Abstract
Dynamics of heavy metals in the surface sediments of Mahanadi river estuarine system were studied for three different seasons. This study demonstrates that the relative abundance of these metals follows in the order of Fe > Mn > Zn > Pb > Cr > Ni ≥ Co > Cu > Cd. The spatial pattern of heavy metals supported by enrichment ratio data, suggests their anthropogenic sources possibly from various industrial wastes and municipal wastes as well as agricultural runoff. The metal concentrations in estuarine sediments are relatively higher than in the river due to adsorption/accumulation of metals on sediments during saline mixing, while there is a decreasing trend of heavy metal concentrations towards the marine side. The temporal variations for metals, such as Fe, Mn, Zn, Ni and Pb exhibit higher values during monsoon season, which are related to agricultural runoff. Higher elemental concentrations are observed during pre-monsoon season for these above metals (except Ni) at the polluted stations and for metals, such as Cr, Co and Cd at all sites, which demonstrate the intensity of anthropogenic contribution. R-mode factor analysis reveals that “Fe–Mn oxy hydroxide”, “organic matter”, “CaCO3”, and “textural variables” factors are the major controlling geochemical factors for the enrichment of heavy metals in river estuarine sediment and their seasonal variations, though their intensities were different for different seasons. The relationships among the stations are highlighted by cluster analysis, represented in dendrograms to categorize different contributing sites for the enrichment of heavy metals in the river estuarine system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rivers play an important role in population development, supplying water for humans, agriculture and industrial consumption. Water and sediment quality which is vital for the survival and well being of living resources, especially in the river and estuarine systems, is governed by many factors, including heavy metals (Pizarro et al. 2010). River sediments also play a significant role in the remobilization of contaminants including heavy metals under favorable conditions and in interactions between water and sediment (Ikem et al. 2003). Water chemistry only assesses the effluent impact at the time of sampling, while the river sediments geochemistry gives a cumulative assessment of the pollution. River sediments represent reservoirs, or sinks for the accumulation of contaminants derived from both natural and anthropogenic sources (Alagarsamy and Zhang 2010; Sánchez-García et al. 2010; Lourino-Cabana et al. 2011; Sundaray et al. 2011a; Martins et al. 2012). Sediment geochemistry is the study of chemical constituents of the sediments and chemical changes. The distributions of the heavy metals are controlled by a number of physical, chemical, and biological factors. Study of the distribution and concentrations of heavy metals in the sediments may reveal the geochemical nature of the sediments and their sources, natural as well as anthropogenic (Forstner and Wittmann 1979; Silva et al. 2009; Nobi et al. 2010). As a combined result of these factors, metal concentrations in the sediment change in space and time (Zhan et al. 2010; Huang et al. 2011; Oshisanya et al. 2011).
Multivariate statistical techniques such as factor analysis (FA) and cluster analysis (CA) are the appropriate tool for a meaningful data reduction and interpretation of multi-constituent chemical and physical measurements. FA, which includes principal component analysis (PCA) is a very powerful technique applied to reduce the dimensionality of a data set consisting of a large number of inter-related variables, while retaining as much of variability as possible present in data set. This reduction is achieved by transforming the data set into a new set of variables, the principal components (PCs), which are orthogonal (non-correlated) and are arranged in decreasing order of importance. Mathematically, the PCs are computed from covariance or other cross-product matrix, which describes the dispersion of the multiple measured parameters to obtain eigen values and eigenvectors. Principal components are the linear combinations of the original variables and the eigenvectors (Wunderlin et al. 2001). Besides considerable data reduction, entire data set’s variability is described through few VFs/PCs without losing much information. Further, grouping of the studied variables according to their common features by VFs helps in data interpretation (Vega et al. 1998; Morales et al. 1999; Helena et al. 2000; Simeonov et al. 2003; Singh et al. 2004; Sundaray et al. 2006; Sundaray 2010).
Cluster analysis groups objects (cases) into classes (clusters) on the basis of similarities within a class and dissimilarities between different classes (Vega et al. 1998). The class characteristics are not known in advance but may be determined from the analysis. Unlike FA that normally uses limited PCs for display purposes, CA uses all the variance or information contained in the original data set.
The Mahanadi river system is the third largest in the peninsula of India and the largest river of Orissa state. The river carries a pollution load from industrial and agricultural areas of Orissa, India (Sundaray et al. 2006, 2009, 2011a, b, 2012a). In our earlier studies, we focused on behavior and distribution patterns of nutrients in Mahanadi river and estuarine waters (Sundaray et al. 2005), evaluation of spatial and temporal variations in the water quality of the Mahanadi river and estuarine system using multivariate statistical technique (Sundaray et al. 2006), application of factor and CA for characterization of Mahanadi river and estuarine water systems (Panda et al. 2006a), evaluation of water quality of Mahanadi river and estuarine system with reference to suitability for agricultural purposes (Sundaray et al. 2009), geochemical speciation and risk assessment of heavy metals in Mahanadi river estuarine system (Sundaray et al. 2011a), application of WQI technique for the classification of water quality: Mahanadi river-estuarine system (Sundaray et al. 2011b) and dynamics and quantification of dissolved heavy metals in the Mahanadi river estuarine system (Sundaray et al. 2012a). Except one study (Sundaray et al. 2011a), all of these studies were mainly concerned with water quality of Mahanadi river estuarine system. Few research projects have been carried out on the sediment quality of the Mahanadi river system only (Chakrapani and Subramanian 1990a, b, 1993a, b); Konhauser et al. 1997). Most studies were mainly concerned with a short stretch of river course or some specific season and almost no study has been performed about spatio-temporal dynamics of heavy metals in the sediments of the entire river estuarine systems.
The objectives of the present work are (1) to assess the spatio-temporal dynamics of heavy metals in sediment samples, (2) to compare their concentrations with the standards for sediment quality and levels in other studies, (3) to identify major geochemical factors responsible for the enrichment of heavy metals in river estuarine sediment samples with the aid of R-mode factor analysis.
Study area description
Geographical setting
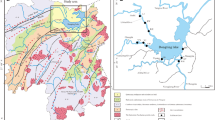
The Mahanadi river basin (80°30′–86°50′E and 19°20′–23°35′N) extends over an area approximately 141,600 km2, has a total length of 851 km and an annual runoff of 50 × 109 m3 with a peak discharge of 44,740 m3 s−1. The basin is characterized by a tropical climate with average annual rainfall of 142 cm with 90 % occurring during the south west monsoon (Sundaray et al. 2006; Chakrapani and Subramanian 1990a). The river begins in the Baster Hills of Madhya Pradesh, flows over different geological formations of Eastern Ghats and adjacent areas and joins the Bay of Bengal after divided into different branches in the deltaic area. The main branches of River Mahanadi meet Bay of Bengal at Paradip and Nuagarh (Devi estuary) (Fig. 1). The tidal estuarine part of the river covers a length of 40 km. Based on the physical characteristics, the estuary has been characterized as a partially mixed coastal plain estuary.
Geology of the basin
The basin geology is characterized by the pre-cambrians of Eastern Ghats consisting of rock types as khondalites, charnockites, leptynites, granites, gneisses, etc., the limestones sandstones and shales of the Gondwanas, and the costal tracts constituted by the recent deltaic alluvium of the river with littoral deposits. The basin lithology consists of granite suite (34 % of the basin area), khondalite suite (7 %), charnockite suite (15 %), limestone, shale of lower Gondwana (17 %), sandstone, shale of upper Gondwana (22 %) and coastal alluvium (5 %). A part of the richest mineral belt of the sub-continent consisting of Fe ore, coal, lime-stone, dolomite, bauxite, Pb and Cu deposits fall within the basin (Chakrapani and Subramanian 1990a; Sundaray et al. 2011a).
Anthropogenic setup of the basin
River Mahanadi serves as a major source of domestic water supply of cities like Cuttack (population about 0.50 million), Sambalpur (population about 0.20 million) and Paradip (population about 0.15 million) apart from a number of rural and minor urban settlements (Fig. 1). The river serves as a major source for fisheries and agricultural irrigation in the state of Orissa, India. It also serves as a major source of domestic water supply of the Cuttack, Sambalpur cities and indirectly to Paradip city through Taladanda canal. Subsequently, the river receives back the untreated domestic waste water from Sambalpur, Bauda, Cuttack, Choudwar, Jagatpur and Paradip cities of Orissa state and effluents from some industrials (fertilizer, paper, textile distilleries and others) directly during its coarse (Radhakrishna 2001). It also receives large amount of agricultural runoff along its coarse. Human influences are pronounced at Sambalpur, Cuttack and Paradip, where the proliferation of industries and sewer discharges are prominent.
Materials and methods
Under the sediment quality monitoring programme of the Mahanadi river basin, samples from 31 locations of Mahanadi river estuarine system, starting from Hirakud reservoir to the estuary points, i.e. at Paradip and Nuagarh (Fig. 1) were taken using a portable type Peterson’s grab sampler. Each sample (vol. 5 × 5 × 3 cm apx.) was obtained by mixing sediments randomly collected (three times) at each sampling point. Samples were brought to the laboratory packed in polyethylene air tight bags. All the samples were carried to laboratory and preserved in a refrigerator at 3–4 °C. Before analysis and characterization, samples were air-dried and homogenized. Powdered samples were digested in triplicate in Teflon beakers with HClO4–HF–HNO3. The concentrations of metals (Fe, Mn, Zn, Cu, Co, Cr, Ni, Pb and Cd) were determined by AAS (Perkin Elmer AAS 3110) in flame mode. All the samples were analyzed in triplicate with blank similarly treated for metal analysis. The precision and accuracy of the methods were systematically and routinely checked by USGS reference sample No. GXR, where it has been found that the precision (coefficient of variation of five replicate analysis) were 3 % for Cu, Cr and Fe and 4 % for Pb, Cd, Co, Ni, Mn and Zn. A portion of the sample was taken for textural analysis (sand, silt and clay percentages). The estimation of organic matter in sediments was determined by Walkley and B1ack method (Trivedi and Goel 1984). The amount of CaCO3 (including other carbonates) in the bulk sediment samples was estimated by the rapid titration method of Hutchinson and Melclennan (Trivedi and Goel 1984).
Results and discussion
The maximum, minimum, mean concentrations along with standard deviations of analyzed metal ions (Fe, Mn, Cu, Ni, Co, Pb, Zn, Cr and Cd) in the sediment samples of Mahanadi river estuarine system are presented in Table 1. The metal concentration in the sediments of some major rivers and estuaries of India compared with our present study are incorporated in Table 2. Owing to varying fluxes of matter into Mahanadi, the concentration of metal ions in the sediments is variable along the course of the river. The spatial and temporal variations of individual metals are depicted in Fig. 2.
Spatial and temporal dynamics of heavy metals
Iron (Fe)
Iron is the most abundant element in the earth’s crust. Oxidation of iron (II) to oxides and hydroxides is one of the most likely reactions to occur when the pH of the river water is raised by mixing with sea water (Boyle et al. 1977; Mayer 1982). The formation of these oxides is considered to be very important in controlling the uptake of other metals from solution. The Mahanadi river sedimentary iron value varies from 0.98 to 6.30 mg (metal)/g (sediment) (avg. 2.85 mg/g), 2.70 to 5.26 mg/g (avg. 3.78 mg/g) and 1.96 to 5.78 % (avg. 3.15 mg/g) for pre-monsoon, monsoon and post-monsoon seasons respectively. Similarly in case of estuarine sediments, Fe concentration varies from 1.02 to 6.41 mg/g (avg. 3.83 mg/g), 3.43 to 5.45 mg/g (avg. 4.44 mg/g) and 3.21 to 5.87 mg/g (avg. 4.51 mg/g) for the respective three seasons. Downstream stations (St No. 4, 5, 6, 16, 17, and 27) of two major urban settlements recorded relatively higher iron contents. Its concentration in the sediments of the estuarine system is always greater than in that of the river during pre-monsoon and post-monsoon seasons (Boyle et al. 1977; Mayer 1982). In the estuarine system, station No. 25 shows the highest concentration, which is attributed to combined impact of effluent load from fertilizer plants like PPL, sewage of Paradip port township situated on the upstream of the creek. The enrichment is also enhanced by the impact of sea water. A maximum concentration is recorded in silty clay sediments and a minimum in coarse sandy sediments. It is observed that the sediment of the Hirakuda reservoir (St. No. 1) has comparatively much higher trace element content, which is attributed to the accumulation of elements by finer particles present in the reservoir.
The weathering of ferruginous laterite and transportation of weathering products through fresh water have resulted in an increased iron concentration during monsoon season compared to pre-monsoon and post-monsoon seasons (Padma and Periakali 1999). Further, during the monsoon season, the river carries a relatively high percentage of finer particles, provides a better site for adsorption of heavy metals and may cause enrichment of metals (Singh 1999). Trace elements are enriched in the river channel sediments attributing the important contribution made by country rocks to the delta (Koshy 2002).
The iron content in the sediment of river Mahanadi is slightly higher than some other Indian river systems like Ganga, Cauvery, Gomati, Narmada, Tapti, Damodar, Brahamaputra and Brahamani, but it is similar (Narmada) or slightly lower than rivers like Godavari and Krishna (Table 2). In the estuarine system, it is observed that there is a decreasing trend of iron towards the marine side, which might be due to the flocculation of dissolved Fe at low salinity. The flocculation of dissolved Fe decreases with increase in salinity (Sholkovitz 1987). This process observed in many estuaries (Zhiquing et al. 1987; Balakrishna et al. 1997). This observation is clearly visible in the Devi estuary; however, in case of Mahanadi estuary the impact of anthropogenic contributions are dominant. Nayak et al. (2004) observed a decreasing trend of trace metals towards the coastal environment. The sedimentary iron of Mahanadi estuaries are somewhat similar to that reported from other Indian estuaries (Table 2).
Manganese (Mn)
Manganese is an essential element in biological systems. Manganese contamination is relatively insignificant except for the common biological toxicity effects found in plants exposed to excess of natural manganese in the soil. In toxic environment, it is present mainly as manganese (IV) oxides and oxy hydroxides. Under anoxic conditions, manganese (IV) compounds are readily reduced to manganese (II) and the process is considered to be both chemical and biochemical in nature (Koshy 2002). It can occur in the sediments as discrete oxide particles, coatings on particles and as manganese (II) adsorbed on organic materials (Feely et al. 1983). Manganese content of the sediments is mainly derived from the primary source rocks through which the river is flowing. The sediments show tremendous fluctuations in their manganese contents, which range from 364 to 1,146 μg/g during pre-monsoon, 602 to 2,500 μg/g during monsoon and 412 to 1,025 μg/g during post-monsoon season in the Mahanadi river (fresh water), and 345–1,546, 1,235–2,315 and 398–1,326 μg/g during the respective three different seasons in estuarine regions.
An increasing trend of manganese content is found in downstream stations of major townships irrespective of seasons. Seralathan and Seetaramaswamy (1987) observed an increasing trend of Mn towards downstream stations and attributed this to the increasing oxidative precipitation of Mn2+ as MnO2 and Mn(OH)2. In the middle reaches of the river decreasing concentrations of Mn may be due to the decreasing oxidative precipitation.
Like temporal variations of Iron, during monsoon periods accumulation of manganese in the sediment is relatively higher (Fig. 2). This likely is due to the settling of the monsoon sediments (carrying manganese from soils). Similar to iron, the spatial variation of manganese shows a decreasing trend towards the marine side in case of Devi estuaries, which might be due to the increasing effect of salinity (Sholkovitz 1987). However, in case of Mahanadi estuary, the impact of anthropogenic contributions is dominant.
The manganese concentration in the sediments of river Mahanadi is lower than in some major Indian rivers like Godavari, Krishna, Cauvery and Tapti (Table 2), but it is slightly higher than the average in Indian rivers. In comparison with world river sediment average, Mahanadi sediment has relatively lower manganese content.
Zinc (Zn)
Zn is essential for man, animals and plants but in high concentrations it is potentially toxic to plants and/or warm-blooded animals and man. Therefore, its addition to soil or plants, which are used for food, is highly harmful (Ohnessorge and Wilhem 1991). Sources of Zn in the environment are usually from natural and anthropogenic sources, including from industrial effluents, domestic effluents, urban storm water runoff and spoil heaps. Zinc occurs in nature as blende, sulphide, silicate, zincite (ZnO), etc. Zinc salts are relatively non-toxic, but heavy doses (165 mg) for a period of 26 days cause vomiting, renal damage and cramps (Kudesia 1990).
The sedimentary zinc content of Mahanadi river ranges from 42.6 to 138.3 μg/g during pre-monsoon, 67.5 to 136.2 μg/g during monsoon and 45.0 to 122.5 μg/g during post-monsoon season, whereas in the estuarine sediments it is from 52.0 to 233.2, 73.3 to 113.7 and 63.48 to 171.9 μg/g for the respective seasons. The seasonal variation (Fig. 2) shows that zinc concentration is maximum during monsoon season probably on account of the input of more terrigenous materials gathered from its catchments. However, in the polluted stations, high values are observed during pre-monsoon season which can be attributed to anthropogenic activities. An increase in the amount of zinc is observed in the downstream stations of major urban settlements, which may be due to accumulation of terrigenous materials coming through drainages and washing of clothes using detergents. In the estuarine region, comparatively lower values of zinc are observed in Devi estuary (St. No. 31), which may be due to de-sorption of metal under the increasing influence of saline water. Similar type of observation was also recorded by Koshy (2002) for river Padma, Kerala. However, in case of Mahanadi estuary, input of organic wastes into the estuary, which comes from municipal sewage as well as fertilizer plants like PPL and Oswal, contributes to the increase in Zn content in sediments (Alagarsamy 1991). The organic matter contents of the sediment influence the concentration of zinc in river Mahanadi. The average values of Zn found in the sediments of river Mahanadi is relatively higher than that found in other rivers in India as well as Indian river average (Table 2). Although it was lower than worldwide river average and also surface rock value (Table 2).
Chromium (Cr)
Chromium occurs in a number of oxidation states, but Cr(III) and Cr(VI) are of main biological relevance. Cr(III) is an essential nutrient for man in amounts of 50–200 μg/day and necessary for the metabolism of insulin and essential for animals. Although Cr(VI) compounds are considered as most toxic and are assumed to cause cancer (Cowi 2002). Chromium in the environment is mainly particulate bound with relatively low mobility and bioavailability. Like Ni, chromium is also toxic to man and can reach the food chain via plant uptake from contaminated soil (Hazlett et al. 1987).
The chromium concentration in the Mahanadi river estuarine sediment ranges from 18.2 to 132.1 μg/g in pre-monsoon, 16.9 to 50.0 μg/g in monsoon and 20.8 to 96.9 μg/g in post-monsoon seasons during study period. The temporal variations of Cr content in the Mahanadi river estuarine system show that there is increasing trend of Cr concentration from monsoon to pre-monsoon. Like other trace metals, irrespective of the seasons, Cr content increases downstream of major townships, this is attributed to anthropogenic contribution. The sediment of river Mahanadi shows low value of chromium as compared to the mean values of other rivers (Table 2).
Copper (Cu)
Copper is one of the several heavy metals which are essential to life, despite being as inherently toxic as non-essential heavy metals exemplified by Pb and Hg (Scheinberg 1991). Continuous ingestion of copper from food or water induces chronic copper poisoning in man and causes a kind of disease called ‘Wilsons disease’ (Dara 1993). Under natural conditions, copper finds its way into surface water form sulfides, sulphates, carbonates and other compounds. In addition, this metal is associated with organic matters, oxides of iron and manganese, silicate, clays and other minerals (Koshy 2002).
The copper concentrations are observed from 10.58 to 38.07, 9.38 to 23.13 and 7.86 to 24.38 μg/g during pre-monsoon, monsoon and post-monsoon season, respectively, with an average value of 17.40, 14.22 and 14.12 μg/g for the three different seasons. The highest copper content is recorded for sediments at Athurbanki creek station (38.07 μg/g) during pre-monsoon, while the lowest value is found at Sambalpur up station (7.86 μg/g) during post-monsoon season.
When the seasonal average for Cu in sediment is computed, a decreasing trend towards the marine side is observed. However, in case of estuarine region, copper concentration (except for Atherbanki) shows an increasing trend from monsoon to pre-monsoon period. The values of copper concentrations in the sediment of the Mahanadi river and also estuaries are lower to those reported from various other river and estuarine sediments in India, as well as lower than world river average, Indian river average and average shale (Table 2).
Cobalt (Co)
The cobalt concentration in the Mahanadi river sediments varies between 20.18 and 35.40 μg/g, during pre-monsoon, 18.30 and 32.75 μg/g during monsoon, 15.62 and 31.25 μg/g during post-monsoon periods, whereas it varies in the range of 18.10–38.23, 19.25–34.45, and 15.25–45.50 μg/g for the respective seasons in the estuarine sediments. In generally, cobalt concentration is highest in pre-monsoon and showed decreasing trend towards monsoon and post-monsoon (Fig. 2).
In the estuarine system (especially Devi estuary), it is observed that there is a decreasing trend of cobalt towards the marine side, which may be due to the increasing effect of salinity (Sholkovitz 1987). However, in case of Mahanadi estuary, the impact of anthropogenic contributions are more dominant. Nayak et al. (2004) observed a decreasing trend of trace metals towards the coastal environment. Irrespective of the spatial variations (Fig. 2), Atharbanki creek (St. No. 25) has the highest concentration of cobalt, which is attributed to high anthropogenic load.
No strongly elevated concentrations of Co are observed, although it is apparently enriched above natural values. The sediment of the Mahanadi river is enriched in heavy metal content in comparison to the world river average, continental rocks and average soil, but depleted in comparison to the other river basins in India along with Indian river average (Table 2).
Nickel (Ni)
Nickel is an essential micronutrient for microorganisms, animals and humans, but not to plants (Gupta et al. 2001). However, in excess concentration, it is toxic to man and can reach the food chain via plant uptake from contaminated soil (Hazlett et al. 1987).
There is a general trend observed for nickel, whose concentration decreases from monsoon to pre-monsoon. Its concentrations in the Mahanadi river estuarine sediments show generally higher values than Cu, but lower than Zn. The values of nickel in the sediments of the Mahanadi river estuarine system are observed in the range 12.90–46.85, 20.20–42.20 and 18.20–38.42 μg/g during pre-monsoon, monsoon and post-monsoon seasons, respectively, for the investigated periods with an average value of 22.26, 28.59 and 23.90 μg/g during the respective three different seasons.
The spatial distribution of nickel is similar to that of other trace metals (Fig. 2). Sedimentary nickel content of Mahanadi river is relatively lower than that found in other rivers in India, as well as Indian river average, world river average and also surface rock value (Table 2).
Lead (Pb)
Lead is found in soil, vegetation, animals, food, water and air in the vicinity of highway. Lead is not essential for plant or animal life and in the environment it is known to be toxic to plants, animals and microorganisms. Lead also to causes adverse effects at the levels of exposure that produce no clinically detectable symptoms and only a few years ago, it was thought to be safe (ATSDR 1993) although it is toxic to most forms of life (USEPA 1995). In the present study, lead concentration varies from 25.75 to 113.43, 28.13 to 98.13 and 21.56 to 79.53 μg/g during pre-monsoon, monsoon and post-monsoon seasons, respectively. It occurs in nature at an average surface rock of 20 μg/g (Martin and Meybeck 1979). Like soils in the terrestrial system, sediments are the primary sinks for lead in the aquatic environment. Its contents in river, estuarine and other coastal sediments have been much altered by man’s activities. The average Pb levels in Indian river sediment is about 11 μg/g (Subramanian 1987), which is much lower than the world average values, i.e. 150 μg/g (Martin and Meybeck 1979). Table 2 showed that in comparison with world river sediment average, Mahanadi sediment has relatively much lower lead content. However, it is higher than that found in other rivers in India as well as Indian river average. Nolting et al. (1996) observed that the low and constant Pb concentrations indicating the minor importance of anthropogenic input from the Laptev Sea in contrast to other areas of world as anthropogenic inputs are considered to be the major source of elevated Pb concentrations in marine sediments (Nolting et al. 1999). The seasonal variation (Fig. 2) shows that sedimentary lead concentration is higher during monsoon season followed by pre-monsoon. Irrespective of seasons, highest values of Pb are recorded at Kathojodi down (St. No. 27), which is attributed to the atmospheric deposition of aerosols containing Pb from the nearby highway in Cuttack. Singh (2001) reported high Pb contents in the sediments of Yamuna river at Delhi due to highway traffic.
Cadmium (Cd)
As many of the cadmium compounds are water soluble, cadmium is more mobile in water and soil environment. Its compounds are generally more bioavailable and tend to bioaccumulate. In the environment cadmium is reported to be toxic to animals and microorganisms and it is not essential for plant or animal life. The higher concentrations of sedimentary cadmium are found at station 25 (Atharbanki creek) and 24 (near Oswal down), which are mainly due to the proximity of the effluent discharge point from fertilizer plants like PPL and Oswal, respectively. One of the major sources of Cd in soil is phosphatic fertilizers (Alan 1996). The concentration of cadmium in Mahanadi sediment is reported to be in the range of minimum 0.95, 0.68 and 0.87 μg/g and maximum of 3.38, 2.85, 2.97 μg/g for pre-monsoon, monsoon and post-monsoon seasons, respectively. Higher Cd content is observed during pre-monsoon season, followed by post-monsoon. The sediment of the Mahanadi river is enriched in Cd content in comparison to the continental rocks, average soil and also world river average (Table 2).
Summary of heavy metal distribution patterns
The summary of heavy metal distribution pattern in river and estuarine sediments during three different seasons is presented in Table 3.
Enrichment ratios (ER)
The metals inputs into the aquatic systems may be from natural or/and anthropogenic sources. Metal enrichment as a result of contamination can be measured in a number of ways. An enrichment ratio (ER) is used as the tool to quantify the enrichment of these heavy metals within the same environment (Sundaray 2010; Sundaray et al. 2012a). In the present study, the ER has been used to identify the enrichment of these metals in the sediments of Mahanadi river estuarine system with respect to local background value (Sundaray 2010; Sundaray et al. 2012a).
The enrichment ratios for heavy metals in sediments have been calculated as follows:
where C x = concentration of metal ‘x’, C b = background concentration of metal ‘x’.
The concentrations at the upstream station (St. No. 1) are used as the local background value because it is considered uninfluenced by industrial and municipal activities (Sundaray et al. 2005, 2012a; Sundaray 2007). ER > 1 has been termed as enrichment and ER < 1 as depletion with respect to background value.
The ERfor heavy metals in sediments of Mahanadi river estuarine system is presented in Table 4.
Comparatively higher values of ER are observed in some stations like St. No. 5, 16, 23, 25 and 27. These sites are influenced by anthropogenic activities (Sundaray et al. 2006) and are probably responsible for relatively high enrichment of Fe, Pb and Cd values. Chen et al. (2001) observed that Pb and Cd discharges are common through sewage outlets. The enrichment ratios for metals are found to be highest at Atherbanki creek, this is due to the input of these metals from the fertilizer plants and municipal waste (Table 4). No remarkable variation in Cu and Co has been shown in the study area, which may be related to lithology of the surrounding soil. In the estuarine region, higher values of ER for metals like Fe and Mn are observed at stations Nos. 20 and 29, which is probably due to the flocculation of dissolved metals during the estuarine mixing (Sundaray et al. 2012b) as these stations are the starting point of estuarine region. The flocculation of dissolved Fe decreases with increase in salinity (Sholkovitz 1987).
Factor analysis
To identify the major factors responsible for the enrichment of heavy metals in river estuarine sediment samples, statistical (factor) analysis was carried out using SPSS 10.0 statistical software.
R-mode factor analysis with rotation has been carried out for three different seasons separately. In addition to the nine analysed metals (Fe, Mn, Zn, Cr, Cu, Co, Ni, Pb and Cd), organic matter, CaCO3 in the sediments and textural parameters, such as sand, silt and clay were also used as variables (total 15) for FA, as these parameters are significantly contributing towards the dynamics of heavy metals in the sediments (Sundaray 2007; Sundaray et al. 2011a).
There are three, four and four factors or PCs explaining a total cumulative variance of 85.44, 80.67 and 56.78 % for pre-monsoon, monsoon and post-monsoon seasons, respectively (Table 5) and which is sufficient to give a good idea of the data structure.
Pre-monsoon season
Factor 1 contributes 61.49 % of total variance is strongly associated with Co, Mn, Fe, Pb, Ni, Cd along with moderate loadings of Zn, Cu, Cr and organic matter. Fe and Mn are considered as main carrier of heavy metals as a part of crystal lattice or particle coating on ferromanganese oxides (Dassenakis et al. 1997). Thus, in this factor,Fe–Mn oxy-hydroxide is playing a major role in scavenging the heavy metals like Co, Pb, Ni, Cd along with Zn, Cr and Cu and hence termed as “Fe–Mn oxy hydroxide factor” (Arakel and Hongjan 1992). However, the possibility of organic matter contribution cannot be ruled out.
The second factor, which represents 13.02 % of the total variance, is found to have strongly associated with clay, silt, organic matter, CaCO3 and heavy metals ions like Zn, Cr, Cu along with Cd and Ni. In this factor, textural parameters clay and silt are playing an important role for distribution of organic matter as well as heavy metal ions like Zn, Cr, Cu along with Ni and Cd. Most of these metals finds their way in to the river water from various industrial/municipal effluents and subsequently get precipitated and adsorbed by finer particles. Clay and silt particles have relatively high metal contents, due to their greater active surfaces on which metals are adsorbed (Biksham et al. 1991). The scavenging action of clay minerals for Zn and Cr has already been reported (Sahu et al. 1998; Panda et al. 1999, 2006b). However, the possibility of carbonaceous precipitation and/or organic matter contribution cannot be over ruled. It has been reported that organic matter in sediments is a major reservoir for trace metals. Considering the above observations, factor 2 may be termed as ‘clay silt-organic matter-CaCO3 factor”.
Factor 3, which represents “textural factor”, is explaining 10.93 % of total variance, where gravel and sand are inversely correlated with each other.
Monsoon season
In case of total stations of monsoon season, the eigen value greater than unity represents four factors, out of which the first factor explains 47.82 % of the variance. In this factor, clay, silt, organic matter and CaCO3 along with toxic metal Cd are associated. Unlike the pre-monsoon season, in this period (monsoon season) textural parameters like clay and silt content do not play important role for fixation of metal ions (except Cd up to some extent).
The correlations of toxic metals like Pb, Cr, Ni, Cu, Cd, Zn and Co are reflected in factor 2, which represents 14.38 % of total variance. This factor is related to a common source and may be contributed by runoff from the agricultural field using fertilizer. Factor 3 contributes 11.79 % of the total variance and in this factor Fe–Mn oxy-hydroxide play a major role in the fixation of both Co and Zn. Factor 4 is a textural factor indicating negative correlation of sand with gravel, which explains 6.68 % the of total variance.
Post-monsoon season
In case of post monsoon seasons, there are four factors explaining 86.78 %, of total cumulative variance, respectively.
Factor 1, which is about 55.31 % of total variance indicating the association of silt, clay, organic matter, CaCO3, Co, Cr and Cd. The textural parameters, such as silt and clay portion of the sediments as well as organic matter and CaCO3 are playing an important role for distribution and enrichment of the above toxic metals in the sediments. This factor may be termed as “silt–clay-organic matter-CaCO3 factor”.
In the second factor Fe–Mn oxy-hydroxide seems to play main role for enrichment of metal ions like Cu Ni, Zn and Co, which may be termed as “Fe–Mn oxy hydroxide factor”. This factor is responsible for 13.97 % of total variance.
Factor 3 explains 10.32 % of total variance showing inter correlation of Zn, Pb, Cr and Cd, which may be related to a common source. Like monsoon season, sand and gravel are separately grouped in the last factor, i.e. factor 4, explaining 7.19 % of total variance. These two textural parameters are inversely correlated with each other.
Cluster analysis
The relationship among stations is obtained through CA using Ward’s method (linkage between groups) and synthesized by the dendrogram plots (distance cluster combine) and shown in Fig. 3 for three different seasons.
For sediment constituents, CA is rendered as dendrograms (Fig. 3), where all 31 sampling sites of the river estuarine system are grouped into three (pre-monsoon season), two (monsoon season) and two (post-monsoon season) statistically significant clusters. The possible groupings are summarized in Table 6.
It is clearly visualized from Fig. 3 that pre-monsoon season consist of higher number of groups (three) than other two seasons. This may be due to the fact that during pre-monsoon the influence of anthropogenic activities is clearly visible from the separate group. However, during other two seasons, the land drainage by rain water is dominated.
In case of pre-monsoon season, the first group of stations may be regarded as less contaminated or lithogenic contribution. Group II is related to stations having moderately significance level of contamination or moderately sedimentation. However, the third group is considered as stations (St. Nos. 23, 27, 16, 24 and 25) having higher significance level of contamination.
Like pre-monsoon season, the first group of both monsoon and post-monsoon seasons are considered as less contaminated stations or lithogenic contribution. However, group II of both monsoon and post-monsoon seasons are related to stations having comparatively higher significance level of contamination and moderately sedimentation.
Conclusion
The spatial distribution pattern indicates large inputs possibly from two major fertilizer plants, municipal sewage from three major towns as well as agricultural runoff. The higher elemental concentration were associated with the stations located downstream from municipal discharge points of Sambalpur, Cuttack township and industries effluent discharge point near Paradip, which confirms that anthropogenic activities influenced its chemical composition. These anthropogenic contributions of various industrial and municipal waste in the river basin are reflected by high ER data. The metal concentrations in estuarine sediments are relatively higher than in the river due to adsorption/accumulation of metals on sediments during saline mixing. However, the metal concentrations in sediments of the Mahanadi estuary are higher than that of the Devi estuary, which may be due to the addition of large amount of solid waste produced by different industries and Paradip town situated near the estuary. It is found that higher concentration of trace elements along with organic matters are found, where finer grain fraction are comparatively high. The temporal variations may be due to either anthropogenic causes, such as agricultural practices (fertilizers and biocides) or wastewater discharge, or natural causes such as, textural factors (silt and clay), pH, redox condition, or river flow. The concentrations of heavy metals are more or less comparable with other Indian river estuarine studies. The concentrations of Fe, Mn, Zn and Pb in the present study are higher than the Indian river average.
FA suggests that factors such as “Fe–Mn oxy hydroxide”, “organic matter”, “CaCO3”, and “textural variables” are significantly responsible for the dynamics of heavy metals in the sediments of Mahanadi river estuarine system during all the seasons; however, their intensities were different for different seasons. The results of CA classified into three groups during pre-monsoon and two groups each during monsoon and post-monsoon seasons depending on contributing sites for the enrichment of heavy metals in the river estuarine system.
References
Alagarsamy R (1991) Organic carbon in the sediments of Mandovi estuary, Goa. Indian J Marine Sci 20:221–222
Alagarsamy R, Zhang J (2010) Geochemical characterisation of major and trace elements in the coastal sediments of India. Environ Monit Assess 161:161–176
Alan W (1996) Soil and environment. Cambridge University Press, Cambridge
Arakel AV, Hongjan T (1992) Heavy metal geochemistry and dispersion pattern in coastal sediments, soils and water of Kedron Brook flood plain area, Brishbane, Australia. Environ Geol Water Sci 20(3):219–231
Athalye RP, Mishra V, Quadros G, Ullal V, Gokhale KS (2001) Heavy metals in the Abiotic and Biotic Compontts of Thane creek, India. Ecol Environ Conserv 7(3):319–325
ATSDR (1993) Toxicological profile for lead. TP. 92-12. Atlanta GA: agency for toxic substances and disease registry
Balakrishna K, Manjunatha BR, Shankar R (1997) A laboratory study of the flocculation of dissolved Heavy metals in Kali river during estuarine mixing, West coast of India. J Geol Soc India 50:753–758
Biksham G, Subramanian V (1988) Elemental composition of Godavari sediments (Central and Sourthen Indian sub-continent). Chem Geol 70:275–286
Biksham G, Subramanian V, Van Griken R (1991) Heavy metal distribution in the Godavari river basin. Environ Geol Water Sci 17:117–126
Boyle E, Edmond JM, Sholkovitz ER (1977) On the mechanism of iron removal in estuaries. Geochim Cosmochim Acta 41:1313–1324
Chakrapani GJ, Subramanian V (1990a) Preliminary studies on the geo-chemistry of the Mahanadi basin, India. Chem Geol 81:241–253
Chakrapani GJ, Subramanian V (1990b) Factors controlling sediment discharge in the Mahanadi river basin. India J Hydrol 117:169–185
Chakrapani GJ, Subramanian V (1993a) Heavy metals distribution and fractionation in sediments of the Mahanadi river basin, India. Environ Geol 22:80–87
Chakrapani GJ, Subramanian V (1993b) Rates of erosion and sedimentation in the Mahanadi river basin. India J Hydrol 149:39–48
Chen Z, Kostaschuk R, Yang M (2001) Heavy metals on tidal flats in the Yangtze Estuary, china. Environ Geol 40(6):742–749
COWI (2002) Heavy metals in waste, European commission DG ENV. E3, Project ENV. E.3/ETU/2000/0058, COWI A/S publications, Denmark
Dara SS (1993) A text book of environmental chemistry and pollution control. S. Chand & Co. Ltd, New Delhi, p 210
Dassenakis M, Scoullos M, Gaitis A (1997) Trace metals transport and behaviour in the Mediterranean estuary of Acheloos River. Mar Pollut Bull 34(2):103–111
Feely RA, Massoth GJ, Paulson AJ, Gendron JF (1983) Possible evidence for enrichment of trace-elements in the hydrous manganese oxide phases of suspended matter from an urbanized embayment. Estuar Coast Shelf Sci 17:693–708
Forstner U, Wittmann GTW (1979) Metal Pollution in Aquatic Environment. Springer-Verlag, Berlin, p 486
Gupta DC, Perers E, Yadava RN (2001) Concentration of heavy toxic metals in waters of the area around Mandideep industrial complex and its impact on human health. In: Kumar A, Ecology of polluted waters, vol 2. A.P.H.Publishing corporation, New Delhi, pp 515–536
Hazlett PW, Rutherford GK, Vanloon GW (1987) Metal contaminants in surface soils and vegetation as a result of nickel/copper smelting at comston, Ontario. Canada. Reclamat Reveg Res. 2:123–137
Helena B, Pardo R, Vega M, Barrado E, Fernandez JM, Fernandez L (2000) Temporal evolution of groundwater composition in an alluvial (Pisuerga river, Spain) by principal component analysis. Water Res 34:807–816
Huang Y, Zhu W, Le M, Lu X (2011) Temporal and spatial variations of heavy metals in urban riverine sediment: An example of Shenzhen River, Pearl River Delta, China. Quat Int doi:10.1016/j.quaint.2011.05.026
Ikem A, Egiebor NO, Nayavor K (2003) Trace elements in water, fish and sediment from Tuskegee lake, Southeastern USA. Water Air Soil Pollut 149:41–57
Konhauser KO, Powell MA, Fyfe WS, Longstaffe FJ, Tripathy S (1997) Trace element geochemistry of river sediment Orissa state, India. J Hydrol 193:258–269
Koshy M (2002) Study of metals in the sediments of river Padma. Acta Ciencia Indica 28 C(4):243–254
Kudesia VP (1990) Water pollution. Pragati prakashan, Merrut
Lourino-Cabana B, Lesven L, Charriau A, Billon G, Ouddane B, Boughriet A (2011) Potential risks of metal toxicity in contaminated sediments of Deûle river in Northern France. J Hazard Mater 186:2129–2137
Martin JM, Meybeck M (1979) Elemental mass—balance of material carried by major World Rivers. Mar Chem 7:173–206
Martins R, Azevedo MR, Mamede R, Sousa B, Freitas R, Rocha F, Quintino V, Rodrigues AM (2012) Sedimentary and geochemical characterization and provenance of the Portuguese continental shelf soft-bottom sediments. J Mar Syst 91:41–52
Mayer LM (1982) Aggregation of colloidal iron during estuarine mixing: kinetics, mechanism, and seasonality. Geochim Cosmochim Acta 46(12):2527–2535
Mohan PM (1995) Enrichment factor: a noble method to present the trace elemental concentration in Vellar estuary. Indian J Mar Sci 24:13–15
Morales MM, Marti P, Liopis A, Campos L, Sagrado S (1999) An environmental study by factor analysis of surface sea waters in the gulf of Valencia (western Mediterranean). Anal Chim Acta 394:109–117
Nayak BB, Acharya BC, Panigrahy PK, Panda UC (2004) Assessment of heavy metals contamination in the coastal sea of Orissa India. Pollut Res 23(4):791–803
Nobi EP, Dilipan E, Thangaradjou T, Sivakumar K, Kannan L (2010) Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands India. Estuar Coast Shelf Sci 87:253–264
Nolting RF, van Dalen M, Helder W (1996) Distribution of trace and major elements in sediment and pore waters of the lena Delta and Laptev sea. Mar Chem 53:258–299
Nolting RF, Ramkema A, Everaarts JM (1999) The geochemistry of Cu, Cd, Zn, Ni and Pb in sediment cores from the continental slope of the Bancd, Arquin (mauritani). Cont Shelf Res 19:665–691
Ohnessorge FK, Wilhem M (1991) Zinc. In: Merian E (ed) Metals and their compounds in the environment. Occurrence, analysis and biological relevance, VCH Weinheim, New York, Base. Cambridge, p 1330
Oshisanya KI, Unyimadu JP, Shelle ROD, Nubi OA, Ladigbolu IA, Oguguah NM, Olumodeji OO, Adeleye AO, Fashade AO (2011) Seasonal Variation of Heavy Metals In Sediment And Water Of Lagos Lagoon. J Am Sci 7(3):384–387
Padma S, Periakali P (1999) Physico-chemical and geochemical studies in Pulicat lake, East Coast of India. Indian J Marine Sci 28:434–437
Panda UC, Sahoo KC, Mohapatra DM, Das CR (1999) Bulk and Partition Analysis of Heavy metals in sediments of Bahuda Estuary, East Coast of India. Indian J Mar Sci 28:102–105
Panda UC, Sundaray SK, Rath P, Nayak BB, Bhatta D (2006a) Application of factor and cluster analysis for characterization of river and estuarine water systems—A case study: Mahanadi River (India). J Hydrol 331:434–445
Panda UC, Rath P, Sahu KC, Majumdar S, Sundaray SK (2006b) Study of Geochemical Association of Some Trace Metals in the Sediments of Chilika Lake: a Multivariate Statistical Approach. Environ Monit Assess 123:125–150
Pizarro J, Vergara PM, Rodríguez JA, Valenzuel AM (2010) Heavy metals in northern Chilean rivers: spatial variation and temporal trends. J Hazard Mater 181(1–3):747–754
Pradhan B, Gouda R, Panigrahy RC (1998) Distribution of some heavy metals in the sediments of Rushikulya estuary, east coast of India. J Environ Poll 5(4):323–327
Radhakrishna I (2001) Saline Fresh Water Interface Structure in Mahanadi delta region, Orissa India. Environ Geol 40(3):369–380
Ramesh R, Subramanian V, Van Grieken R (1990) Heavy metal distribution in sediments of Krishna river basin, India. Environ Geol 15:207–216
Rath P (2003) Statistical approach to study the trace metals in the industrial belt of Angul and Talcher, Orissa. Ph.D. Thesis, Utkal University, Bhubaneswar, India
Sahu KC, Panda UC, Mohapatra DM (1998) Geochemistry and minerology of sediments in rushikulya estuary, East Coast of India. Chem Environ Res 7(1&2):77–92
Sánchez-García L, Andrés JR, Martín-Rubí JA (2010) Geochemical signature in offshore sediments from the Gulf of Cádiz inner shelf: sources and spatial variability of major and trace elements. J Mar Syst 80:191–202
Scheinberg HI (1991) Copper. In: Merian E (ed) Metals and their compounds in the environment: Occurrence, Analysis and Biological relevance. VCH Weinheim, Cambridge, pp 803–851
Seetharamaiah J, Seetaramaswamy A (1987) Geochemistry of modern deltaic sediments of the Cauvery river, east coast of India. Indian J Marine Sci 16:31–38
Sholkovitz ER (1987) Flocculation of dissolved organic and inorganic matter during the mixing of river water and seawater. Geochim Cosmochim Acta 40(7):831–845
Silva N, Haro J, Prego R (2009) Metals background and enrichment in the Chiloe. Interior Sea sediments (Chile). Is there any segregation between fjords, channels and sounds? Estuar Coast Shelf Sci 82:469–476
Simeonov V, Stratis JA, Samara C, Zachariadis G, Voutsa D, Anthemidis A, Sofoniou M, Kouimtzis TH (2003) Assessment of the surface water quality in Northern Greece. Water Res 37:4119–4124
Singh AK (1999) Elemental composition of Damodar River sediments, A tributary of the Lower Ganga India. J Geol Soc India 53:219–231
Singh M (2001) Heavy metal pollution in freshly deposited sediments of the Yamuna river (the Ganges river tributary): a case study from Delhi and Agra urban centres India. Environ Geol 40(6):664–671
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—a case study. Water Res 38:3980–3992
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges India. J Hydrol 312(1–4):14–27
Subarmanium V, Van’t Dack L, Van Grieken RV (1985) Chemical comparison of river sediments from the Indian sub continent. Chem Geol 48:271–279
Subramanian V (1987) Environtal Geochemistry of Indian River Basins. A review. J Geol Soc India 29:205–220
Subramanian V, Van Grieken RV, Van’t Dack L (1987) Heavy metal distribution in the sediments of Ganges and Brahmaputra Rivers. Environ Geol 9:93–103
Subramanian V, Jha PK, Van Grieken R (1988) Heavy metal in the Ganges Estuary. Mar Pollut Bull 19(6):290–293
Sundaray SK (2007) Water quality assessment of Mahanadi River, Orissa, India, using multivariate statistical approach. Ph.D. Thesis, Utkal University, Bhubaneswar, India
Sundaray SK (2010) Application of multivariate statistical techniques in hydrogeochemical studies—a case study: Brahmani–Koel River (India). Environ Monit Assess 164:297–310
Sundaray SK, Panda UC, Nayak BB, Bhatta D (2005) Behaviour and Distribution Pattern of Nutrients in River-Estuarine Waters of Mahanadi, Orissa, India. Asian J Water Environ Pollut 2(1):77–84
Sundaray SK, Panda UC, Nayak BB, Bhatta D (2006) Multivariate statistical techniques for the evaluation of spatial and temporal variation in water quality of Mahanadi River—Estuarine System (India)—a case study. Environ Geochem Health 28:317–330
Sundaray SK, Nayak BB, Bhatta D (2009) Environmental studies on river water quality with reference to suitability for agricultural purposes: Mahanadi river estuarine system, India—A case study. Environ Monit Assess 155:227–243
Sundaray SK, Nayak BB, Lin SW, Bhatta D (2011a) Geochemical speciation and risk assessment of heavy metals in river estuarine sediments—a case study: Mahanadi basin, India. J Hazard Mater 186:1837–1846
Sundaray SK, Nayak BB, Bhatta D (2011b) Application of WQI technique for the classification of water quality: Mahanadi river- estuarine system, India – a Case Study. Asian J Water Environ Pollut 8(2):113–125
Sundaray SK, Nayak BB, Kanungo TK, Bhatta D (2012a) Dynamics and quantification of dissolved heavy metals in the Mahanadi River Estuarine system, India. Environ Monit Assess 184:1157–1179
Sundaray SK, Nayak BB, Lee BG, Lin SW, Bhatta D (2012b) Experimental comparison of the flocculation and removal of dissolved heavy metals in the estuaries. Environ Monitor Assess (in press)
Trivedi RK, Goel PK (1984) Chemical and biological methods for water pollution studies. Environmental publications, Karad
Turekian KK, Wedepoh KH (1961) Distribution of the elements in some major units of the earth’s crust. Bull Geol Soc Am 72:175–192
USEPA (1995) Process design manual. Land application of sewage sludge and domestic seepage, US Environmental protection Agency, Office of Research and development, EPA-625/R-95/001
Vaithiyanathan P, Ramanathan A, Subramanian V (1993) Transport and distribution of heavy metals in Cauvery river. Water Air Soil Pollut 71(1–2):13–28
Vega M, Pardo R, Barrado E, Deban L (1998) Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res 32:3581–3592
Wunderlin DA, Diaz MP, Ame MV, Pesce SF, Hued AC, Bistoni MA (2001) Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquia River basin (Cordoba-Argentina). Water Res 35:2881–2894
Zhan S, Peng S, Liu C, Chang Q, Xu J (2010) Spatial and temporal variations of heavy metals in surface sediments in Bohai Bay, North China. Bull Environ Contam Toxicol 84:482–487
Zhiquing L, Jianlu Z, Jinsi C, Renquan H (1987) Flocculation of dissolved Fe, Al, Mn, Si, Cu, Pb and Zn during estuarine mixing. Acta Oceanol Sinica 6:568–576
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sundaray, S.K., Nayak, B.B., Lee, BG. et al. Spatio-temporal dynamics of heavy metals in sediments of the river estuarine system: Mahanadi basin (India). Environ Earth Sci 71, 1893–1909 (2014). https://doi.org/10.1007/s12665-013-2594-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2594-6