Abstract
An analytical method was developed for the measurement of 18 novel halogenated flame retardants in house dust. Sample preparation was based on ultrasound-assisted solvent extraction and clean up with solid phase extraction (SPE). Sample extracts were analyzed by gas chromatography-mass spectrometry (GC/MS) operated in electron capture negative ion (ECNI) chemical ionization mode. Baseline data from 351 fresh (active) dust samples collected under the Canadian House Dust Study (CHDS) revealed that five out of 18 target chemicals were present with detection frequencies higher than 90 %. Median (range) concentrations for these five compounds were as follows: 104 (<1.5–13,000) ng/g for 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EHTBB), 8.5 (<1.7–2390) ng/g for 1,2-bis(2,4,6-tribromophenoxy)ethane (BTBPE), 10.2 (<1.7–430) ng/g for hexabromobenzene (HBB), 2.9 (<1.2–1410) ng/g for syn-dechlorane plus (syn-DP) and 5.6 (<1.9–1570) ng/g for anti-dechlorane plus (anti-DP). A comparison of two sampling methods in a subset of 40 homes showed significant positive correlations between samples of “active” dust and samples taken directly from the household vacuum cleaner for all target compounds having median values above their corresponding method detection limits (MDLs). In addition, the method was also applied to the analysis of the targeted compounds in National Institute of Standards and Technology (NIST) standard reference material (SRM 2585, organic contaminants in house dust). Results from the current study could contribute to the potential certification of target chemicals in SRM 2585.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various safety regulations require that flame retardants (FRs) be added to products such as upholstered furniture, textiles, electronics, and building materials. During the past decades, polybrominated diphenyl ethers (PBDEs) have been used extensively. However, due to their persistence in the environment, bioaccumulation in the biota, and potential adverse impacts on both the environment and human health, major commercial PBDE formulations (e.g., Penta-BDE, Octa-BDE, and Deca-BDE) were banned or voluntarily withdrawn from the market in many countries (European Court of Justice 2003, 2008; Government of Canada 2008; USEPA 2010). Therefore, other alternative FRs including non-PBDE halogenated FRs have been introduced with a growth rate of about 5 % per year (Covaci et al. 2011). The total volume for such non-PBDE halogenated “novel” FRs (NFRs) has been estimated to be from 100,000 to 180,000 tons per year (Harju et al. 2009). However, little is known about the chemical compositions, uses, exposure levels, and health effects of such non-PBDE NFRs. A total of 18 target NFRs (Table 1) have been selected in this study, including 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EHTBB or TBB), 1,2-bis(2,4,6-tribromophenoxy)ethane (BTBPE), hexabromobenzene (HBB), and dechlorane plus (DP). A recent review paper listed some physical properties (e.g., log Kow, water solubility, vapor pressure) for several novel brominated flame retardants (Covaci et al. 2011). Based on their similar molecular structures and physical chemical properties to PBDEs, they may have similar environmental fates to PBDEs. Although novel or emerging, these substances have been detected in various environmental matrices including indoor dust in several recent studies (Tables 5 and 6), human maternal serum, and breast milk (Zhou et al. 2014a, b). House dust is a repository for organic compounds and could be a major route of human exposure to indoor pollutants. For example, Lorber (2008) estimated that the US adult resident’s exposure to PBDEs was mainly from indoor dust. Therefore, a simple, sensitive, and robust analytical method is necessary to generate statistically robust baseline concentrations of these NFRs in house dust to help assess human exposure to NFRs in the indoor environment. Several non-PBDE NFRs are currently being assessed by various regulatory agencies including Health Canada, under the Chemicals Management Plan, and the US EPA (Government of Canada 2013; USEPA 2013).
However, the analysis of organics in dust is challenging due to the complex matrix composition. House dust contains hundreds, if not thousands, of organic compounds with various chemical and physical properties (Wise et al. 2006). Therefore, the use of certified reference materials for indoor dust is beneficial for evaluating the accuracy of the results generated from different analytical methods. The National Institute of Standards and Technology (NIST) has produced a standard reference material for house dust (SRM 2585, organic contaminants in house dust) and provided certified values for several classes of compounds including polybrominated biphenyl ether (PBDE) congeners (NIST 2010; Wise et al. 2006). Nevertheless, there are no certified values for target NFRs. Thus, another goal of the current study was to measure the concentrations of target NFRs in SRM 2585, which may contribute to a potential certification of these NFRs in the reference material.
House dust is often collected by vacuuming. In the current study, two sampling methods were used: a sample of fresh, or “active”, dust (FD) collected by technicians following a protocol developed for the Canadian House Dust Study (CHDS) and a composite sample taken from typical household vacuums (HD) collected by residents during routine house cleaning (Rasmussen et al. 2011, 2013). Previous studies have shown that FD and HD samples could provide comparable results for some compounds, including most synthetic musk compounds (Kubwabo et al. 2012), phthalates (Kubwabo et al. 2013), triclosan and parabens (Fan et al. 2010), and organophosphate flame retardants (Fan et al. 2014). Thus, the third goal of this study was to compare the FD and HD sampling methods for target NFRs to investigate whether the two sampling methods could provide comparable results for target NFR compounds and evaluate whether the cost-effective and non-intrusive HD method is a valid alternative to the FD method.
Material and methods
Materials
Target analytes (18 non-PBDE FRs) and their acronyms are listed in Table 1; their chemical formula and structures are illustrated in Figure S1. All chemical standards were purchased from Wellington Laboratories Inc. (Guelph, Ontario, Canada) with the exception of 6-Fluoro-2,2′,4,4′-tetrabromodiphenylether (F1BDE-47) (Chiron AS, Norway) and 2,3,3′,4,4′-Pentabromodiphenyl ether (BDE-105) (AccuStandard Inc., New Haven, CT). Individual stock solutions were prepared in toluene, and working standard solutions were prepared by mixing individual standard solutions and diluting in toluene to the appropriate concentrations. GC-grade solvents (i.e., hexane, acetone, and toluene) were purchased from EMD Chemicals Inc. (Mississauga, ON, Canada). NIST SRM 2585 (organic contaminants in house dust) was obtained from NIST (Gaithersburg, MD, USA).
House dust sample collection and preparation
The CHDS was designed to provide statistically representative national baseline estimates for indoor environmental concentrations of chemicals found in typical urban households. Sampling was conducted between 2007 and 2010 across 13 cities in Canada. The first priority of the current study was to provide nationally representative Canadian information on selected NFRs in house dust to inform risk management activities, and another priority was the comparison and evaluation of two dust sampling techniques. Details of participant recruitment, dust sampling, and sample preparation under the CHDS protocol (e.g., sieving, shipping, and storage) were described in detail previously (Rasmussen et al. 2011, 2013) and briefly summarized here. During the home visit, two types of dust samples were collected. Technicians obtained existing household vacuum dust (HD) samples from the vacuum systems used by the study participants, which included a variety of models of canister vacuums and traditional bag vacuum cleaners. Fresh dust (FD) samples were collected by technicians using a Pullman-Holt High-Efficiency Particulate Air (HEPA) vacuum sampler (model 102 ASB-12PD), in which dust particles follow a direct pathway from the floor to the vacuum bag, without passing internal mechanical parts, thus avoiding potential contamination of the sample. The bag is specified to capture 99.97 % of all particles ≥0.3 μm. Wet areas in the home (i.e., kitchens, bathrooms, laundry rooms) were avoided to protect the integrity of the FD sample. Participants were requested to abstain from cleaning floor surfaces for a period of 7 days before the scheduled fresh dust sampling, which allowed sufficient mass of fresh dust to accumulate. All HD and FD vacuum bags were sealed in paper and then double-bagged on site during the home visit, by the technician, prior to being shipped to Health Canada for preparation and analysis. All dust samples were air-dried and sieved, and the fraction with particle sizes <80 μm was used for analysis. This size fraction was selected because it provides a homogeneous sample with good reproducibility and has been shown to be relevant for indoor environmental pathways (Rasmussen et al. 2013). Sieved dust samples were kept frozen in gas tight amber glass jars to prevent potential photolysis. Statistical analysis was conducted using SPSS Statistics v.19 and MS Excel 2010. Evaluation of the data distributions indicated that non-parametric tests were appropriate. The Spearman rank and Wilcoxon signed rank tests were used to compare HD and FD results from 40 homes, for compounds with median concentrations greater than their corresponding method detection limits (MDLs).
Sonication extraction and sample cleanup
Dust samples (0.1 g ± 0.003 g) were accurately weighed and transferred to 10-mL glass centrifuge tubes. Twenty-five microliters of the internal standard mixture solution, which contained F1BDE-47 (0.2 ng/μL), BDE-105 (0.2 ng/μL), 13C-syn-dechlorane plus (1.0 ng/μL), and 13C-anti-dechlorane plus (1.0 ng/μL), was spiked into the sample. The tube was vortexed for 10 s, sonicated for 5 min, and vortexed again to re-suspend the dust pellets. Such vortexing and sonication were repeated two more times, and then the tube was centrifuged for 5 min at 3000 rpm. The clear supernatant was transferred to a 4-mL amber vial. This completed one extraction cycle. The extraction cycle was repeated two more times and the supernatant combined. The combined extract was concentrated by a gentle stream of nitrogen at room temperature and then solvent exchanged to hexane (1 mL) prior to sample cleanup on a Florisil SPE cartridge (500 mg/6 cc, UCT, from Bristol, PA). The SPE cartridge was preconditioned with 2 × 5 mL of hexane. The concentrated extract was loaded onto the cartridge. The vial was rinsed with hexane (2 × 0.5 mL), and the rinse was also added to the cartridge. The target compounds were eluted with 2 × 5.0 mL of hexane. The eluent was evaporated at room temperature with a gentle stream of nitrogen to just dryness. The residue was reconstituted in 175 μL of toluene. After 25 μL of recovery standard BDE-77 (0.2 ng/μL) was added, the final solution was subjected to gas chromatography-mass spectrometry (GC/MS) analysis.
GC/MS instrumental analysis
Sample extracts were analyzed using an Agilent 6980 gas chromatographer (GC) coupled with a Waters Quattro micro triple quadrupole mass spectrometer (Waters Corp., Milford, MA, USA) operated in electron capture negative ionization (ECNI) mode. Methane was used as reagent gas. The ion source and GC interface temperatures were set at 180 and 280 °C, respectively. The GC column was a Zebron ZB-5HT (15 m × 0.25 mm i.d., 0.10 μm of film thickness) from Phenomenex (Torrance, CA, USA). The carrier gas was helium with a constant flow of 1 mL/min. The oven temperature was initially held at 80 °C for 2 min, ramped to 200 °C at 4 °C/min, held at 200 °C for 1 min, ramped to 300 °C at 25 °C /min, held at 300 °C for 2 min, ramped up to 330 °C at 35 °C/min, and finally held at 330 °C for 10 min. The GC injector was equipped with a programmable-temperature vaporizer inlet (PTV) which was operated in solvent vent mode. The initial inlet temperature was held at 90 °C for 0.04 min, ramped to 295 °C at 700 °C/min, and held at 295 °C thereafter till the end of the GC/MS analysis. Vent pressure was set at 10 kPa with vent flow of 75 mL/min ending at 0.02 min. Purge flow was 50 mL/min after 1.25 min. The injection volume was 2 μL. Selected ion monitoring (SIM) mode was employed to monitor two ions for each individual compound, one for quantitation and the other for confirmation (Table 1).
Results and discussion
Method performance, validation, and QA/QC
A few recent studies have reported methods for the analysis of PBDEs and some non-PBDE “novel” flame retardants (NFRs) in dust samples (Ali et al. 2011, 2012a; Cristale and Lacorte 2013; Dodson et al. 2012; Fromme et al. 2014; Ionas and Covaci 2013; Shoeib et al. 2012; Stapleton et al. 2008; Van den Eede et al. 2012). The general procedure includes solvent extraction of organics from dust samples, sample cleanup using solid phase extraction (SPE), and separation and detection of analytes by GC/MS or LC/MS. In this study, a mixed solvent of hexane/acetone (3 × 1.5 mL, v/v, 1:1) was used by sonication extraction. The extraction efficiency after 2 cycles was higher than 95 % for all the target analytes, but three extraction cycles were used to ensure higher recoveries of all target analytes. For sample cleanup for the analysis of NFRs, a few recent studies used the SPE method with different adsorbents including deactivated alumina (Shoeib et al. 2012), neutral silica (Ionas and Covaci 2013), acidified silica (Dirtu et al. 2012; Geens et al. 2010), deactivated silica (Lankova et al. 2015), Florisil (Dodson et al. 2012; Van den Eede et al. 2012), deactivated Florisil (Stapleton et al. 2008), and polymeric sorbents such as Oasis HLB (from Waters Inc., Milford, MA, USA) (Ionas and Covaci 2013). We briefly tested three different sorbents for SPE cleanup: Florisil (500 mg/6 cc, UCT, from Bristol, PA), silica (500 mg/6 cc, from Sigma-Aldrich, Oakville, ON), and HLB (500 mg/6 cc, from Waters Inc., Milford, MA). We found that Florisil SPE gave us better efficiency for sample cleanup of the target analytes. Sample cleanup coupled with separation and detection by GC/ECNI-MS provided good separation of all target analytes in dust samples (Figures S2 and S3). For GC/MS analysis, some NFRs may undergo thermal decomposition or isomeric conversion in the GC injection liner at high temperature. For example, 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane (TBECH) can convert among the four isomeric forms at elevated temperatures. Tomy et al. (2008) found that β-TBECH could convert to other TBECH isomers (i.e., α−, γ−, and δ−). When the mixture of α − and β − TBECH was injected at 295 °C for GC/MS analysis in this study, it was found that more than 30 % of α − and β − TBECH were converted to γ − and δ − TBECH; likewise, more than 40 % of γ − and δ − TBECH were converted to α − and β − TBECH. In addition, such conversions were not consistent. Therefore, the best injection temperature for TBECH should be below 150 °C. In this study, a programmable temperature vaporization (PTV) injector was used. The PTV temperature was initially set at 90 °C for 0.04 min, then ramped to 295 °C at 700 °C/min, and held at 295 °C thereafter during the GC run which ensured all target compounds evaporated in the injection liner and were transferred to the GC column. Such PTV injection minimized the thermal conversion of TBECH (Fig. 1) and possibly other target compounds as well.
Six different concentration levels of target analytes in toluene were used to determine retention time and check the linearity range of the method. The calibration curve was linear (R 2 > 0.993) over a concentration range of 0.5 to 250 pg/μL for each target analyte with the exception of EHTBB (5–2500 pg/μL), BTBPE (2.5–1250 pg/μL), syn-DP and anti-DP (2.5–1250 pg/μL). Solvent-washed dust, free of target analytes, was used as a dust surrogate to determine the method detection limits (MDLs), recoveries, and precision. The estimation of MDL for each analyte was based on the US EPA Regulation 40 CFR Part 136 (Appendix B) method (USEPA 1986). Eight (8) replicates of solvent-washed dust samples spiked with a mixture of target compounds at 2.5 ng/g level were processed through the entire sample preparation and instrumental analysis procedures. The MDLs ranged from 0.5 to 1.9 ng/g (Table 2). Since the concentrations of NFRs vary greatly for different compounds and samples, recoveries were investigated at four different spiking levels spanning from 10 to 100 ng/g (Table 2), and at each level, replicate analysis (n = 4) was performed. The overall recoveries for each analyte are listed in Table 2, ranging from 61 % for PBBB to 112 % for β-TBECH; the average recoveries of the majority of target analytes were over 80 %. Method precision was evaluated with replicate analysis (n = 4) of solvent-washed dust samples spiked with target analytes and analyzed on three different days. The overall inter-day variations (RSD, %) were lower than 15 % for all the analytes except PBBA (19 %), indicating that the method was robust. Sample analysis was performed by batch. Each batch consisted of two method blanks, three QC samples, and 18 samples with two duplicates. The relative percent difference (RPD) for duplicate analyses was less than 20 %. If the QC sample results deviated from the spiked concentrations more than 20 % for the majority of target compounds or no baseline separation could be achieved for α − and β − TBECH, then maintenance of the GC/MS system (e.g., change injection liner, trim column head, clean ion source, or even change the GC column) would be performed. No target analytes were detected in method blanks, and thus, the reported data were not blank-corrected.
NFR concentrations in SRM 2585
NIST SRM 2585 was analyzed for target analytes. Six replicates along with two matrix blanks were performed for the analysis. There are no certified values for target NFR compounds in SRM 2585 (Table 3). Internal standards were spiked prior to sample extraction, and their recovery recoveries monitored. The average recoveries of the internal standards were found to be satisfactory: F1BDE-47 (88 ± 2.3 %), BDE-105 (97 ± 6.2 %), 13C10-syn-DP (90 ± 2.6 %), and 13C10-anti-DP (81 ± 3.8 %). A few recent studies reported only EHTBB and BTBPE in SRM 2585 and the results were often associated with large uncertainties (Table 3). Therefore, comparison of our results with those reported by other studies was inconclusive; nevertheless, we calculated the average concentrations of EHTBB and BTBPE using the values reported by other studies and this study. The EHTBB concentration measured in this study was 39 ± 4.8 ng/g, 11 % off the average (35 ± 5.0 ng/g); the BTBPE concentration was 38 ± 5.9 ng/g, −14 % off the average (44 ± 16 ng/g). Such discrepancies emphasize the need for more independent analyses. Other major NFRs detected in SRM 2585 include syn-DP (18.7 ± 1.5) ng/g, anti-DP (44.1 ± 7.9) ng/g, PBEB (7.7 ± 0.6) ng/g, and ATE (6.0 ± 2.2) ng/g. TBpX, BATE, (γ + δ)-TBECH, TBCT, PBT, PBEB, DPTE, PBBB, and PBBA were either not detected or their concentrations were below their corresponding MDLs. To the best of our knowledge, this study is the first to report 15 more NFRs in SRM 2585 other than EHTBB, BTBPE, and HBB. The results of this study could potentially contribute to the certification of these NFRs in NIST SRM 2585.
Comparison of FD and HD samples for NFRs in dust
Out of a total of 18 compounds in Table 4, four compounds (i.e., (γ + δ) − TBECH, DPTE, PBBB, and PBBA) were not detectable in either FD or HD samples, and four compounds (TBpX, BATE, PBT, and PBEB) had median values below MDL in both datasets. Statistical comparisons of the remaining 10 compounds are summarized in Table 4. Non-parametric tests (Spearman rank and Wilcoxon signed rank) were used for these comparisons as previous work showed that the Canadian House Dust Study (CHDS) dataset cannot be assumed to be normally distributed (Rasmussen et al. 2011, 2013). The comparisons in Table 4 demonstrate a general consistency between FD and HD results, which indicates that, for the studied compounds, household vacuum samples provide cost-effective and informative results which are comparable to those of fresh dust samples. The Spearman rank correlations are significant and positive between FD and HD for all 10 compounds: at the 99 % confidence intervals (CIs) for eight compounds and at the 95 % CIs for two compounds (Table 4). A comparison of medians using the Wilcoxon rank test indicates that there is no significant difference between median values of FD and HD samples (p > 0.05; Wilcoxon rank) with the exception of TBCT for which the FD median is significantly higher than the HD median (p = 0.042; Table 4).
Concentrations in house dust
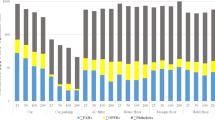
Although PBDEs have been investigated extensively in various environmental matrices, including house dust, data on the occurrence of non-PBDE halogenated FRs in indoor dust are less common. In this study, a subset of 351 randomly selected FD samples collected under the CHDS was analyzed. EHTBB and BTBPE were the two major compounds detected in this study with detection frequencies higher than 90 %. EHTBB was identified to be one major component in Firemaster 550 (Stapleton et al. 2008), which might be designed to replace penta-BDE formulation in polyurethane foam. BTBPE has been advertised as a replacement for octa-BDE mixtures and is used as an additive in thermoplastic and thermosetting plastic systems and may be used as a replacement for octa-BDE mixtures (Danish EPA 2006). The worldwide production/usage of BTBPE was estimated to be 16,710 tons in 2001, but there is no production information available for EHTBB (Covaci et al. 2011). In this study, EHTBB had a detection frequency of 95 % (n = 351), with median concentration (range) of 104 (<1.7–13,000) ng/g. BTBPE was detected in 93 % of the samples, with median concentration of 8.5 (<1.7–2390) ng/g. Similar concentrations of EHTBB and BTBPE were found in another Canadian study, where 116 dust samples were collected in 2007–2008 in Vancouver, BC, Canada (Shoeib et al. 2012). They were also detected in every sample collected in the USA, including San Francisco, Boston, and Durham (Dodson et al. 2012; Stapleton et al. 2008, 2014). Such high detection frequencies of EHTBB and BTBPE in Canada and the USA suggest their widespread use in North America (Table 5). These two compounds were also detected in other regions out of North America, but with relatively lower detection frequencies and concentrations (Table 5).
HBB, syn-DP, and anti-DP were detected in this study with frequencies higher than 90 % (Table 6). HBB is used as an additive flame retardant in polymers, plastics, textiles, wood, and paper (Bruchajzer et al. 2004). In this study, HBB was detected in 94 % of the samples, with median concentration of 10.2 (<0.5–430) ng/g, higher than that of 3.7 (<0.02–130) ng/g reported in the Vancouver study (Shoeib et al. 2012). Dechlorane plus is an additive flame retardant used in electrical connectors and in commercial wire and cable (Oxy Co. 2013). Commercial DP formulation contains two major isomers (syn- and anti-), which were well resolved during GC/MS analysis in this study. Both isomers were detected with frequencies higher than 90 %. The median concentrations of 2.9 (<1.2–1400) ng/g for syn-DP and 5.6 (<1.9–1570) ng/g for anti-DP ng/g in this study were similar to those reported by other studies listed in Table 6 (Dodson et al. 2012; Shoeib et al. 2012), but were relatively lower than 14 (2.3–5680) ng/g for total DP (i.e., sum of syn-DP and anti-DP) reported by Zhu et al. (2007), who found DPs in every sample (n = 69) collected in the city of Ottawa, Canada in 2002–2003. Other NFR analytes have seldom been studied in dust samples (Table 6). TBECH is an additive FR used mainly in expandable polystyrene beads used for thermal insulation in housing. It is also used as a FR for extruded polystyrene foam and for adhesives in fabric and vinyl lamination, electrical cable coatings, high-impact plastic parts of appliances, and in some construction materials (Arsenault et al. 2008). Technical TBECH consists of near equimolar amounts of two diastereomers: α − and β − TBECH (Arsenault et al. 2008). Annual US production/imports of TBECH were 10,000–500,000 lb from 1986–2002 (USEPA 2002). In this study, α − and β − TBECH were detected in 58 % of the samples with median concentrations of 1.17 (<0.9–79) ng/g and 0.94 (<0.62–70) ng/g, respectively. Dodson et al. (2012) also detected α − and β − TBECH in dust samples in San Francisco, but with lower detection frequency and lower concentrations (Dodson et al. 2012) (Table 6). Other analytes which were also detected in this study with high frequency included TBCT (70 %), ATE (55 %), and PBB (53 %); however, their respective concentrations were low (Table 6). DPTE, PBBB, and PBBA were not detected in any samples analyzed in this study.
Conclusions
A new method was developed to measure 18 non-PBDE halogenated flame retardants in house dust. The method demonstrated good sensitivities and recoveries for the target compounds. EHTBB, HBB, BTBPE, syn-, and anti-DP were the major compounds detected and quantified in house dust samples. Two vacuum sampling methods provided comparable results, indicating that dust sampling from household vacuum bags is a cost-effective and informative alternative to the standardized sampling of fresh dust by qualified technicians. The analytical method was also applied to the analysis of target compounds in NIST standard reference material (SRM 2585, organic contaminants in house dust). The results obtained from SRM 2585 could inform a potential certification of concentrations of these novel flame retardants in the reference material.
References
Ali N, Harrad S, Muenhor D, Neels H, Covaci A (2011) Analytical characteristics and determination of major novel brominated flame retardants (NBFRs) in indoor dust. Anal Bioanal Chem 400:3073–83
Ali N, Dirtu AC, Van den Eede N, Goosey E, Harrad S, Neels H, Mannetje t A, J C, Douwes J, Covaci A (2012a) Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere 88:1276–82
Ali N, Van den Eede N, Dirtu AC, Neels H, Covaci A (2012b) Assessment of human exposure to indoor organic contaminants via dust ingestion in Pakistan. Indoor Air 22:200–11
Ali N, Ali L, Mehdi T, Dirtu AC, Al-Shammari F, Neels H, Covaci A (2013) Levels and profiles of organochlorines and flame retardants in car and house dust from Kuwait and Pakistan: implication for human exposure via dust ingestion. Environ Int 55:62–70
Arsenault G, Lough A, Marvin C, McAlees A, McCrindle R, MacInnis G, Pleskach K, Potter D, Riddell N, Sverko E, Tittlemier S, Tomy G (2008) Structure characterization and thermal stabilities of the isomers of the brominated flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane. Chemosphere 72:1163–1170
Bruchajzer E, Frydrych B, Szymanska JA (2004) Effect of repeated administration of hexabromobenzene and 1,2,4,5-tetrabromobenzene on the levels of selected cytochromes in rat liver. Int J Occup Med Environ Health 17:347–53
Co O (2013) Product stewardship summary: dechlorane plus. Oxy Occidental Chemical Corporation, Dallas, Texas, USA, http://www.oxy.com/OurBusinesses/Chemicals/ResponsibleCare/Documents/Dechlorane%20Plus.pdf. Accessed 25 Mar 2015
Covaci A, Harrad S, Abdallah MA, Ali N, Law RJ, Herzke D, de Wit CA (2011) Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environ Int 37:532–56
Cristale J, Lacorte S (2013) Development and validation of a multiresidue method for the analysis of polybrominated diphenyl ethers, new brominated and organophosphorus flame retardants in sediment, sludge and dust. J Chromatogr A 1305:267–75
Danish EPA (2006) Deca-BDE and alternatives in electrical and electronic equipment. http://www2.mst.dk/common/Udgivramme/Frame.asp?http://www2.mst.dk/udgiv/publications/2007/978-87-7052-349-3/html/default_eng.htm. Accessed 10 July 2015
Dirtu AC, Ali N, Van den Eede N, Neels H, Covaci A (2012) Country specific comparison for profile of chlorinated, brominated and phosphate organic contaminants in indoor dust. Case study for Eastern Romania, 2010. Environ Int 49:1–8
Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA (2012) After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol 46:13056–13066
European Court of Justice. (2003) Marketing and use of certain dangerous substances and preparations (pentabromodiphenyl ether, octabromodiphenyl ether) DIRECTIVE 2003/11/EC. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32003L0011. Accessed 8 May 2015
European Court of Justice. (2008) Restriction of use of certain hazardous substances—Decabromodiphenyl ether (‘DecaBDE’) Case C-14/06, 2008. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:62006CJ0014. Accessed 10 June 2015
Fan X, Kubwabo C, Rasmussen P, Jones-Otazo H (2010) Simultaneous quantitation of parabens, triclosan, and methyl triclosan in indoor house dust using solid phase extraction and gas chromatography-mass spectrometry. J Environ Monit 12:1891–7
Fan X, Kubwabo C, Rasmussen PE, Wu F (2014) Simultaneous determination of thirteen organophosphate esters in settled indoor house dust and a comparison between two sampling techniques. Sci Total Environ 491–492:80–86
Fromme H, Hilger B, Kopp E, Miserok M, Volkel W (2014) Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and “novel” brominated flame retardants in house dust in Germany. Environ Int 64:61–8
Geens T, Ali N, Roosens L, Neels H, Covaci A (2010) Analytical characteristics of several new brominated flame retardants. Talanta 81:1865–9
Government of Canada. (2008) Polybrominated diphenyl ethers regulations (SOR/SOR/2008-218). http://www.ec.gc.ca/lcpe-cepa/eng/regulations/detailReg.cfm?intReg=108. Accessed 10 Apr 2015
Government of Canada. (2013) Certain organic flame retardants substance grouping. http://www.chemicalsubstanceschimiques.gc.ca/group/flame_retardant-ignifuges/index-eng.php. Accessed 5 Mar 2015
Harju M, Heimstad ES, Herzke D, Sandanger T, Posner S, Wania F (2009) Emerging “new” brominated flamer retardants in flame retarded products and the environment. The Norwegian Pollution Control Authority, Oslo, Norway. http://www.miljodirektoratet.no/old/klif/publikasjoner/2462/ta2462.pdf. Accessed 20 Mar 2015
Ionas AC, Covaci A (2013) Simplifying multi-residue analysis of flame retardants in indoor dust. Int J Environ Anal Chem 93:1074–1083
Kubwabo C, Fan X, Rasmussen P, Wu F (2012) Determination of synthetic musk compounds in indoor house dust by gas chromatography–ion trap mass spectrometry. Anal Bioanal Chem 404:467–477
Kubwabo C, Rasmussen PE, Fan X, Kosarac I, Wu F, Zidek A, Kuchta SL (2013) Analysis of selected phthalates in Canadian indoor dust collected using household vacuum and standardized sampling techniques. Indoor Air 23:506–514
Lankova D, Svarcova A, Kalachova K, Lacina O, Pulkrabova J, Hajslova J (2015) Multi-analyte method for the analysis of various organohalogen compounds in house dust. Anal Chim Acta 854:61–9
Lorber M (2008) Exposure of Americans to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol 18:2–19
NIST (2010) Standard reference material 2585—organic contaminants in house dust. National Institute of Standards and Technology, Gaithersburg, https://www-s.nist.gov/srmors/. Accessed 8 Mar 2015
Rasmussen PE, Beauchemin S, Chénier M, Levesque C, MacLean LCW, Marro L, Jones-Otazo H, Petrovic S, McDonald LT, Gardner HD (2011) Canadian house dust study: lead bioaccessibility and speciation. Environ Sci Technol 45:4959–4965
Rasmussen PE, Levesque C, Chénier M, Gardner HD, Jones-Otazo H, Petrovic S (2013) Canadian House Dust Study: population-based concentrations, loads and loading rates of arsenic, cadmium, chromium, copper, nickel, lead, and zinc inside urban homes. Sci Total Environ 443:520–529
Sahlstrom L, Sellstrom U, de Wit CA (2012) Clean-up method for determination of established and emerging brominated flame retardants in dust. Anal Bioanal Chem 404:459–66
Shoeib M, Harner T, Webster GM, Sverko E, Cheng Y (2012) Legacy and current-use flame retardants in house dust from Vancouver, Canada. Environ Pollut 169:175–182
Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF (2008) Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol 42:6910–6
Stapleton HM, Misenheimer J, Hoffman K, Webster TF (2014) Flame retardant associations between children’s handwipes and house dust. Chemosphere 116:54–60
Tomy GT, Pleskach K, Arsenault G, Potter D, McCrindle R, Marvin CH, Sverko E, Tittlemier S (2008) Identilication of the novel cycloaliphatic brominated flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane in Canadian Arctic beluga (Delphinapterus leucas). Environ Sci Technol 42:543–549
USEPA. (1986) EPA Regulation 40 CFR Part 136 (Appendix B) Appendix B to Part 136 -- Definition and Procedure for the Determination of the Method Detection Limit -- Revision 1.11. US Environmental Protection Agency (EPA). http://www.ecfr.gov/. Accessed 05 Mar 2014
USEPA (2002) Non-confidential inventory update reporting production volume information. Toxic Substances Control Act (TSCA) Inventory. http://www.epa.gov/oppt/iur/tools/data/2002-vol.htm. Accessed 24 Feb 2015
USEPA. (2010) An exposure assessment of polybrominated diphenyl ethers (PBDEs) EPA/600/R-08/086F. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=210404. Accessed 27 Mar 2015
USEPA. (2013) EPA announces chemicals for risk assessment in 2013, focus on widely used flame retardants US Environmental Protection Agency (EPA). http://www.epa.gov/oppt/existingchemicals/pubs/2013wpractivities.html. Accessed 10 Mar 2015
Van den Eede N, Dirtu AC, Ali N, Neels H, Covaci A (2012) Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta 89:292–300
Wang J, Ma YJ, Chen SJ, Tian M, Luo XJ, Mai BX (2010) Brominated flame retardants in house dust from e-waste recycling and urban areas in South China: implications on human exposure. Environ Int 36:535–41
Wise S, Poster D, Kucklick J, Keller J, VanderPol S, Sander L, Schantz M (2006) Standard reference materials (SRMs) for determination of organic contaminants in environmental samples. Anal Bioanal Chem 386:1153–1190
Zhou SN, Buchar A, Siddique S, Takser L, Abdelouahab N, Zhu J (2014a) Measurements of selected brominated flame retardants in nursing women: implications for human exposure. Environ Sci Technol 48:8873–80
Zhou SN, Siddique S, Lavoie L, Takser L, Abdelouahab N, Zhu J (2014b) Hexachloronorbornene-based flame retardants in humans: levels in maternal serum and milk. Environ Int 66:11–7
Zhu J, Feng YL, Shoeib M (2007) Detection of dechlorane plus in residential indoor dust in the city of Ottawa, Canada. Environ Sci Technol 41:7694–8
Acknowledgments
We thank Christine Levesque for the sample preparation and inventory, Dave Gardner for assistance with statistical analysis, Water and Earth Science Associates Ltd for participant recruitment and vacuum sampling, Dr. Hongtao Shang and Xinlong Xia for technical assistance and discussion, and Angelina Buchar (Existing Substances Risk Assessment Bureau, Health Canada) and Shabana Siddique (Cancer Care Ontario, Canada) for the internal review of the manuscript. This study was jointly funded by Health Canada and the Chemicals Management Plan (CMP), Government of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Canadian House Dust Study (CHDS) was approved by Health Canada’s Research Ethics Board.
Additional information
Responsible editor: Constantini Samara
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 429 kb)
Rights and permissions
About this article
Cite this article
Fan, X., Kubwabo, C., Rasmussen, P.E. et al. Non-PBDE halogenated flame retardants in Canadian indoor house dust: sampling, analysis, and occurrence. Environ Sci Pollut Res 23, 7998–8007 (2016). https://doi.org/10.1007/s11356-015-5956-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5956-7