Abstract
The concentrations of trace metals (Cd, Pb, Cu, Cr, Fe, Ni, Zn and Co) were studied in soil profiles of eight municipal waste dump sites in Warri metropolis. The results indicate that concentrations of trace metals varied widely among the different dumpsites and decreased with depths in a studied soil profile. The accumulation pattern for the heavy metals in the soil profiles follows the order: Fe > Pb > Mn > Co > Cr > Zn > Ni > Cu > Cd. The concentrations of metals found in these sites was below the Canadian remediation criteria for land for agricultural, residential and commercial/industrial purposes except for the cadmium levels in some sites that exceeded criterion values for agricultural and residential. In such sites, remedial actions are required before any form of chosen development can take place. The levels of heavy metals found in these dump sites were higher than that of the control site. The examined metals showed no significant correlation with soil physicochemical properties at the surface horizon, which is suggestive evidence that these metals arise from anthropogenic input.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Municipal solid waste heaps have become landmarks in several major cities in Nigeria, blocking motorways and threatening to cause disease epidemics and flooding. The enforcement of the existing regulations on solid waste disposal remains a problem in Nigeria (Iwegbue et al. 2006b).
In most Nigerian cities, different waste is not sorted and at times special waste (clinical, industrial and institutional) are co-deposited in the municipal dumps. There is no doubt that these dump sites contribute significant amounts of pollutants to the soil ecosystem. At times, Government clears these dump sites and commences developmental projects such as schools, playgrounds, parks, markets and other public utilities without proper assessment of the extent of contamination of such land. People who use the facilities constructed on such contaminated sites are at great risk of exposure to contaminants such as heavy metals, PCBs, pesticides and other toxic organics. Apart from these, leachate emanating from these dump sites could contaminate ground and surface water systems. In addition, the coastal nature of Warri and its environs makes it worthwhile for continuous monitoring of metals in these dump sites in order to protect the coastal aquatic system from pollution arising from land-based anthropogenic activities.
Contamination of heavy metals in the environment is of major interest because of their toxicity, persistence and threat to human life and the environment (Purves 1985). Contrary to other pollutants such as petroleum hydrocarbon, heavy metals persist in the environment as metal atoms; however, their speciation might change as soil conditions change. Moreover, unexpected rapid mobilization of heavy metals could result in environmental catastrophe, menacing health and welfare by poisoning water and food sources (Stigliani and Anderberg 1993).
The objectives of the present study were to determine the concentration of heavy metals and some physicochemical characteristics of soil profiles of municipal waste dumps with a view to providing information on the extent of contamination, risk to human environment and guidance for redevelopment.
Materials and methods
Description of study area
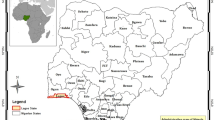
Warri is one of the major industrial cum coastal towns in the Niger Delta region of Nigeria. It has a port, a petroleum refining industry, a steel complex and several oil fields in its environs. Warri lies at 4°17′60°N, 6°15°E (Fig. 1). The area enjoys a tropical climate, with well-demarcated rainy and dry seasons. The dry season stretches from November to April while the rainy season is usually from May to October. The average rainfall is 2,500 mm while mean hourly rainfall is 100 mm. The minimum ambient temperature recorded is 18°C, while the maximum is 35°C, with a minimum ambient of 20°C and a mean maximum ambient of 31°C (Opute 2003). Warri occupies a low lying area with height generally less than 6 m above sea level. The area is drained by the Warri River and its networks of tributaries and creeks, emptying into the sea. The drainage pattern is dentritic with tributaries branching without a preferred orientation (Olobaniyi and Owoyemi 2006). Warri is underlain by a sequence of sedimentary formation with a thickness of about 8,000 m, including, from bottom to top, Akata formation, Agbada formation, Benin formation and the Somebreiro-Warri Deltaic Plain sands (Allen 1965; Reyment 1965; Short and Stauble 1967).
The Akata formation rests unconformably on the migmatite-gniess basement complex and forms the basal unit of the Niger Delta stratigraphic pile. This formation consists of an open marine facies unit dominated by high pressured carbonaceous shale’s. The formation ranges in age from Palacocene to Eocene and its thickness could exceed 1,000 m. The Agbada formation consists of a sequence of alternating deltaic sands and shales. It is Eocene to Oligocene in age and exceeds 3,000 m in thickness. This formation is the oil-reservoir in the Niger Delta basin. The Benin formation is Oligocene to Pleistocene in age. It consists predominantly of freshwater continental friable sands and gravel that are of excellent aquifer properties, with occasional interrelation of shales. The thickness of the Benin formation is variable but generally exceeds 2,000 m (Olobaniyi and Owoyemi 2006). Directly underlying the Warri area and the topmost of the sedimentary formation is the Quaternary to Recent alluvium, the Somebreiro-Warri Deltaic Plain sands (Wigwe 1975). This formation consists of fine to medium and coarse-grained unconsolidated sands that are often feldspathic (with 30–40 wt% feldspars) and occasionally gravely. This sequence is locally stratified with peat and lenses of soft and plastic clay, that could be sandy, and shale. The formation generally does not exceed 120 m in thickness and it is predominantly unconfined (Olobaniyi and Owoyemi 2006).
Sampling and analysis
Soil samples were collected from eight municipal wastes dump sites in Warri and its environs. The municipal waste dump sites have footprint area of 2,500–22,500 m2 with estimated waste volumes of 4,750–42,750 m3. The sites are designated as follows: site 1 = Refinery road waste dump; site 2 = Okere market road dump site; site 3 = Ogunu dump site; site 4 = NPA road dump site; site 5 = Giniwa dump site; site 6 = Enerhen road dump site; site 7 = Sapele road dump site; and site 8 = Udu bridge dump site. Site 9 is a control site at Osubi forest located 4 km from the Warri municipality. The control site has no history of waste dumping or farming. At each site, a quadrant was constructed around the dump site. The sizes of the quadrants varied with dump site area. For example, a quadrant of 50 × 50 m was constructed around the dump site with a footprint area of 2,500 m2. The quadrant was further divided into 9 cells of 16.6 × 16.6 m, and soil samples were collected from each cell at depths of 0–15, 15–30, 30–45 and 45–60 cm. A total of 9–15 samples were collected from each site depending on the footprint areas of the waste dump sites. On reception in the laboratory, the sample were air-dried, sieved to pass a 2-mm mesh and stored at 4°C until required for analysis.
The soil pH was measured in a 1:2.5 (v/v) ratio of soil and water suspension with a digital pH following the procedure of Radojevic and Bashkin (1999). Total organic matter content was determined by the Walkley and Black method (Radojevic and Bashkin 1999). Cation exchange capacity was determined as the sum of basic cation extractable by 1 M NH4Ac and exchangeable acidity (Radojevic and Bashkin 1999). Particle size distribution was determined by hydrometer method for silt and clay and dry sieving for sand fractions (Reeuwijk 1995). For heavy metal determination, 1.0 g of the soil sample was weighed into a 50-mL Teflon beaker and 9 mL of 65% (v/v) HNO3, 4 mL of 50% (v/v) HF, 1 mL of 37% v/v HCl and 2 mL of 75% v/v HClO4 were then added and digested at 135°C for 2 h. The digest was slowly evaporated until the white fumes of HClO4 appeared. The digest was filtered and made up to 25 mL with high purity water. The sample solutions were analyzed for Cd, Cu, Cr, Ni, Fe, Pb, Co and Zn using atomic absorption spectrophotometry (AAS) (Unicam Pye SP 2000) with a hollow cathode lamp as light source.
Matrix matching, standard addition and background correction were used to overcome interferences. After every four determinations, blanks and certified reference material were also run to determine the precision and instrumental uncertainties. All sample runs were replicated and the coefficient of variation between replicate analyses was less than 5%. Analar grade or its equivalent grade reagents were used throughout the work. Stock standard solutions of the metals were prepared by dissolving ultra pure metals/compound (99.9% pure) obtained from Aldrich. The percentage recovery for metals from the certified reference material were 93.4 for Cd, 85.7 for Fe, 89.7 for Cu, 93.4 for Co, 96.7 for Mn, 89.3 for Cr, 98.3 for Ni and 96.3 for Pb.
Enrichment factor
The enrichment coefficient of the metals in the soil profiles was qualified by the equation described by Baut-Menard and Chesselet (1979).
where C 1 M e = the examined metal content in the examined environment; C 2 M e = the examined metal content in the reference environment; C 1n = the reference element content in the examined environment; and C 2n = the examined reference element content in the reference environment.
A reference element is often a conservative one, such as the most commonly used elements: Al, Fe, Me, Mn, Sc, T, etc. (Quevauviller et al. 1989; Isakson et al. 1997; Lee et al. 1998; Reimann and de Caritat 2000; Bergamaschi et al. 2002; Hernandez et al. 2003; Conrad and Chisholm-Branse 2004; Mishra et al. 2004; Yougming et al. 2006; Nwajei and Iwegbue 2007). Therefore, iron is applied as a reference element because it is the most naturally abundant element in soil. It is pertinent to note that the enrichment coefficient is a convenient measure of geochemical trends and is used for comparisons between areas and over time. The enrichment coefficient gives an insight into differentiating an anthropogenic source from natural origin. An enrichment factor (EF) value close to 1 indicates crustal origin while values greater than 10 point to a non-crustal source (Nolting et al. 1999; Yougming et al. 2006). Five contamination categories are recognised based on enrichment coefficient values (Sutherland 2000; Loska and Wiechula 2003; Table 1).
Contamination/pollution index
The contamination/pollution (C/P) index was derived by employing the contamination/pollution index as defined by Lacatusu (2000).
The target (reference) values of metals were obtained using the standard table formulated by the Department of Petroleum Resources of Nigeria (DPR 2002) for maximum allowed concentrations of metals in soil (Table 2). A C/P index value greater than unity (1) defines a pollution range and when the value is less than unity defines contamination ranges. The significance of the C/P index is given in Table 3.
Results and discussion
General characteristics
Table 4 present the pH, particle size distribution, cation exchange capacity and percentage organic matter of the soil profiles of municipal waste dumps. The pH of the soil profiles decreased with increasing depths of the profiles. The pH value for all sites and depths ranged from 5.1 to 8.3. We have earlier reported similar pH range for soil profiles of automobile mechanic waste dumps in Port Harcourt, Nigeria (Iwegbue et al. 2006a, b). pH is very critical for most metals, since metal availability is relatively low when the pH is around 6.5–7 with the exception of Mo, Se and As; the mobility of metals decreases with increasing pH because the formation of insoluble hydroxide, carbonate and organic complexes (Yu et al. 1997). As shown in Table 4, the sand fraction is the predominant particle size in all sites and depths. The clay and silt fraction was less than 36% in all sites and depths. No regular trend in particle size distribution with depth. There is no clear evidence of organic matter increased with depths of the profiles. Similarly, cation exchange capacity (CEC) also showed a corresponding increase with depth. The cation exchange capacity is directly related to the capacity of adsorbing heavy metals, since the adsorption behaviour depends on the combination of the soil properties and the specific characteristics of the element (Barry et al. 1995). The CEC increases with increasing depths of profile, possibly due to an increase of clay content with depth. The CEC reported herein indicates that subsurface samples have the greater capacity for metal retention. In contrast, in this study, metals are retained more on the surface horizons than the subsurface horizons.
Heavy metal distribution
The vertical distribution of metals in the soil profiles of municipal waste dump sites are presented in Table 5. Analysis of variance (p < 0.05) showed significant variability in the concentrations of metals with depths and sites. This variability is associated with soil physicochemical characteristics and contaminant supplies. The concentrations of metals at these sites showed a decline with depths of the profiles. The concentrations of metals found at these sites were higher than concentrations of the metals found at the control site. The concentrations of metals found at the control sites were 0.93 mg kg−1 for Cr, 1.8 mg kg−1 for Cu, 4.8 mg kg−1 for Zn, 2.6 mg kg−1 for Pb, 1893 mg kg−1 for Fe, 29.7 mg kg−1 for Mn, 3.5 mg kg−1 for Co, and 4.1 mg kg−1 for Ni, while Cd was below the limit of detection. Similarly, Bamgbose et al. (2000) reported metal concentrations of 6.74, 4.94, 10.41, 1.60, 0.81 and 0.49 μg/g for Zn, Pb, Mn, Cu, Cd and Cr, respectively, at their control site in southwestern Nigeria. Higher concentrations of metals in soils of waste dumps in comparison with that of the control site indicate that municipal waste dumps contribute significant amounts of metals to the soil environment. The concentration profiles of the metals in the soil follow the order Fe > Pb > Mn > Co > Cr > Zn > Ni > Cu > Cd. The levels of metals obtained in this study were compared with both domestic and international guidelines (Table 2). The Dutch “target values” based on natural (background) soil levels and on negligible risk concentrations used in the Netherlands for soil protection (Lame and Leenaer 1998) are similar to the Department of Petroleum Resources target values in Nigeria. However, other international guidelines for metals in soils include Canadian soil quality criteria (CCME 1991), the Australian Ecological Investigation levels (EIL) and maximum concentration of toxic metal in soils permitted under the European Community regulation (Kabata-Pendias and Pendias 1992) are presented in Table 2 (above) for comparison. It is pertinent to note that guidelines for redevelopment for contaminated land is not currently available in Nigeria. Hence, the Canadian soil quality criteria were used for such comparison. The Australian EIL for urban setting is based on consideration of phytotoxicity levels, and soil data (Zarcinas et al. 2004). The Canadian soil quality criteria are based on level above which remedial action is necessary before such lands can be used for either agriculture, residential and industrial purposes. In general, the concentrations of metals in most of the sites were below these international guideline values.
The concentrations of copper in the soils of dump sites ranged between 2.50 and 82.12 mg kg−1. Sites 2 and 8 have significant higher mean levels (p < 0.05) of copper at 0–15 cm depths in comparison with levels found in other sites and depths. The concentrations of copper found in the soil profiles of the municipal waste dumps were higher than values of copper reported for municipal waste dumps in Agbor (Osakwe and Egharevba 2008). However, the levels of copper observed in the present study were lower than levels reported for soil profiles at automobile waste dumps in Nigeria (Iwegbue et al. 2006b; Ilemobayo and Ipinmoroti 2008). Similar levels of copper have been reported in soil profiles in the vicinity of a copper smelter in Poland (Kabala and Singh 2001). The target value for copper in soil is 35 mg kg−1 (Department of Petroleum Resources 2002). Only 0–15 cm depths at sites 1, 5 and 8 have concentrations above the DPR target value.
The highest mean concentration of Pb was observed at the 0–15 depth of site 6 (109.9 mg kg−1) whereas the lowest lead concentration was found in site 8. Lead concentrations spanning between 0.09 and 1.0 mg kg−1 (Nwajei et al. 2007), 2.30–3.76 mg kg−1 (Osakwe and Egharevba 2008) 57.60–443.36 mg kg−1 (Bamgbose et al. 2000), 316.2–560 mg kg−1 (Iwegbue et al. 2006b), and 14.23–37.98 (Iwegbue et al. 2009) have been reported in soils of municipal waste dumps and other contaminated sites in Nigeria. The concentrations found in the present study were similar to the range reported by Bamgbose et al. (2000) but were relatively higher than levels observed by Nwajei et al. (2007) and Osakwe and Egharevba (2008). The Department of Petroleum Resources (DPR) target value for lead in soil is 85 mg kg−1. The concentrations of lead in the 0–15 cm depths exceeded the DPR target value except for sites 7 and 8.
The concentrations of cadmium spanned between not detected (nd) and 6.80 mg kg−1. Cadmium was not detected at deeper sections of the soil profile at most sites. However, higher mean concentrations of cadmium were observed in sites 1 and 5. The concentrations of Cd observed in sites 1, 4 and 5 exceeded the DPR target values of cadmium in soils. Njoku and Ayoka (2007) reported cadmium levels below detection limit in soils of a municipal waste dump in Owerri, southeastern Nigeria. The levels of cadmium observed in sites 1, 4 and 5 were similar to values reported for soils of municipal waste dumps in southwestern Nigeria (Bamgbose et al. 2000). Low levels of cadmium were observed in this study in comparison with levels previously reported for automobile waste dumps (Iwegbue et al. 2006a). Sites 1, 4 and 5 have cadmium concentrations above the Canadian environmental quality criteria for agricultural and residential purposes. Like other urban centres where the demand for suitable land for development exceeds the availability, such contaminated sites may be used in the future for either residential or industrial, construction of children’s playgrounds, schools, etc. However, in developing countries like Nigeria, the government in most cases clears these sites and goes ahead with redevelopment projects without any form of assessment. People using such land may be faced with great environmental hazards (Iwegbue et al. 2009). For example, sites 1, 4 and 5, 7 and 8 require remedial action before any form of chosen development can proceed on such land.
However, it is pertinent to mention that merely exceeding the natural concentration does not, cause deleterious biological effects. Negative effects of elevated concentrations occur only where their critical (threshold) levels are exceeded. For example, the maximum concentrations of the potentially toxic elements in soil permitted under European community regulation are Pb (300), Cd (3.0), Zn (30) Ni (75) and Cu (140) (Kabata-Pendias and Pendias 1992).
The mean concentrations of nickel in all sites and depths ranged between 0.06 and 21.51 mg kg−1. The highest mean concentration of nickel was observed at 0–15 cm depth of site 1. The concentrations of nickel found at these sites were below the DPR target value for nickel in soil. The concentrations of nickel at our sites were higher than values reported for nickel in soils in the vicinity of fertilizer processing plants (Aina and Sridhar 2004), foam manufacturing industrial sites (Oviasogie and Omoruyi 2007), and municipal waste dumps at Owerri, southeastern Nigeria (Njoku and Ayoka 2007).
The concentration of zinc in the present study spanned between 4.64 and 40.61 mg kg−1 in all sites and depths, with site 8 having higher levels relative to other sites. The levels of zinc observed in the present study were lower than levels reported for soils of some contaminated sites in Nigeria (Bamgbose et al. 2000; Iwegbue et al. 2006a; Inuwa et al. 2007; Ilemobayo and Ipinmoroti 2008; Oviasogie and Omoruyi 2007) and in some other parts of the world (Asaah et al. 2005; Kashem et al. 2007). However, we have observed similar levels in soil around oilfields in Nigeria (Iwegbue et al. 2006a) and much lower zinc levels in soils around industrial waste dump sites (Aina and Sridhar 2004) and municipal waste dumps (Njoku and Ayoka 2007).
The highest mean levels of chromium in waste dumps were found at 0–15 cm depth of sites 1 and 5. Overall, the concentration of chromium showed a remarkable decline with depths of the profile. The concentrations of chromium observed in the present study were similar to values reported for municipal waste dumps in southwestern Nigeria (Bamgbose et al. 2000) and were higher than the range of 1.59–7.61 mg kg−1 reported for soil profiles of municipal waste dumps in Agbor, Nigeria (Osakwe and Egharevba 2008). The main sources of chromium in these dump sites include textile materials, photographic materials, chromium steels, ceramics and paper.
The concentrations of manganese spanned between 28.9 and 101.2 mg kg−1 in all sites and depths, with highest mean levels found in the top sections of sites 1 and 5. Bamgbose et al. (2000) reported manganese levels ranging between 77.26 and 187.55 mg kg−1 in soils from municipal waste dumps at Abeokuta southwestern Nigeria. Similarly, Nwajei et al. (2007) reported manganese concentrations ranging from 6.96 to 17.84 mg kg−1 in soils of municipal waste dumps in Onitsha, southeastern Nigeria. The concentrations of manganese observed in our sites were similar to the lower limit of the range reported by Bamgbose et al. (2000) and were rather higher than than the values we have previously reported for soils of the Onitsha municipal dumps and some sites in northern Nigeria (Gungshik et al. 2007; Gungshik and Mohammed 2007).
Cobalt concentration in the soil profiles of the dump sites ranged between 0.36 and 57.50 mg kg−1, with a higher concentration of (p < 0.05) observed in the soil profile of site 1 relative to other sites. The concentrations of cobalt in the soil profile of most sites exceeded the DPR target value of 20 mg kg−1. However, sites 7 and 8 and subsoils of some other sites have concentrations below the DPR target value (see Table 2). It is pertinent to mention that soil profiles of site 1 showed relatively higher levels of some of the examined metals in comparison with other sites (e.g., Cd, Cr, Cu, Mn, Co and Ni). Higher concentrations of these metals at site 1 may possibly be due to its proximity to industrial areas and the co-deposition of industrial waste in this municipal waste dump.
The concentrations of iron in the soil profiles of the municipal waste dumps studied ranged between 2,311 and 7,130 mg kg−1, with the profile of site 4 having the highest concentrations. The major sources of iron in these sites include metallic scraps being co-deposited with domestic wastes. The concentrations of iron observed in the present study were lower than levels reported for soils around an oil field (Iwegbue et al. 2006b) and those found in the automobile spare parts market (Nwajei and Iwegbue 2007).
Contamination/pollution index and enrichment factor
Assessment of soil for heavy metal pollution based on absolute metal content value provides inadequate information on the significance of the value obtained with the intrinsic soil feature and how the value is related to maximum allowable limits for each. The presence of one metal can significantly affect the impact that another has on organisms. This effect can be synergistic, additive or antagonistic (Eisler 1993). The contamination/pollution (C/P) index was calculated as the ratio between metal effectively measured by chemical analysis to the reference value. The Department of Petroleum Resources (2002) target values and the conversion formula (Lacatusu 2000) used for the C/P index vary from one country to another based on the chosen criteria. The calculated C/P index values were interpreted according to the scheme provided in Table 3. The C/P indices in Table 6 showed a decline with the depths of profiles. The C/P indices indicated that examined sites fitted into the “very slight contamination” to “severe pollution” ranges, with Pb in the “slight pollution range”, cadmium in the “moderate pollution” to “very severe pollution” ranges and cobalt in the ‘slight pollution’ range. However, copper in 0–15 cm depth of some sites (e.g., 1, 5 and 8) fitted into the slight pollution to “moderate pollution” range. Because of possible occurrence of synergy among metals even at low concentrations, the index values defining only the pollution range were totalled, obtaining in this case the values of multiple pollution with heavy metals for each site and depth, and also interpreted according to the scheme (Table 3). The values of multiple pollution index with heavy metals (Table 7) indicate that the degree of pollution with respect to sites follow the order: 1 > 5 > 4 > 3 > 6 > 8 > 2 > 7, with significant contributions from copper, lead, cobalt and cadmium.
The calculated enrichment (EF) is displayed in Table 8, which shows the distribution of each element’s EF. It is clear from Table 8 that all the elements except for manganese and iron have mean enrichment factors higher than 2 at 0–15 cm depths. Iron and manganese have enrichment factors slightly greater than unity, further confirming their mainly natural source. On the other hand, other elements (Cd, Cr, Pb, Cu) have enrichment factors higher than 10 at 0–15 cm depth in most sites. These were considered to originate mainly from anthropogenic sources. It seems, therefore, that EFs can also be effective tools to differentiate a natural origin from an anthropogenic source in this study.
The mean EFs decrease in the order of Cd > Pb > Cr > Cu > Co > Zn > Fe > Mn which can also be seen as the degree of order of their overall contamination of the soils of the dump sites.
The EF values decreased with increasing depths which indicates that 0–15 cm depth showed significant enrichment relative to other depths. In overall, most sites and depths have enrichment factor values in the “deficiency to minimal enrichment” and “very high enrichment” ranges. However, cadmium in some sites have enrichment factors greater than 40 (i.e. in the extremely high enrichment range).
The correlation patterns of the metals at the surface horizon showed no significant relationship with soil physicochemical characteristics which is suggestive of the evidence that concentrations at the surface horizon were due to anthropogenic input (Table 9). However, significant correlations exist between Cr, Cu, Mn, Ni and Cd in the surface horizons while Ni showed a positive correlation with Cd and Pb with Co. The correlation of chromium with copper, cadmium, nickel, and manganese can be explained either by toxicity or chemical similarity. However, the positive correlation showed that Cd, Cr, Ni and Cu come from the same source. The major sources of these metals in the municipal waste dumps include copper–cadmium alloy, nickel–cadmium batteries, PVC stabiliser and photovoltaic cells, etc.
A principal component analysis (PCA) was also applied to the datasets. PCA provides a means of reducing the complexity of the total metal datasets. Principal component loading value provides information on the relationship among the variables to provide pollutant sources (Meglen 1992). Factor loading with VARIMAX rotation was calculated (Tables 10, 11, 12, and 13). In this study, PCA was applied to study correlation among the heavy metals.
Three principal components or groups of metals were extracted from the depth profiles except for 0–15 cm depth interval, where two principal components were extracted. The composition of these factors are different for each of the depth intervals. In the 0–15 cm depth interval, PC 1 (or factor 1, expressing about 54.78% of the total variance) includes metals that are mainly from industrial emission/source (Cr, Cu, Ni and Cd) (Yougming et al. 2006), and PC 2 explaining about 24.70% of the total variance include Pb and Co which are derived chiefly from domestic and municipal waste into the environment, as suggested by their persistent level (Asaah et al. 2005). PCA of heavy metals from the 15–30 cm depth interval resulted in the first two groups (30.63 and 23.04%) accounting for 54.02% of the total variance in the metal dataset. In this depth, three components were extracted which contributed most of the metals studied except Cd, Ni and Zn which are mainly from anthropogenic input. Therefore, this indicates that the source of Cd, Ni and Zn was different from other metals. For the 30–45 cm depth interval, PCA results also showed that three components were extracted and accounted for 52.0 and 20.46% of the total variance. PC 1 had significant positive loadings in Cr, Pb, Fe and Mn, respectively. This indicates the affinity of Pb and Cr for the surface of Fe–Mn oxides (Ryan et al. 2002; Gadh et al. 1993; Jardao and Hickless 1989). This also indicates that Fe–Mn oxide surfaces are natural scavenger for these metals and therefore these elements are associated with indigenous clay minerals in the soil or that contamination has been associated with soil types that have higher Mn and Fe (Zarcinas et al. 2004). PC 2 had significant positive loading in Cu and Zn, and PC 3 had significant loadings in Fe and Co, respectively.
Furthermore, three principal components were extracted from the dataset of the 45–60 cm depth intervals. PC 1 expressing about 40.77% of the total variance includes Ni, Mn, Co and Cd, and PC 2 explaining about 29.78% of the total variance includes Cr, Pb and Zn, respectively.
Conclusion
The experimental data revealed that the concentrations of the examined metals varied widely among the different dump sites and decreases with depths in a given soil profile. The concentrations of metals found in these sites were below the Canadian remediation criteria for land for agricultural, residential and industrial/commercial purposes except for cadmium in a few sites. Such sites require remedial action before any form of the chosen development could proceed. There was no significant correlation between the metals and the soil physicochemical characteristics, which is suggestive of evidence that these metals arise from anthropogenic inputs (waste deposit). Overall, the concentrations of metals found in these sites pose no risk to the environment; however, the metal content of these dump sites requires constant monitoring since unexpected rapid mobilisation could result in serious environmental problems requiring protecting of the adjacent coastal water system from contamination arising from land-based activities.
References
Allen, J. R. L. (1965). Late quaternary Niger delta and adjacent areas: sedimentary environment and lethofacies. Bulletin of AAPG, 49, 547–600.
Alloway, B. J. (2005). Bioavailability of trace metal in soil. In O. Selinus, J. A. Centeno, R. B. Finkelman, R. Fuge, U. Lindh, & P. Smedly (Eds.), Essentials of medical geology. Impacts of the natural environment & public health (pp. 347–372). London: Elsevier Academic Press.
Aina, G. R. E., & Sridhar, M. K. C. (2004). Soil quality near a chemical fertilizer industry at Port-Harcourt. Nigeria AJEAM-RAGEE, 8, 19–26.
Asaah, V. A., ABimbola, A. F., & Surh, G. E. (2005). Heavy metal concentrations and distribution in surface soils of the Bassa Industrial zone 1. Dovala Cameroon. Arabian Journal of Science and Engineering, 31(2A), 147–158.
Bamgbose, O., Odukoya, O., & Arowolo, T. O. A. (2000). Earthworm as bioindicator of heavy metal pollution in dumpsite of Abeokuta city, Nigeria. Revista de Biologia Tropical, 48(1), 229–234.
Barry, G. A., Chudek, P. J., Best, E. K., & Moody, P. W. (1995). Estimating sludge application rates to land based on heavy metal and phosphorus sorption characteristics of soil. Water Research, 29, 2031–2034. doi:10.1016/0043-1354(95)00027-I.
Baut-Menard, R., & Chesselet, R. (1979). Variable influence of flux on trace metal chemistry of ocean suspended matter. Earth and Planetary Science Letters, 42, 398–411.
Bergamaschi, L., Rizziv, E., Valcuvia, M. G., Verza, G., Profuno, A., & Gallorni, M. (2002). Determination of trace element and evaluation of their enrichment factors in Himalayan Lichens. Environmental Pollution, 120(1), 137–144. doi:10.1016/S0269-7491(02)00138-0.
Conrad, C. F., & Chisholm-Branse, C. J. (2004). Partial survey of trace metal contaminations in the sediments of the Elizabeth River, Vinginia. Marine Pollution Bulletin, 49, 319–324. doi:10.1016/j.marpolbul.2004.02.019.
Canadian Council of Ministers of the Environment (CCME). (1991). Interim Canadian environmental quality criteria for contaminated sites. Report CCEM EPC-C534, Winnipeg, Manitoba.
Department of Petroleum Resources (DPR). (2002). Environmental guidelines and standards for the petroleum industries in Nigeria Department of Petroleum Resources, Ministry of Petroleum and Mineral Resources, Abuja-Nigeria.
Eisler, R. (1993). Zinc hazards to fish, wildlife and invertebrates; a synoptic review. US fish and wildlife service, Biological Report 10 publication USFWS. Washington, DC, 20240.
Gadh, R., Tandon, S. N., Mathur, R. P., & Singh, O. V. (1993). Speciation of metal in Yamuna river sediment. The Science of the Total Environment, 136, 229–242. doi:10.1016/0048-9697(93)90311-S.
Gungshik, J. K., & Mohammed, J. D. (2007). Impacts of mining on trace metal content of soil. African Journal of Natural Sciences, 10, 21–24.
Gungshik, J. R., Lohdip, Y. N., & Bewarang, E. S. (2007). Speciation of trace metals in topoils in Jos industrial areas. African Journal of Natural Sciences, 10, 15–19.
Hernandez, L., Probst, A., Probst, J. L., & Ulrich, E. (2003). Heavy metal distribution in some French forest soils, evidence for atmospheric contamination. The Science of the Total Environment, 312, 195–219. doi:10.1016/S0048-9697(03)00223-7.
Ilemobayo, O., & Ipinmoroti, K. (2008). Profile of heavy metals from automobile workshop in Akure, Nigeria. Journal of Environmental Science and Technology, 1(1), 19–26.
Inuwa, M., Abdulraham, F. W., Birnin Yauri, U. A., & Ibrahim, S. A. (2007). Analytical assessment of some trace metals in soils around the major industrial areas in northwestern Nigeria. Trends in Application Science and Research, 2(6), 515–521.
Isakson, J., Oblad, M., Lindgren, E. S., Fridell, M. D., Pacyna, J. M., & Makinen, M. (1997). Perturbation of background aerosol at rural sites in the Nordic countries. Atmospheric Environment, 31(18), 3077–3086.
Iwegbue, C. M. A., Egobueze, F. E., & Opuene, K. (2006a). Preliminary assessment of heavy metals levels of soils of an oilfield in the Niger Delta, Nigeia. International Journal of Environmental Science and Technology, 3(2), 167–172.
Iwegbue, C. M. A., Isirimah, N. O., Igwe, C., & William, E. S. (2006b). Characteristic levels of heavy metals in soil profiles of automobile mechanic waste dumps in Nigeria. Environmentalist, 26, 131–137.
Iwegbue, C. M. A., Williams, E. S., & Isirimah, N. O. (2009). Study of heavy metal distribution in soils impacted with crude oil in southern Nigeria. Soil Sediment Contamination, 18(2), 136–143.
Jardao, C. P., & Hickless, G. (1989). Chemical association of Zn, Cd, Pb and Cu in soils and sediments determined by the sequential extraction. Environmental Technology and Letter, 10, 743–752.
Kabala, C., & Singh, B. R. (2001). Fractionation and mobility of copper, lead and zinc in soil profiles in the vicinity of a copper smelters. Journal of Environment Quality, 30, 485–492.
Kabata-Pendias, A., & Pendias, H. (1992). Trace elements in soil and plants and edn. Boca Raton, FL: CRC Press.
Kashem, M. A., Singh, B. R., Kondo, T., Imarne Hug, S. M., & Kawai, S. (2007). Comparison of extractability of Cd, Cu, Pb and Zn with sequential extraction in contaminated and non contaminated soils. International Journal of Environmental Science and Technology, 4(2), 169–176.
Lacatusu, R. (2000). Appraising levels of soil contamination and pollution with heavy metals. European Soil Bureau Research Report No 4.
Lame, F. J. P., & Leenaer, H. (1998) Target values and background levels in The Netherlands: how to define good soil quality. In contaminated soil ‘98. Proceedings of the sixth international KZK/TNO conference on contaminated soil, 17–21 May 1998, Edinburgh, UK, Vol 2. London UK. Thomas Telford Publishing, pp. 783–784.
Lee, C. L., Fang, M. D., & Hsieh, M. F. (1998). Characterization and distributing of metals in surfacial sediments in southwestern Taiwan. Marine Pollution Bulletin, 36(6), 464–471.
Loska, K., & Wiechula, D. (2003). Application of principle component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemistry, 51, 123–733.
Meglen, R. R. (1992). Examining large databases: a chemometric approach using principal component analysis. Marine Chemistry, 39, 217–237.
Mishra, V. K., Kim, K. H., Kang, C. H., & Cho, K. C. (2004). Wintertime sources and distribution of airborne lend in Korea. Atmospheric Environment, 30(20), 3471–3479.
Njoku, P. C., & Ayoka, A. O. (2007). Evaluation of heavy metal pollutants from soil at municipal solid (MSW) deposit in Owerri, Imo state, Nigeria. Journal of Chemical Society Nigeria, 32(1), 57–60.
Nolting, R. F., Ramkema, A., & Everaarts, J. M. (1999). The geochemistry of Cu, Cd, Zn, Ni and Pb is sediment cores from the continental slope of Banc d’ again (Mauritania). Continental Shelf Research, 19, 665–691.
Nwajei, G. E., & Iwegbue, C. M. A. (2007). Trace metal concentrations in soils in the vicinity of Uwelu motor spare parts market, Benin city, Nigeria. Journal of Chemical Society Nigeria, 32(2), 283–286.
Nwajei, G. E., Iwegbue, C. M. A., & Okafor, M. I. (2007). Heavy metals in surface soils under waste dumps from Onitsha, Nigeria. Journal of Biological Science, 7(2), 405–408.
Olobaniyi, S. B., & Owoyemi, F. B. (2006). Characterization by factor analysis of the chemical facies of groundwater in Deltaic plain sands aquifer of Warri, Western Niger Delta, Nigeria. African Journal of Science and Technology (Science and Engineering Series), 7(1), 73–81.
Opute F. I. (2003). Contribution to the knowledge of Algae of Nigeria. II. Chlorophyceae from the Warri/Forcados Estuaries Part I. the Orders Volvocales and Chloroccocales. Benin Science Digest, 1, 31–52.
Osakwe, S. A., & Egharevba, F. (2008). Sequential fractionation of cadmium, copper, lead and chromium in soils around municipal solid waste dumps in Agbor, Nigeria. Journal of Chemical Society Nigeria, 33(2), 139–147.
Oviasogie, P. O., & Omoruyi, E. (2007). Levels of heavy metals and physicochemical properties of soils in a foam manufacturing industry. Journal of Chemical Society Nigeria, 32(1), 102–106.
Purves, D. (1985). Trace-element contamination of the environment. Amsterdam: Elsevier.
Quevauviller, P., Lavigns, R., & Cortex, L. (1989). Impact of industrial drainage wastes on the heavy metal distribution in the drainage basin and estuary of the Sado River (Portugal). Environment Pollution, 59, 267–286.
Radojevic, M, & Bashkin, V. N. (1999). Practical environmental analysis Royal Society of Chemistry, UK.
Reeuwijk, L. P. (ed). (1995). Procedure for soil analysis. Technical paper 9 5th ed ISRIC, Wageningen, The Netherlands.
Reimann, C., & de Caritat, P. (2000). Intrinsic flaws of element enrichment factors (Ef) in environmental geochemistry. Environmental Science and Technology, 34, 5084–5091.
Reyment, R. A. (1965). Aspect of the geology of Nigeria. Ibadan University Press, 145 pp.
Ryan, P. C., Wall, A. J., Hillier, S., & Clark, I. (2002). Insight to sequential chemical extraction procedure for quantitative XRD: A study of trace metal partitioning in sediment related to frog malformations. Chemical Geology, 184, 337–357.
Short, K. C., & Stauble, A. I. (1967). Outline of the geology of the Niger Delta. Bulletin AAPG, 51, 761–779.
Stigliani, W. M., & Anderberg, S. (1993). Heavy metal pollution I the Rhine Basin. Environmental Science and Technology, 27, 786–793.
Sutherland, R. A. (2000). Bed sediment-associated trace metals in urban stream Oahu, Hawaii. Environmental Geology, 39, 361–627.
Wigwe, G. A. (1975). The Niger delta. Physical. In G. E. K. Ofomata (Ed.), Nigeria in maps: Eastern states (pp. 38–40). Benin: Ehtiope Publ. House.
Yougming, H., Peixuan, D., Junji, C., & Posmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Science of the Total Environment, 355, 176–186.
Yu, T. R., Sun, H. Y., & Zhang, H. (1997). Specific adsorption of cations. In T. R. Yu (Ed.), Chemistry of variable charge soils (pp. 140–174). New York: Oxford University Press.
Zarcinas, B., Pongsakul, P., McLaughlin, M. J., & Cozens, G. (2004). Heavy metals in soil and crops in Southeast Asia. 2 Thailand. Environmental Geochemistry and Health, 26, 359–371.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwegbue, C.M.A., Nwajei, G.E., Ogala, J.E. et al. Determination of trace metal concentrations in soil profiles of municipal waste dumps in Nigeria. Environ Geochem Health 32, 415–430 (2010). https://doi.org/10.1007/s10653-010-9285-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-010-9285-y