ranged from 0.51 to 1.07. Compared with the gradation standards proposed by Abrahim and Parker (2008) (S2G), the present CPI values for all the sites fell into the category of ‘nil to very low contamination’. According to Zhu et al. (2012), when CPI < 1, sediment can be considered as unpolluted; when the CPI > 1, sediment is considered as contaminated by heavy metals. By using Zhu et al.’s (2012) standard, only SM was polluted with heavy metals as its CPI value was >1 while the PS, DT and KJ were considered as unpolluted. This result was in partial agreement with EF, which showed that SM that was severely enriched with As. Zhu et al. (2012) reported the CPI value of the sediment of Xiawan Port ranged from 25.54 to 231.4. In comparison to Zhu et al.’s (2012) study, the CPI from the present study was significantly lower than that from Zhu et al.’s (2012) results. This was due to the Xiawan Port heavily received urban and industrial sewage from an industry zone (Zhu et al. 2012) while the present study concentrated on upstream to midstream of Langat River where the pollutant introduction was not as serious as the large scale of anthropogenic inputs occurred at Hunan province.
Martínez-Martínez et al. (2013) reported that a majority of their samples had exceeded the ‘ultra high contamination’ according to Abrahim and Parker’s (2008) standard. Zhou et al. (2007) found that the range of CPI of their soil samples were 0.89 to 23.68 with all tailing sites and a nearby ore dressing plant site having CPI reached ‘very high contamination’ by Abrahim and Parker’s (2008) standard.
The Er and PERI of the sediment collected at the four sites along the Langat River are presented at Table 6. The Er values ranged from 23.62 to 74.66 for As, 6.33 to 10.23 for Cd, 0.62 to 1.21 for Cr, 1.05 to 1.74 for Cu, 0.11 to 0.31 for Mn, 1.32 to 2.15 for Ni, 3.44 to 4.59 for Pb and 0.32 to 0.48 for Zn. According to the classification by Hakanson (1980) shown in S2E, all of the Er values for Zn, Cu, Pb, Cr and Cd were below 40, hence classified as ‘low ecological risk’ for Zn, Pb, Cr and Cd. For As, PS and DT were categorised as ‘low ecological risk’ (Er < 40), while SM and KJ were categorised as ‘moderate ecological risk’ (40 ≤ Er < 80).
The PERI values ranged from 37.8 to 92.7, showing that combination of all the seven metals of all the sampling sites were categorised as ‘low ecological risk’ (RI < 150 according to Hakanson (1980) (S2F).
Possible sources of heavy metal contamination
Effects of dam discharge
The elevated levels of Co, Cr, Fe, Mn, Ni and Zn in PS might be caused by the periodic release of impounded water from Pangsun Dam reservoir in short distant upstream from PS. Wang et al. (2015) suggested that flushing of dam water may cause high ecological stress on the downstream reaches. This is due to sedimentation of suspended matter, which may adsorb pollutants from flowing water from river and deposited into the reservoir. Flushing of dam water might release these pollutants from the sediment. The sediment transportation of the river decreased following the construction of river dam (Apichitchat and Jung 2015; Fan et al. 2015). This phenomenon has been due to the ability of a dam to trap a portion of incoming sediment load from upper stream source altering the sediment regimes of the river (Fan et al. 2015). Reducing sediment load is likely to reduce the nutrient fluxes in the river and cause erosion of the downstream riverbank. Fan et al. (2015) found that the Cu concentration in reservoir water increased for ‘low-flow period’ while decreased on ‘high-flow period’ and ‘normal-flow period’ after the dam was constructed (post-dam), compared with pre-dam data. Young and Ishiga (2014) noted increased of elemental concentration in 2013 when they studied the impact of gate removal of Arase Dam at Kuma River, Japan. They hypothesised that this phenomenon has been due to periodic flushing in winter causing increase in fine particle fraction.
The anoxic environment of the hypolimnion bottom of the dam reservoir would cause the hypolimnion water to be more capable to dissolve metals, and this could result in elevated metal concentrations (McCully 2001) in the sediments. This acidic characterisation of water is supported the slightly lower water pH value at PS (6.22), which was slightly lower than that at DT and KJ (6.53 and 6.74, respectively), located farther from the dam.
Previous investigations of the concentration of Hg of the sediments of the river dam reservoir have confirmed the contribution of the formation of dam reservoir to the elevated total Hg and bioavailable methyl-Hg levels in the sediment of the dam reservoir (Hall et al. 2005; Teisserenc et al. 2014). For example, Teisserenc et al. (2014) observed drastic increase of total Hg concentration in the sediment of Gabbro lake and Sandgirt lake that coincided with the time of reservoir impoundment. Zhao et al. (2013) found higher metal levels in the sediments near the dam than those far from the dam. These previous reports signified the potential of river dam to accumulate heavy metal pollutants in the sediments.
Levels of Fe, Mn, Ni and Zn were found to be highest in PS among all the four sites but they were dominated by resistant fraction, indicating association with natural origin from the breakdown of the silica lattices of rocks (Badri and Aston 1983; Yap et al. 2002). Therefore, although the total metal concentrations were higher in PS than those in the other sites, most of them were not bioavailable. This deduction was in agreement with the results of the CA of the metal levels between TST (or shell) and sediments. Table 3 showed that all of the CEs of Co, Fe, Mn, Ni and Zn between TST (or shell) and sediments were low (less than 0.70).
Factor loadings for the ten metals in the sediments (Table 4a) grouped Co, Cr, Ni and Zn as factor 1. Among the four metals in factor 1, the concentrations of Co, Ni and Zn were found to be highest in PS, showing the effect of the discharge of dam impoundment on the factor loadings of the metals in the sediment. However, factor loadings of Co, Fe, Mn, Ni and Zn based on TST of C. javanica (Table 4b) were found not to be loaded into a single factor. The discrepancy of factor loadings between sediments and TST might be a result of differences in the metabolism, rates of accumulation and depuration of the five metals. Moreover, the above five metals were essential elements with important biological needs to the organism. For factor loadings based on the shells of C. javanica (Table 4c), four metals (Co, Fe, Mn, and Zn, with the exception of Ni) were loaded into factor 1. However, As, Cr and Cu (loaded into factor 1) were not affected by dam discharge.
Despite higher levels of Co, Fe, Mn, Ni and Zn found at PS, all of the geochemical indexes (I geo, EF, C f and Er) showed that discharge of dam impoundment did not pose significant ecological risk to the surrounding environment.
Effects of agricultural activities: pesticides and fertilisers
The highest concentrations of As and Cd were found in SM because it is located at the upstream of Semenyih River, which is a recreation site with oil palm plantation in the surroundings. The higher As concentration in SM might be caused by the possible use of sodium arsenite as pesticide/herbicide at the plantation (Aris et al. 2015; Osman 2014). Cd can be associated with phosphate fertiliser, originating from the Cd in phosphorous-ore (Osman 2014). Therefore, the use of phosphate fertiliser also may contribute to the elevated levels of As and Cd in oil palm plantation (Fuge 2013; Tao et al. 2016; Zaharah et al. 1997). Alloway (1995) suggested that high Cd in some soils and surface waters in agricultural areas had been linked to the application of phosphate fertiliser.
Organic As compound dominated the pesticide production. Therefore, it is believed that the elevated As levels in the sediment were originated from the use of pesticides in oil palm plantation. However, it can be argued that the environmental distribution of inorganic As and its speciation in the sediment might be different depending on the AAs such as the use of phosphate fertiliser (Osman 2014) around the site.
The geochemical fractions of As and Cd in SM showed different pattern in the distributions of metal concentrations. The As level in the sediment is consistently concentrated in RES fraction. In contrast, the distribution of Cd is concentrated in all three non-resistant fractions (EFLE, AR and OO) with small percentages of RES fractions (0.98–7.51%). This is in agreement with those reported by Saleem et al. (2015), Díaz-de Alba et al. (2011) and Marmolejo-Rodrıguez et al. (2007) that concluded that Cd was being among the most labile metal. However, RES fractions for As and Cd in SM were the lowest among the four sites. The results showed that higher levels of As and Cd in SM were followed by higher percentages of non-resistant fractions of As and Cd when compared to the other sites (PS, DT and KJ). These results could be related to agricultural activities surrounding SM.
The results showed significant positive CEs of As between clam TST and sediment but not for Cd. The inconsistency of the Cd level in TST and sediment might be caused by the fluctuating DO levels in the flowing river water. Tran et al. (2001) reported the water oxygenation level could be influenced Cd accumulation pattern in C. fluminea. The DO levels (mg/L) in all the four sites differed greatly, ranging from 3.35 at KJ to 9.11 at DT. Therefore, mechanism in C. javanica to Cd exposure could be influenced by ever changing DO levels.
The I geo value of As in SM reached ‘very strongly polluted level’ while EF value also reached ‘severe enrichment’ category. The C f also showed that As in SM is ‘very high contamination’. When the toxic-response factor was being taken into the equation, the potential risk of As will be ‘moderate potential ecological risk’. It must be noted that SM consisted mainly of RES As in the sediment, indicating the actual impact of As on the surrounding biota might be minimal. These indexes indicated that the use of pesticides and fertiliser had contributed to the elevation of As in SM but posed no ecological risk. Owing to the inherently low Cd concentration, all of the ecological risk indexes for Cd in SM are minimal, with the exception of I geo with ‘unpolluted to moderately polluted’.
Effects of urban domestic wastes
The highest concentrations of Pb and Cu were found in KJ, which could be related to domestic wastes. Pb, Cu and Zn are known to be originated from paint, fuel additives, tyres and particulates from moving parts (Wang et al. 2013), which can be extensively found in urban site at KJ. Municipal wastes had been found to have elevated levels of heavy metals (Duc et al. 2013; Iwegbue et al. 2010; Yuksel 2015) while Lee et al. (2012) argued that Pb and Cd levels could be associated with the incineration of municipal wastes. Duc et al. (2013) reported that untreated domestic wastewater from Hanoi metropolitan had great impact on the Nhue-Day River System.
The elevated Cu concentrations in the sediments in KJ may be contributed by the vehicular activities because Cu could be released from Cu wiring thrust bearing and brakes (Fuge 2013). Although Pb additive to fuel had been banned, the Pb pollution in the urban dust and soil was a legacy of its former usage (Fuge 2013). The weathering of the old paint or renovation of the buildings, with the scrapping of old paint, could be a source of Pb in soil. The Pb-contaminated soils might be eventually merged with river waters at KJ by surface runoff. Li-qun et al. (2015) found that the Cu, Zn and Pb contamination was concentrated in the highly populated urban periphery, also supporting the higher levels of Cu and Pb in KJ.
High percentages of non-resistant factions of the metals studied, except for As, Cd and Fe, indicated that most metals in KJ were potentially more bioavailable than the other sites. Within the non-resistant fractions, both Cu and Pb were concentrated in OO fraction, which was considered to be originated from domestic and industrial origins. The effluents of some industries such as food processing industry were known to release sludge rich in OO matter. The high metal concentrations in OO fraction in KJ might also be due to the low DO level in KJ. Purushothaman and Chakrapani (2007) argued that high-flow and abundant oxygenated condition may accelerate the oxidation or decomposition of the matter resulting in the release of metals into the water layer. However, Wan et al. (2012) and Shafie et al. (2014) reported that the potential impact of heavy metals was not serious in Langat River and that it was generally unpolluted by Cd, Ni and Cr in regard to surface sediment. This agrees with the results of PERI in the present study, which showed no alert in the area studied, including KJ.
Conclusion
In conclusion, higher levels of Co, Fe, Mn, Ni and Zn in PS were attributed by the discharge of Pangsun Dam impoundment. Higher levels of As and Cd in SM could be a result of the usage of pesticides and phosphate fertiliser in the plantation while higher levels of Pb and Cu in KJ could be attributed to domestic wastes as well as the usage of motor vehicle. Based on CA, positive and significant correlations of As, Co, Cr, Fe, Mn, Ni, Pb and Zn between surface sediment and TST were found. This indicated that the clam TST was a potential and good biomonitor for the eight metals in the surrounding habitat. Positive and significant correlations of As, Cd, Cr, Fe and Mn between surface sediment and shell were found, indicating the shell of C. javanica was able to reflect the above five metals as well.
The SQGs and PERI revealed that Cd, Co, Cr, Cu, Fe, Mn, Ni and Zn were not likely to cause adverse effect to the Langat River ecosystem, while As in SM showed various degrees of pollution, and Pb was found as ‘minor to moderate pollution’, ‘low contamination’ and ‘low ecological risk’. Based on PERI, the urban site at KJ, the effect of dam at PS and the possible usage of pesticide and phosphate fertiliser in SM were not likely to cause adverse effect to the river ecosystem with ‘low ecological risk’. However, further studies are needed to mitigate, control and manage the sustainable usage of the river water in the future.
References
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments form Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238
Alloway BJ (1995) Heavy metals in soils, 2nd edn. Blackie Academic & Professional, London
Aoun M, Arnaudguilhem C, El Samad O, Khozam RB, Lobinski R (2015) Impact of a phosphate fertilizer plant on the contamination of marine biota by heavy elements. Environ. Sci. Poll. Res. 22:14940–14949
Apichitchat S, Jung K (2015) Hydrological simulation for impact assessment of Kaeng Sue Ten dam in Thailand. KSCE J Civil Eng 19(7):2325–2332
Aris AZ, Lim WY, Looi LJ (2015) Natural and anthropogenic determinants of freshwater ecosystem deterioration: an environmental forensic study of the Langat River Basin, Malaysia. In: Environmental management of river basin ecosystems, ed. Ramkumar, Mu., Kumaraswamy, K., Mohanraj, R., pp. 455–476. Springer
Aris AZ, Looi LJ (2015) Estuaries ecosystems health status—profiling the advancements in metal analysis. In Advances in Coastal and Marine Resources. Springer. Pages 429–453
Atafar Z, Mesdaghinia A, Nouri J, Homaee M, Yunesian M, Ahmadimoghaddam M, Mahvi AH (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160:83–89
Badri MA, Aston SR (1983) Observation on heavy metal geochemical associations in polluted and non-polluted estuarine sediments. Environ Poll Ser B 6:181–193
Binta Hasan A, Kabir S, Selim Reza AHM, Nazim Zaman M (2013) Enrichment factor and geo-accumulation index of trace metals in sediments of the ship breaking area of Sitakund Upazilla (Bhatiary–Kumira), Chittagong, Bangladesh. J Geochem Exp 125:130–137
Birth G (2003) A scheme for assessing human impacts on coastal aquatic environments using sediments, eds., Woodcoffe, C. D., Furness, R. A. Sydney, Australia: Coastal GIS
Buat-Menard P, Chesselet R (1979) Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sci Lett 42:398–411
Cardoso PG, Grilo TF, Reis AT, Coelho JP, Pereira E, Pardal MA (2015) Field transplantation of the bivalve Scrobicularia plana along a mercury gradient in Ria de Aveiro (Portugal): uptake and depuration kinetics. Sci. Tot. Environ. 512–513:55–61
CCME (Canadian Council of Ministers of the Environment) (1999) Sediment quality guidelines for the protection of aquatic life. (http://st-ts.ccme.ca/en/index.html) retrieved 28 June 2015
Cheng WH, Yap CK (2015) Potential human health risks from toxic metals via mangrove snail consumption and their ecological risk assessments in the habitat sediment from Peninsular Malaysia. Chemosphere 135:156–165
Cristina MMD, Maria H, Pestana D, Luiz D (1986) Geochemical partitioning of heavy metals in sediments of three estuaries along the coast of Rio de Janeiro (Brazil). Sci Tot Environ 58(1–2):63–72
Dhanakumar S, Solaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotox Environ Saf 113:145–151
Díaz-de Alba M, Galindo-Riano MD, Casanueva-Marenco MJ, García-Vargas M, Kosore CM (2011) Assessment of the metal pollution, potential toxicity and speciation of sediment from Algeciras Bay (south of Spain) using chemometric tools. J. Hazard. Mat. 190:177–187
Duc TA, Loi VD, Thao TT (2013) Partition of heavy metals in a tropical river system impacted by municipal waste. Environ Monit Assess 185:1907–1925
Emsley J (2001) Manganese. Nature’s building blocks: an A–Z guide to the elements. Oxford University Press, Oxford, UK, pp. 249–253
Fan H, He D, Wang H (2015) Environmental consequences of damming the mainstream Lancang-Mekong River: a review. Earth Sci Rev 146:77–91
Foster P, Cravo A (2003) Minor elements and trace metals in the shells of marine gastropods from a shore in tropical East Africa. Wat. Air Soil Poll. 145:53–65
Fu J, Zhao CP, Luo YP, Liu CS, Kyzas GZ, Luo Y, Zhao DY, An SQ, Zhu HL (2014) Heavy metals in surface sediments of the Jialu River, China: their relations to environmental factors. J Hazard Mat 270:102–109
Fuge R (2013) Anthropogenic Sources. In Essentials of Medical Geology, ed. Selinus, O., pp 59–74. Springer
George R, Martin GD, Nair SM, Chandramohanakumar N (2013) Biomonitoring of trace metal pollution using the bivalve molluscs, Villorita cyprinoides, from the Cochin backwaters. Environ Monit Assess 185:10317–10331
Giordano R, Musmeci L, Ciaralli L, Vernillo I, Chirico M, Piccioni A, Costantini S (1992) Total contents and sequential extractions of mercury, cadmium, and lead in coastal sediments. Mar. Poll. Bull. 24(7):350–357
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentary approach. Wat Res 14:975–1001
Hall BD, St. Louis VL, Rolfhus KR, Bodaly RA, Beaty KG, Paterson MJ, Peech Cherewyk KA (2005) Impacts of reservoir creation on the biogeochemical cycling of methyl mercury and total mercury in boreal upland forests. Ecosystems 8:248–266
Han FX, Su Y, Monts DL, Plodinec MJ, Banin A, Triplett GE (2003) Assessment of global industrial-age anthropogenic arsenic contamination. Naturwissenschaften 90:395–401
Harguinteguy CA, Noelia Cofré M, Fernández-Cirelli A, Luisa Pignata M (2016) The macrophytes Potamogeton pusillus L. and Myriophyllum aquaticum (Vell.) Verdc. as potential bioindicators of a river contaminated by heavy metals. Microchem J 124:228–234
Haris H, Aris AZ (2013) The geoaccumulation index and enrichment factor of mercury in mangrove sediment of Port Klang, Selangor, Malaysia. Arab J Geosci 6:4119–4128
Hassanien MA, El Shahawy AM (2011) Environmental heavy metals and mental disorders of children in developing countries. In Environmental Heavy Metal Pollution and Effects on Child Mental Development: Risk Assessment and Prevention Strategies. NATO Science for Peace and Security Series C: Environmental Security Volume 1. Ed. Simeonov, L.I., Kochubovski, M.V. and Simeonova B.G. Springer
Iqbal J, Tirmizi SA, Shah MH (2013) Statistical apportionment and risk assessment of selected metals in sediments from Rawal Lake (Pakistan). Environ Monit Assess 185(1):729–743
Iwegbue CMA, Nwajei GE, Ogala JE, Overah CL (2010) Determination of trace metal concentrations in soil profiles of municipal waste dumps in Nigeria. Environ Geochem Health 32:415–430
Kochubovski M (2011) Heavy metals as persistent problem for Balkan countries. In Environmental Heavy Metal Pollution and Effects on Child Mental Development: Risk Assessment and Prevention Strategies. NATO Science for Peace and Security Series C: Environmental Security Volume 1. Ed. Simeonov, L.I., Kochubovski, M.V. and Simeonova B.G. Springer
Landis WG, Yu MH, (2003) Introduction to environmental toxicology: impacts of chemicals upon ecological systems, Third Edition. CRC Press LLC
Lee CS, Lim YW, Kim HH, Yang JY, Shin DC (2012) Exposure to heavy metals in blood and risk perception of the population living in the vicinity of municipal waste incinerators in Korea. Environ. Sci. Poll. Res. 165:349–363
Lim WY, Aris AZ, Tengku Ismail TH (2013) Spatial geochemical distribution and sources of heavy metals in the sediment of Langat River, western Peninsular Malaysia. Environ Forensic 14:133–145
Li-qun C, Yeboah S, Cheng-sheng S, Xiao-dong C, Ren-zhi Z (2015) GIS-based assessment of arable layer pollution of copper (Cu), zinc (Zn) and lead (Pb) in Baiyin District of Gansu Province. Environ Earth Sci 74:803–811
Lopes C, Herva M, Franco-Uría M, Roca E (2011) Inventory of heavy metal content in organic waste applied as fertilizer in agriculture: evaluating the risk of transfer into the food chain. Environ Sci Poll Res 18:918–939
Loska K, Cebula J, Pelczar J, Wiechula D, Kwapulinski J (1997) Use of enrichment factors together with geoaccumulation indexes to evaluate the content of Cd, Cu and Ni in the Rybnik Water Reservoir in Poland. Wat Air Soil Pollut 93:347–365
Ma X, Zuo H, Tian M, Zhang L, Meng J, Zhou X, Min N, Chang X, Liu Y (2016) Assessment of heavy metals contamination in sediments from three adjacent regions of the Yellow River using metal chemical fractions and multivariate analysis techniques. Chemosphere 144:264–272
Ma Y, Qin Y, Zheng B, Zhang L, Zhao Y (2015) Seasonal variation of enrichment, accumulation and sources of heavy metals in suspended particulate matter and surface sediments in the Daliao river and Daliao river estuary. Northeast China Environ Earth Sci 73:5107–5117
Maanan M (2008) Heavy metal concentrations in marine molluscs from the Moroccan coastal region. Environ Pollut 153:176–183
Marie V, Baudrimont M, Boudou A (2006) Cadmium and zinc bioaccumulation and metallothionein response in two freshwater bivalves (Corbicula fluminea and Dreissena polymorpha) transplanted along a polymetallic gradient. Chemosphere 65:609–617
Marmolejo-Rodrıguez AJ, Prego R, Meyer-Willerer A, Shumilin E, Cobelo-Garci A (2007) Total and labile metals in surface sediments of the tropical river-estuary system of Marabasco (Pacific coast of Mexico): influence of an iron mine. Mar Poll Bull 55:459–468
Martínez-Martínez S, Acosta JA, Faz Cano A, Carmona DM, Zornoza R, Cerda C (2013) Assessment of the lead and zinc contents in natural soils and tailing ponds from the Cartagena-La Unión mining district, SE Spain. J Geochem Explor 124:166–175
McCully P (2001) Rivers no more: the environmental effects of dams. In: silenced rivers: the ecology and politics of large dams. ZedBooks, UK
Milestone (2014) ETHOS One. The best choice in microwave sample preparation. Milestone. Available online at http://www.anamed.com.tr/milestone/wp-content/uploads/2014/10/ETHOSOne-CAT288EN-003.pdf (Assessed on 06 August 2016)
Muhammad Ali BN, Lin CY, Cleophas F, Abdullah MH, Musta B (2015) Assessment of heavy metals contamination in Mamut river sediments using sediment quality guidelines and geochemical indices. Environ Monit Assess 187:4190
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. GeoJournal 2:108–118
Nemati K, Abu Bakar NK, Abas MR, Sobhanzadeh E (2011) Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J Hazard Mater 192:402–410
Osborne JW (2002) Notes on the use of data transformations. Pract Assess Res Eval 8(6)
Osman KT (2014) Soil pollution. In: soil degradation, conservation and remediation. Springer, Netherland
Ozaki H, Watanabe I, Kuno K (2004) Investigation of the heavy metal sources in relation to automobiles. Wat Air Soil Poll 157:209–223
Pandey M, Tripathi S, Pandey AK, Tripathi BD (2014) Risk assessment of metal species in sediments of the river Ganga. Catena 122:140–149
Purushothaman P, Chakrapani GJ (2007) Heavy metals fractionation in Ganga River sediments. India Environ Monit Assess 132(1):475–489
Rocha GHO, Lini RS, Barbosa F Jr, Batista BL, de Oliveira Souza VC, Nerilo SB, Bando E, Mossini SAG, Nishiyama P (2015) Exposure to heavy metals due to pesticide use by vineyard farmers. Int Arch Occup Environ Health 88:875–880
Rosales-Hoz L, Carranza-Edwards A, Lopez-Hernandez M (2000) Heavy metals in sediments of a large, turbid tropical lake affected by anthropogenic discharges. Environ Geol 39:3–4
Rudnick RL, Gao S (2003) Composition of the continental crust. In: Rudnick, R.L. (Eds.) The Crust, vol. 3.Elsevier. pp. 1–64
Saleem M, Iqbal J, Shah MH (2015) Geochemical speciation, anthropogenic contamination, risk assessment and source identification of selected metals in freshwater sediments—a case study from Mangla Lake, Pakistan. Environ Nanotechnol Monit Manage 4:27–36
Sarmani SB (1989) The determination of heavy metals in water, suspended materials and sediments from Langat River, Malaysia. Hydrobiologia 176–177(1):233–238
Schi KC, Weisberg SB (1999) Iron as a reference element for determining trace metal enrichment in Southern California coast shelf sediments. Mar Environ Res 48:161–176
Schwartz MS, Benci JL, Selote DS, Anuj K, Sharma AK, Chen AG, Dang H, Fares H, Vatamaniuk OK (2010) Detoxification of multiple heavy metals by a half-molecule ABC transporter, HMT-1, and coelomocytes of Caenorhabditis elegans. PLoS One 5(3) e9564
Shafie N, Aris AZ, Hazzeman H (2014) Geoaccumulation and distribution of heavy metals in the urban river sediment. Int J Sediment Res 29:368–377
Song Y, Ji J, Yang Z, Yuan X, Mao C, Frost RL (2011) Geochemical behavior assessment and apportionment of heavy metal contaminants in the bottom sediments of lower reach of Changjiang River. Catena 85(1):73–81
Sundaray SK, Nayak BB, Lee BG, Bhatta D (2014) Spatio-temporal dynamics of heavy metals in sediments of the river estuarine system: Mahanadi basin (India). Environ Earth Sci 71:1893–1909
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ Geol 39(6):611–627
Tao HH, Slade EM, Willis KJ, Caliman JP, Snaddon JL (2016) Effects of soil management practices on soil fauna feeding activity in an Indonesian oil palm plantation. Agric Ecosyst Environ 218:133–140
Taylor SR (1964) Abundance of chemical elements in the continental crust: a new table. Geochim Cosmochim Acta 28:1273–1285
Teisserenc R, Lucotte M, Canuel R, Moingt M, Obrist D (2014) Combined dynamics of mercury and terrigenous organic matter following impoundment of Churchill Falls Hydroelectric Reservoir, Labrador. Biogeochem 118:21–34
Tran D, Boudou A, Massabuau JC (2001) How water oxygenation level influences cadmium accumulation pattern in the asiatic clam Corbicula fluminea: a laboratory and field study. Environ Toxicol Chem 20(9):2073–2080
USEPA (United States Environmental Protection Agency) (2002) A guidance manual to support the assessment of contaminated sediments in freshwater ecosystems. US EPA-905-B02–001-C
Usero J, Morillo J, Gracia I (2005) Heavy metal concentrations in molluscs from the Atlantic coast of southern Spain. Chemosphere 59:1175–1181
Venkatesha RK, Somashekar RK, Prakash KL (2012) Heavy metal status of sediment in river Cauvery, Karnataka. Environ Monit Assess 184:361–373
Walsh K, Dunstan H, Murdoch RN, Conroy BA, Roberts TK, Lake P (1994) Bioaccumulation of pollutants and changes in population parameters in the gastropod mollusc Austrocochlea constricta. Arch Environ Contam Toxicol 26:367–373
Wan YL, Aris AZ, Mohamad PZ (2012) Spatial variability of metals in surface water and sediment in the Langat River and geochemical factors that influence their water-sediment interactions. Sci World J 14
Wang B, Xia DS, Yu Y, Jia J, Xu SJ (2013) Magnetic records of heavy metal pollution in urban topsoil in Lanzhou, China. Chin Sci Bull 58(3):384–395
Wang Y, Chen P, Cui R, Si W, Zhang Y, Ji W (2010) Heavy metal concentrations in water, sediment, and tissues of two fish species (Triplohysa pappenheimi, Gobio hwanghensis) from the Lanzhou section of the Yellow River, China. Environ Monit Assess 165:97–102
Wang ZY, Lee JHW, Melching CS (2015) Dams and impounded rivers. In: Wang ZY, Lee JHW, Melching CS (eds) River dynamics and integrated river management. Tsinghua University Press, Beijing and Springer-Verlag, pp. 397–465
Won EJ, Kim KT, Choi JY, Kim ES, Ra K (2016) Target organs of the Manila clam Ruditapes philippinarum for studying metal accumulation and biomarkers in pollution monitoring: laboratory and in-situ transplantation experiments. Environ Monit Assess 188:478
Xu ZQ, Ni SJ, Tuo XG, Zhang CJ (2008) Calculation of heavy metals’ toxicity coefficient in the evaluation of potential ecological risk index. Environ Sci Technol 31(2):112–115
Yang Z, Lu W, Long Y, Bao X, Yang Q (2011) Assessment of heavy metals contamination in urban topsoil from Changchun City, China. J Geochem Explor 108(1):27–38
Yap CK, Ismail A, Tan SG, Omar H (2002) Correlations between speciation of Cd, Cu, Pb and Zn in sediment and their concentrations in total soft tissue of green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia. Environ Int 28:117–126
Yap CK, Ismail A, Tan SG, Abdul Rahim I (2003) Can the shell of the green-lipped mussel Perna viridis (Linnaeus) from the west coast of Peninsular Malaysia be a potential biomonitoring material for Cd, Pb and Zn? Estuar Coast Shelf Sci 57:623–630
Yap CK, Hatta Y, Berandah FB, Tan SG (2008) Comparison of heavy metal concentrations (Cd, Cu, Fe, Ni and Zn) in the shells and different soft tissues of Anadara granosa collected from Jeram, Kuala Juru and Kuala Kurau, Peninsular Malaysia. Pertanika J Trop Agric Sci 31(2):205–215
Yap CK, Aziran Y, Cheng WH (2009) Distribution of heavy metal concentrations in the different soft tissues and shells of the bivalve Psammotaea elongata and gastropod Faunus ater collected from Pantai Sri Tujuh, Kelantan. J Sust Sci Manage 4(1):66–74
Yap CK, Edward FB, Tan SG (2010) Similarities and differences of metal distributions in the tissues of molluscs by using multivariate analyses. Environ Monit Assess 165(1–4):39–53
Yap CK, Noorhaidah A, Tan SG (2011) Zn concentrations in the different soft tissues of Telescopium telescopium and their relationships with Zn speciation by sequential extraction in surface sediments: a statistical multiple linear stepwise regression analysis. In: Gastropods: diversity, habitat and genetics. Nova Science Publishers Inc. New York. Eds: Andrea M. Bianchi and Jamie N. Fields, 127–148 pp
Young SM, Ishiga H (2014) Assessment of dam removal from geochemical examination of Kuma River sediment, Kyushu. Japan Environ Monit Assess 186:8267–8289
Yuan X, Zhang L, Li J, Wang C, Ji J (2014) Sediment properties and heavy metal pollution assessment in the river, estuary and lake environments of a fluvial plain, China. Catena 119:52–60
Yuksel O (2015) Influence of municipal solid waste compost application on heavy metal content in soil. Environ Monit Assess 187:313
Zaharah AR, Zulkifli H, Sharifuddin HAH (1997) Evaluating the efficacy of various phosphate fertiliser sources for oil palm seedlings. Nutr Cycl Agroecosys 47:93–98
Zar JH (1996) Biostatistical analysis. 3rd ed. Prentice Hall, New Jersey
Zhao Q, Liu S, Deng L, Dong S, Wang C (2013) Longitudinal distribution of heavy metals in sediments of a canyon reservoir in Southwest China due to dam construction. Environ Monit Assess 185:6101–6110
Zhou J, Dang Z, Cai M, Liu C (2007) Soil heavy metal pollution around the Dabaoshan mine, Guangdong province, China. Pedosphere 17(5):588–594
Zhu H, Yuan X, Zeng G, Jiang M, Liang J, Zhang C, Yin J, Huang H, Liu Z, Jiang H (2012) Ecological risk assessment of heavy metals in sediments of Xiawan Port based on modified potential ecological risk index. Transac Nonfer Metals Soc China 22:1470–1477
Acknowledgments
The authors wish to acknowledge the financial supports to Mr. WKW under MyBrain15 of Malaysia’s MoHe and Graduate Research Fellowship of UPM for his Master study in UPM, which are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Highlights
• Heavy metal pollution in the Langat River by Corbicula javanica transplantation was investigated.
• Generally, the clam is a good biomonitor of metal pollution.
• Anthropogenic activities were identified as three contributors of the metals pollution in the river.
• Based on PERI, only As and Pb could pose a potential ecological risk to the river ecosystem.
• All geochemical indexes showed that overall heavy metal concentrations in the river are still within safe limit.
Rights and permissions
About this article
Cite this article
Wong, K.W., Yap, C.K., Nulit, R. et al. Effects of anthropogenic activities on the heavy metal levels in the clams and sediments in a tropical river. Environ Sci Pollut Res 24, 116–134 (2017). https://doi.org/10.1007/s11356-016-7951-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7951-z