Abstract
To characterize the target organs of the Manila clam Ruditapes philippinarum for use in environmental study, the accumulation of trace metals and three biomarkers was measured in different organs. Exposure with Cu and Pb carried out under laboratory conditions revealed a linear uptake of metals throughout the experimental period in each tissue. In particular, significant increase was observed in gills and mantle. The increase of intracellular reactive oxygen species showed the great potential of gills as a target tissue for both Cu and Pb exposure. The highest activity of glutathione S-transferase and their relative increase in activity were also observed in gills. Metallothionein-like protein levels, however, increased greatly in the digestive gland and mantle during Cu and Pb exposure, respectively, although all tissues, except the foot, showed significant changes after 24 h of metal exposure. In the field study, the highest concentration of metals was recorded in the gills and mantle, accounting for over 50 % of the total accumulated metal in all sites. Additionally, Cu and Pb increased significantly in these two organs, respectively. However, the order of accumulation rate in laboratory exposure was not concomitant with those of the lab-based study, suggesting that different routes of metal uptake and exposure duration induce distinct partitioning of metals and regulating system in R. philippinarum. These series of exposure studies demonstrated that gills, mantle, and digestive gland in R. philippinarum are potential target tissues in environmental monitoring study using metal concentrations and biomarkers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The trace metal pollution in marine ecosystems has been of great concern due to the increased anthropogenic release of metals into the environment. Metals can be easily assimilated into the aquatic organisms from the water and sediment by the means of food web (Alibabić et al. 2007; Burger et al. 2007). Once a trace metal has been assimilated by the aquatic organism, it cannot be easily degraded or excreted (Reynoldson 1987; Tessier and Campbell 1987), although some metals can be regulated by specialized mechanisms for detoxification (Rainbow and Dallinger 1993; Bonneris et al. 2005). Thus, the transfer and accumulation of metals to higher trophic level organisms through food web has repercussions for the entire aquatic ecosystem (Chen et al. 2000). To study trace metal pollution in aquatic environments, quantitative analysis of trace metals in organisms has been considered a principle and simple method to assess the pollution levels associated with bioavailability (Boening 1999; Fernández-Tajes et al. 2011; Ra et al. 2011). Moreover, the use of organisms to monitor the level of trace metals in marine environments allows comparison of the levels of trace metals over space and time (Rainbow 1995). For example, the Mussel Watch Program initiated in 1986 by the National Oceanic and Atmospheric Administration has supplied ample information on bivalve health in local and regional environments (Maruya et al. 2013).
Furthermore, another current and promising approach associated with pollution assessment and environmental monitoring includes biomarkers that measure the subcellular effects of organisms exposed to pollutants (De Luca-Abbott et al. 2005; Valavanidis et al. 2006; Choi et al. 2011; Won et al. 2012). Biomarkers widen our knowledge on toxic effects of various pollutants on marine organisms in their natural environment by providing valuable information on metal bioavailability (Depledge et al. 1995). Recently, the transplantation experiments have also been considered as a useful method to evaluate environmental pollution and its biological impact (Martel et al. 2003; Gaber et al. 2008; Ra et al. 2011). For example, the results obtained using caged mussels and clams in the assessment of environmental pollution gradient areas were more reliable compared to other approaches used in previous studies (Martel et al. 2003; Ra et al. 2011).
Many field studies have demonstrated the potential of bivalves as sentinel organisms to assess pollution levels in marine environments (Rainbow 2006). The Manila clam, Ruditapes philippinarum, a commercially important food resource, is widely distributed in intertidal regions along the western coast of Korea (Ahn et al. 2006). Moreover, their distribution in sediments and feeding habit associated with filter feeding allow their use as good indicator species for pollution monitoring in both the sediment and water in coastal areas (Ahn et al. 2006; Choi et al. 2011). Accumulation of trace metals in soft tissues of Manila clam, studied at several sites in Korea, demonstrated that this species is a suitable model for assessing spatial distribution of metals in coastal areas (Ahn et al. 2006). Furthermore, a significant increase in total amount of trace metals was observed in the Manila clam exposed to polluted sites in our previous long-term exposure test (Ra et al. 2011). In fact, many studies with R. philippinarum had reported the suitability of biomarkers for pollutant monitoring (De Luca-Abbott et al. 2005; Martín-Díaz et al. 2007; Ra et al. 2011; Won et al. 2012). However, a small study on R. philippinarum identified the target organs that can be utilized for trace metal monitoring using biomarker in the environment.
In relation to metal accumulation and biomarker studies using aquatic organisms, limited results have been obtained from the total soft body, although it has been reported that muscle tissue, compared to other tissues such as liver, kidney, and gill tissues, usually has low levels of trace metals (Jezierska and Witeska 2006). Hence, there was no clear trend in metal accumulation in total soft tissues. Similarly, no significant correlation in Pb concentration between the whole fish and specific organs has been reported in both herring and perch (Boalt et al. 2011).
Thus, the aim of this study was to identify a candidate organ of R. philippinarum for bioaccumulation of trace metals and a biomarker in biomonitoring studies. First, this was conducted in lab-based exposure tests. The Cu and Pb were selected as target elements based on the previous studies that showed high concentrations of the two elements in the sediment and the exposed clams (Ra et al. 2011). Next, the newly accumulated metals, metallothionein-like protein (MTLP) levels, activity of glutathione S-transferase (GST), and reactive oxygen species (ROS) levels were analyzed from seven different organs (gill, mantle, digestive gland, siphons, adductor muscle, and foot) of the metal-exposed R. philippinarum to identify potential organs suitable for biomarker study. Additionally, the transplantation exposure was conducted to compare accumulation of eight elements (chrome, Cr; cobalt, Co; nickel, Ni; copper, Cu; zinc, Zn; arsenic, As; cadmium, Cd; and lead, Pb) in different organs.
Materials and methods
Test organism
Individuals of the Manila clam were gathered from the Sunjae Island, located off the west coast of Korea, and sorted by size (length range 31.2–36.3 mm, average 32.7 mm). For in-situ incubation, 50 specimens were moved directly to each site by installing cages. For exposure test under laboratory conditions, R. philippinarum were transferred to the laboratory and were maintained at artificial seawater (18 °C, 30 practical salinity units, pH 7.8–8.0) until it used for lab exposure test (4 days) for adaptation.
Experimental procedure of the lab exposure test
Thirty individuals were exposed to three concentrations of Cu and Pb (0.1, 0.5, and 2 mg/L) spiked seawater (20 L, pH 7.8–8.1, 18 °C) for 24 h under laboratory condition. The short exposure and high doses were selected based on our recent study that showed no significant increase in metals after a short-term waterborne exposure (Won et al. 2012). The exposure concentrations were sublethal and determined according to our previous research (unpublished data). Standard solutions, Cd(NO3)2, 2–3 % HNO3, 1000 mg/L Cd and Pb(NO3)2, 2–3 % HNO3, 1000 mg/L Pb, (Merck, Darmstadt, Germany) were used as stock solutions for water spike. After 24-h exposure, clams were collected to analyze metal content, GST activity, MTLP content, and ROS levels.
Experimental procedure of the in-situ exposure
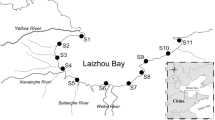
The clams gathered from the pristine areas were placed in cages and transplanted into three different sites in the Lake Shihwa, the polluted environment located in the west coast of Korea (Fig. 1). Fifty specimens were placed in each cage and incubated for 8 weeks. Every 2 weeks, ten individuals were gathered. Six individuals were used to measure metal accumulation.
Trace metal analysis
For metal analysis, Manila clam were dissected to seven different tissues: gills, siphon, adductor muscle, mantle, foot, and digestive gland, using a plastic knife. Each tissue was cleaned with distilled water and freeze dried. The homogenized dried samples were completely digested with suprapure HNO3 (Merck, Germany) at 180 °C for 24 h (Ra et al. 2011). After digestion, samples were dried and dissolved with 2 % HNO3 following the protocol described by Ra et al. (2011). Metal concentration was measured using an inductively coupled plasma mass spectrometer (Thermo Elemental X7; Thermo Scientific, Waltham, MA, USA). For quality control of metal analysis, SRM2976 (mussel tissue from the National Institute of Standard and Technology, USA) and ERM CE-278 (mussel tissue from the European Reference Materials) were used as a reference material (Online resource 1).
Biomarkers
Dissected organs from three individuals were homogenized and analyzed for biomarkers duplicates (three individuals were pooled for each sample). The activity of GST and the level of MTLPs in Cu- and Pb-exposed R. philippinarum were measured following the spectrophotometric methods as described in previous studies (Regoli et al. 1997, Viarengo et al. 1997, Won et al. 2012). For GST analysis, GST assay kit (Sigma Aldrich, St. Louis, MO, USA) was used following the manufacturer’s protocols with minor modification. Briefly, the four volumes of sample buffer (product code S2444, Sigma Aldrich) were used as a homogenizing buffer. Then, the homogenized samples were centrifuged at 18,000×g for 90 min to eliminate cellular debris. The supernatants, cytosol fraction, were used to evaluate enzyme activity. Finally, GST activity was assayed as the increase in absorbance at 340 nm due to the conjugation of glutathione to 1-chloro-2,4-dinitrobenzene (final concentrations of each regent were 10 mM) using kinetic analysis of a spectrophotometer. The MTLP contents were measured spectrophotometrically by measuring the absorbance of cysteine at 412 nm. Briefly, the dissected organs from three clams were homogenized individually in buffer (1/4:w/v) with 0.5 M sucrose, 20 mM Tris–HCl (pH 8.6), 6 μM leupeptin (Sigma Aldrich) and 0.5 mM phenylmethylsulphonyl fluoride (PMSF, Sigma Aldrich) as antiproteolytic agents and 0.01 % β-mercaptoethanol (Sigma Aldrich) as a reducing agent, respectively. The homogenates were then centrifuged at 30,000×g for 20 min. The supernatant was treated with ethanol/chloroform. For 1 mL of each supernatant, 1.05 mL cold ethanol and 80 μL chloroform were added. The samples were centrifuged at 6000×g for 10 min (4 °C), and the supernatants were reacted with 37 % HCl with cold ethanol. For precipitating, the samples were kept at freezer (−20 °C) for 1 h and centrifuged (6000×g, 10 min, 4 °C). The MTLP-containing pellet was washed with 87 % ethanol and 1 % chloroform in a homogenizing buffer to remove soluble thiols. Then, the pellet was centrifuged and totally dried under N2 gas. Ellman’s regent (DTNB, 5,5-dithiobis-2-nitrobenzoic acid, pH8) was added to the sample, and the absorbance was measured at 412 nm using a spectrophotometer. Glutathione was used as standard.
Eight specimens were used to measure ROS levels in tissues for each concentration of metals. Dissected tissues (gill, siphon, foot, mantle, digestive gland, and adduct muscle) from four specimens were pooled and homogenized in cell lysis buffer. The analysis was repeated ten times for each tissue (each treatment, n = 8). Cellular ROS levels were measured by ROS assay kit (OxiSelect™ Intracellular ROS Assay Kit, Cell Biolabs, INC.) following manufacturer’s protocols.
Data analysis
The increments of metal concentrations and ROS levels were expressed as single values calculated from the mean value. The increment was calculated as follows:
\( \mathrm{Increment}\ \left(\%\right)=\frac{Cf- Ci}{Ci}\times 100 \),
where Cf and Ci indicate the final and initial levels, respectively, of trace metals and ROS in R. philippinarum.
The results are expressed as means (standard deviation, 1σ). The homogeneity of variance was conducted according to the Levene’s test. Data were analyzed using one-way analysis of variance, followed by Tukey’s honestly significant difference test (p < 0.05). All statistical analyses were performed using SPSS® version 12.0 software (SPSS Inc., Chicago, IL, USA).
Results and discussion
Accumulated trace metal and the biomarkers in a short-term lab exposure test
Cu and Pb, the most accumulated elements in the previous in-situ study (Ra et al. 2011), were tested in a short-term exposure test. During the experiment, R. philippinarum exposed to seawater spiked with Cu and Pb did not show any acute toxic symptoms. Treatments of clam with these two elements increased the levels of both elements in all tissues in a dose-dependent manner (Fig. 2, Online resource 2). These results demonstrated that the potency of Manila clam for accumulation of significant amounts of trace metals from adjacent environment as either essential or nonessential elements. Interestingly, the trend of organ-specificity measured by the levels of accumulated metals in each tissue is observed with following sequences: gills > mantle > adductor muscle > siphon = foot > digestive gland. Generally, the gills and mantle of bivalves are easily exposed to ionic metals as they are composed of mucous layers correlated with ion-exchange and their close contact with the surrounding water contributes to greater and faster accumulation (Saha et al. 2006; Sakar et al. 2008). Thus, in the freshwater bivalve P. grandis, waterborne exposure to Cd induces significant partitioning of Cd into the gills, while dietary exposure affects the intestine (Copper et al. 2010). In rainbow trout, waterborne Cd exposure also caused much higher accumulation of Cd in the gills compared to the levels observed in dietborne exposure, as indicated by higher concentrations of Cd in the liver, kidneys, and intestinal tissues (Szebedinszky et al. 2001). Thus, the different levels of accumulated metals observed in Cu- and Pb-exposed R. philippinarum suggest that the pollutants partitioning in the environment can be one of the reasons for different trends of metal accumulation in their tissues.
In the six different organs of R. philippinarum, the ROS levels increased after exposure to both Cu and Pb in a dose-dependent manner, suggesting that oxidative stress condition was induced by Cu and Pb exposure (Fig. 3). Furthermore, the increased GST activity in some organs of R. philippinarum demonstrates the activation of the defense system for regulation of oxidative conditions in Cu and Pb exposure (Fig. 4a). The initial ROS levels in each organ decreased in the following order: siphon > digestive gland > gills > mantle > foot > adductor muscle (Fig. 3a). However, the relative increase of ROS levels under Cu and Pb exposure was significantly different (Fig. 3b) among the tissues. Siphon, the organ that had the highest level of ROS showed no significant modulations during both Cu and Pb exposure, while the gills showed the most sensitive alterations in the net increasing levels of the ROS up to fourfold those observed in the adductor muscle (Fig. 3a). Interestingly, the highest activity and significance modulations of GST were observed in the gills and mantle (gills for Cu, gills and mantle for Pb) (Fig. 4a). These results suggest that, due to the specific activities of gills, they are the first organs to be exposed to metals and the main organ involved in metal circulation in this species.
a Effects of Cu and Pb (0, 0.1, 0.5, and 2 mg/L) exposure on reactive oxygen species (ROS) levels in Ruditapes philippinarum. Different letters indicate significant differences at p < 0.05. b The increment (%) of intracellular ROS levels in R. philippinarum exposed to three different concentrations of Cu and Pb
MTLP levels, however, increased greatly in the digestive gland and the mantle during the Cu and Pb exposure, respectively, and all tissues, except the adductor muscle, showed significant changes in MTLP levels after the 24-h exposure (Fig. 4b). Previous studies have used diverse organs of bivalves for detecting physiological changes in environmental monitoring. For example, the expression of acetylcholine esterase in response to pesticides was examined in the adductor muscle of Manila clams due to its systematic function and ease of dissection (Choi et al. 2011), while the digestive gland was a target for measuring some antioxidant enzymes in R. philippinarum when evaluating metals and nonylphenols (Won et al. 2012). The digestive gland is significantly involved in both metabolisms and detoxification of metals (Bonneris et al. 2005). The differences in biomarker responses suggested that diverse mechanisms are involved in the defense of R. philippinarum against metal exposure. These results can be applied in ecotoxicological studies using this species.
Trace metal accumulation in in-situ exposure test
The uptake of the eight different elements was determined in R. philippinarum incubated at three different polluted sites for 8 weeks. During the 8 weeks of incubation, the concentrations of each element increased in a time-dependent manner in all three sites (Online resource 3). In many studies, bivalves have been regarded promising candidates for indicator species of the pollution (Boening 1999; Fernández-Tajes et al. 2011). Particularly, studies conducted in Korea found that R. philippinarum are partially effective sentinel organisms as their metal concentration paralleled that of their adjacent environment (Ahn et al. 2006; Ji et al. 2006). In our previous study, however, R. philippinarum reared in metal-polluted sediment did not show any significant changes in the short-term exposure test conducted under laboratory conditions (Won et al. 2012). Thus, the significant accumulation of trace metals observed over the 4 to 6 weeks of incubation suggested that at least 4 weeks are required for R. philippinarum to accumulate significant levels of metals in a polluted area.
The increment in metal concentrations was also calculated at three different sites (Fig. 5). The increments were in variable ranges, in the order of ten to hundreds, according to the type of element. Among the tested elements, Cu and Pb have significantly increased in the 8-week-long in-situ transplantation. Furthermore, the net accumulation rates of all the elements were the highest in R. philippinarum transplanted to site A, located in the upper region of Lake Shihwa, thus corroborating previous result on environmental monitoring conducted in this area (Kim et al. 2003; Ra et al. 2011). Many studies have reported that the upper region of this artificial lake is more polluted with metals and other emerging pollutants compared to the adjacent regions due to the simultaneous inflow of nonpoint source pollutants (Kim et al. 2003; Ra et al. 2011; Won et al. 2012). Particularly, high increment of Cu and Pb in the gills and mantle, over 200 % in site A, was in accordance with chemical results reporting considerable pollution of this area by Cu and Pb (Won et al. 2012). Thus, the results obtained in this study clearly showed that R. philippinarum reflect the environmental conditions, paralleling the contamination gradient at the three sites. Furthermore, the results also suggest that R. philippinarum have the ability to accumulate metals in their specific tissues and as such this species can be used as a metal monitoring species in marine ecosystems.
Many studies have focused on the total soft tissues of bivalves to measure pollutants accumulated in their body (Romero-Ruiz et al. 2003; Gabr et al. 2008). In this study, however, there was no significant increase for most of the elements in the adduct muscle and foot, while their accumulation in the gills and mantle was approximately 50 % higher than the total accumulation. The correlation efficiency between accumulated metal concentrations and incubation time in each organ was significantly higher than those of compared in total metal concentrations and exposure time in clams (Table 1). In particular, nickel has a high explanation power in the siphon and mantle (correlation efficiency >0.8), although it was not significant in the correlation between the total accumulated concentrations and exposure time. Similarly, in other bivalves such as Crassostrea virginica, Sanguilonaria acuminata, Anadara granosa, Meretrix meretrix, and Pelecyora trigona, the gills have accumulated considerable amounts of metals (Sarkar et al. 2008; Luxama et al. 2010). Furthermore, in bivalves, the adductor muscle is considered a poor indicator of metal monitoring, with low absorptive and secretory function and lower levels of metals compared to other tissues (Sarkar et al. 2008). In clams transplanted to site A, the mantle accumulated the highest levels of metal (69 %) compared to the initial levels, followed by the gills (49 %), siphon (36 %), digestive gland (27 %), foot (29 %), and adduct muscle (21 %). However, the order of the accumulation rate among the different tissues was not concomitant with those observed under laboratory conditions, in which the digestive gland and foot exhibited the lowest accumulation rate. These results are indicative of different exposure routes and exposure times between in-situ and laboratory conditions.
Interestingly, the gills of R. philippinarum at site A of the study area accumulated some Cd (Fig. 6). This is in accordance with a previous study, which reported that freshwater bivalve Pyganodon grandis has a great potential to accumulate Cd in their gills due to the presence of the metal-binding protein, metallothionein, with specificity to Cd (Wang et al. 2011). It has been proposed that the element-specificity and organ-specificity of metal-binding proteins (e.g., MTLPs) determine the allocation of accumulated metals in organisms (Dallinger et al. 1997). Although the present study did not assess MTLPs in the in-situ conditions, we conclude that species-specific and element-specific results explain the different accumulation levels observed in R. philippinarum.
The field and laboratory experiments presented herein show that the gills, mantle, and digestive gland of R. philippinarum have a greater ability to accumulate trace metals and therefore are the promising candidates for target organs for measuring biomarkers in a short-time exposure. Particularly, the mantle and gill showed great potential for accumulation of Cu and Pb in both laboratory and field conditions. Additionally, we observed that accumulated Cu and Pb contents through water phase (laboratory short-term exposure test) can be easily partitioned to muscle parts, including the foot and adductor muscle, than field exposure test that might suggest mixed exposure condition, both water and dietborne exposure. The results from this study contribute to our knowledge and understanding of the potential of Manila clam as metal monitoring species in coastal areas.
References
Ahn, I.-Y., Ji, J., Choi, H. J., Pyu, S.-H., Park, H., Choi, J.-W., et al. (2006). Spatial variation of heavy metal accumulation in Manila clam Ruditapes philippinarum from some selected intertidal flats of Korea. Ocean and Polar Research, 28, 215–224.
Alibabić, V., Vahčić, N., Bajramović, M., et al. (2007). Bioaccumulation of metals in fish of salmonidae family and the impact on fish meat quality. Environmental Monitoring and Assessment, 131(1), 349–364.
Boalt, E., Dahlgren, H., Miller, A., et al. (2011). Cadmium, lead and mercury concentrations in whole fish, liver and muscle of herring (Clupea harengus) and perch (Perca fluviatilis). Report written for Naturvårdsverket (Swedish Environmental Protection Agency). Report Nr 6:2012. 10 pp.
Boening, D. W. (1999). An evaluation of bivalves as biomonitors of heavy metals pollution in marine waters. Environmental Monitoring and Assessment, 55, 495–470.
Bonneris, E., Perceval, O., Masson, S., Hare, L., Campbell, P. G., et al. (2005). Sub-cellular partitioning of Cd, Cu and Zn in tissues of indigenous unionid bivalves living along a metal exposure gradient and links to metal–induced effects. Environmental Pollution, 135, 195–208.
Burger, J., Gochfeld, M., Jeitner, C., Burke, S., Stamm, T., et al. (2007). Metal levels in flathead sole (Hippoglossoides elassodon) and great sculpin (Myoxocephalus polyacanthocephalus) from Adak Island, Alaska: potential risk to predators and fishermen. Environmental Research, 103(1), 62–69.
Chen, C. Y., Stemberger, R. S., Klaue, B., Blum, J. D., Pickhardt, P. C., Folt, C. L., et al. (2000). Accumulation of heavy metals in food web components across a gradient of lakes. Limnology and Oceanography, 45(7), 1525–1536.
Choi, J. Y., Yu, J., Yang, D. B., Ra, K., Kim, K. T., Hong, G. H., Shin, K. H., et al. (2011). Acetylthiocholine (ATC)-cleaving cholinesterase (ChE) activity as a potential biomarker of pesticide exposure in the Manila clam, Ruditapes philippinarum, of Korea. Marine Environmental Research, 71, 1162–1168.
Copper, S., Hare, L., Campbell, P. G., et al. (2010). Subcellular partitioning of cadmium in the freshwater bivalve, Pyganodon grandis, after separate short-term exposures to waterborne or diet-borne metal. Aquatic Toxicology, 100, 303–312.
Dallinger, R., Berger, B., Hunziker, P., Kägi, J. H. R., et al. (1997). Metallothionein in snail Cd and Cu metabolism. Nature, 388, 237–238.
De Luca-Abbott, S. B., Richardson, B. J., McCellan, K. E., Zheng, G. J., Martin, M., Lam, P. K. S., et al. (2005). Field validation of antioxidant enzyme biomarkers in mussels (Perna viridis) and clams (Ruditapes philippinarum) transplanted in Hong Kong coastal water. Marine Pollution Bulletin, 51, 694–707.
Depledge, M. H., Aagaard, A., Györkös, P., et al. (1995). Assessment of trace metal toxicity using molecular, physiological and behavioral biomarkers. Marine Pollution Bulletin, 21, 19–27.
Fernández-Tajes, J., Flórez, F., Pereira, S., Rábade, T., Laffon, B., Méndez, J., et al. (2011). Use of three bivalve species for biomonitoring a polluted estuarine environment. Environmental Monitoring and Assessment, 177, 1–4.
Gabr, H. R., Gab-Alla, A. A.-F. A., et al. (2008). Effect of transplantation on heavy metal concentrations in commercial clams of Lake Timsah, Suez Canal, Egypt. Oceanologia, 50(1), 83–93.
Jezierska, B., & Witeska, M. (2006). The metal uptake and accumulation in fish living in polluted waters. Soil and Water Pollution Monitoring, Protection and Remediation, 69, 3–23.
Ji, J., Choi, H. J., Ahn, I.-Y., et al. (2006). Evaluation of Manila clam Ruditapes philippinarum as a sentinel species for metal pollution monitoring in estuarine tidal flats of Korea: effects of size, sex, and spawning on baseline accumulation. Marine Pollution Bulletin, 52, 447–453.
Kim, K.-T., Kim, E. S., Cho, S. R., Park, J. K., Park, C. K., et al. (2003). Change of heavy metals in the surface sediments of the Lake Shihwa and its tributaries. Ocean Polar Research, 25, 447–457.
Luxama, J. D., Carroll, M. A., Catapane, E. J., et al. (2010). Effects of potential therapeutic agents on copper accumulations in gill of Crassostrea virginica. In Vivo, 31, 32–42.
Martel, P., Kovacs, T., Voss, R., Megraw, S., et al. (2003). Evaluation of caged freshwater mussels as an alternative method for environmental effects monitoring (EEM) studies. Environmental Pollution, 124, 471–483.
Martín-Díaz, M. L., Blasco, J., Sales, D., DelValls, T. A., et al. (2007). Biomarkers study for sediment quality assessment in Spanish ports using the crab Carcinus maenas and the clam Ruditapes philippinarum. Archives of Environmental Contamination and Toxicology, 53, 66–76.
Maruya, K. A., Dodder, N. G., Schaffner, R. A., Weisberg, S. B., Gregorio, D., Klosterhaus, S., Alvarez, D. A., Furlong, E. T., Kimbrough, K. L., Lauenstein, G. G., Christensen, J. D., et al. (2013). Refocusing mussel watch on contaminants of emerging concern (CECs): the California pilot study (2009-10). Marine Pollution Bulletin, 81(2), 334–339.
Ra, K., Kim, K. T., Bang, J. H., Lee, J. M., Kim, E. S., Cho, S. R., et al. (2011). A preliminary study of environmental impact assessment of tidal power plant in Shihwa Lake, Korea: heavy metal accumulation in the transplanted Manila clam (Ruditapes philippinarum). Journal of Coastal Research, SI64, 932–936.
Rainbow, P. S., & Dallinger, R. (1993). Metal uptake, regulation and excretion in freshwater invertebrates. In R. Dallinger & P. S. Rainbow (Eds.), Ecotoxicology of metals in invertebrates, SETAC special publication series (pp. 119–131). Boca Raton, FL: Lewis Publishers.
Rainbow, P. S. (1995). Biomonitoring of heavy metal availability in the marine environment. Marine Pollution Bulletin, 31, 183–192.
Rainbbow, P. S. (2006). Biomonitoring of trace metals in estuarine and marine environment. Australasian Journal of Ecotoxicology, 12, 107–122.
Regoli, F., Nigro, M., Bertoli, E., Principato, G., Orlando, E., et al. (1997). Defenses against oxidative stress in the Antarctic scallop Adamussium colbecki and effects of acute exposure to metals. Hydrobiologia, 355, 139–144.
Reynoldson, T. B. (1987). Interactions between sediment contaminants and benthic organisms. Hydrobiologia, 149, 53–66.
Romero-Ruiz, A., Amezcua, O., Rodríguez-Ortega, M. J., Muñoz, J. L., Alhama, J., Rodríguez-Ariza, A., Gómez-Ariza, J. L., López-Barea, J., et al. (2003). Oxidative stress biomarkers in bivalves transplanted to the Guadalquivir estuary after Aznalcóllar spill. Environmental Toxicology and Chemistry, 22, 92–100.
Saha, M., Sarkar, S. K., Bhattacharya, B., et al. (2006). Interspecific variation in heavy metal body concentrations in biota of Sunderban mangrove wetland, Northeast India. Environmental International, 32, 203–207.
Sarkar, S. K., Cabral, H., Cardoso, I., Bhattacharya, A. K., Satpathy, K. K., Alam, M. A., et al. (2008). Biomonitoring of heavy metals using the bivalve molluscs in Sunderban mangrove wetland, northeast coast of bay of Bengal (India): possible risks to human health. Clean, 36, 187–194.
Szebedinszky, C., McGeer, J. C., McDonald, D. G., Wood, C. M., et al. (2001). Effects of chronic Cd exposure via the diet or water on internal organ-specific distribution and subsequent gill Cd uptake kinetics in juvenile rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 20, 597–607.
Tessier, A., & Campbell, P. G. C. (1987). Partitioning of trace metals in sediments: relationships with bioavailability. Hydrobiologia, 149, 43–52.
Valavanidis, A., Vlahogianni, T., Dassenakis, M., Scoullos, M., et al. (2006). Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and Environmental Safety, 64, 178–189.
Viarengo, A., Ponzano, E., Dondero, F., Fabbra, R., et al. (1997). A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antartic mollusk. Marine Environmental Research, 44, 69–84.
Wang, D., Couillard, Y., Campbell, G. C., Jolicoeur, P., et al. (2011). Changes in subcellular metal partitioning in the gills of freshwater bivalves (Pyganodon grandis) living along an environmental cadmium gradient. Canadian Journal of Fisheries and Aquatic Sciences, 56, 774–784.
Won, E.-J., Hong, S., Ra, K., Kim, K.-T., Shin, K.-H., et al. (2012). Evaluation of the potential impact of polluted sediments using Manila clam Ruditapes philippinarum: bioaccumulation and biomarker responses. Environmental Science and Pollution Research, 19, 2570–2580.
Acknowledgments
This research was supported by a grant from the KIOST [PE99402] funded to Kongtae Ra and partially supported by grant from the National Research Foundation of Korea (NRF-2015R1C1A2A01053437) funded to Eun-Ji Won.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 10622 kb)
Rights and permissions
About this article
Cite this article
Won, EJ., Kim, KT., Choi, JY. et al. Target organs of the Manila clam Ruditapes philippinarum for studying metal accumulation and biomarkers in pollution monitoring: laboratory and in-situ transplantation experiments. Environ Monit Assess 188, 478 (2016). https://doi.org/10.1007/s10661-016-5485-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5485-y