Abstract
A new method for the simultaneous determination of 11 synthetic musks and one fragrance compound in house dust was developed. The nitro musks included musk ketone (MK, 4-tert-butyl-3,5-dinitro-2,6-dimethylacetophenone), musk xylene (MX, 1-tert-butyl-3,5-dimethyl-2,4,6-trinitrobenzene), musk ambrette (1-tert-butyl-2-methoxy-4-methyl-3,5-dinitrobenzene) and musk moskene (1,1,3,3,5-pentamethyl-4,6-dinitroindane). The polycyclic musk compounds were 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-(γ)-2-benzopyran (HHCB), 7-acetyl-1,1,3,4,4,6-hexamethyl-1,2,3,4-tetrahydronaphthalene (AHTN), 4-acetyl-1,1-dimethyl-6-tert-butylindane, 6-acetyl-1,1,2,3,3,5-hexamethylindane, 5-acetyl-1,1,2,6-tetramethyl-3-isopropylindane, 6,7-dihydro-1,1,2,3,3-pentamethyl-4(5H)-indanon. The one macrocyclic musk was 1,4-dioxacycloheptadecane-5,17-dione. The bicyclic hydrocarbon fragrance compound (1,2,3,4,5,6,7,8-octahydro-2,3,8,8-tetramethylnaphthalen-2yl)ethan-1-one (OTNE) and HHCB-lactone (4,6,6,7,8,8-hexamethyl-1H,3H,4H,6H,7H, 8H-indeno[5,6-c]pyran-1-one), a degradation product of HHCB, were also analysed. NIST SRM 2781 (domestic sludge) and SRM 2585 (organic contaminants in house dust) were analysed for these target compounds. The method was applied for the analysis of 49 paired samples collected using two vacuum sampling methods: a sample of fresh or “active” dust (FD) collected using a Pullman–Holt vacuum sampler, and a household dust (HD) sample taken from the participants’ vacuum cleaners. Method detection limits and recoveries ranged from 12 to 48 ng/g and 54 to 117 %, respectively. AHTN, HHCB, OTNE and HHCB-lactone were detected in all samples, with median concentrations of 552, 676, 252 and 453 ng/g for FD samples, respectively; and 405, 992, 212 and 492 ng/g for HD samples, respectively. MX and MK were detected with high frequencies but with much lower concentrations. The two sampling methods produced comparable results for the target analytes. Widely scattered concentration levels were observed for target analytes from this set of 49 house dust samples, suggesting a wide variability in Canadian household exposure to synthetic musks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic musks (Table 1) are often divided into three major classes: (1) nitromusks, including musk ketone (MK, 4-tert-butyl-3,5-dinitro-2,6-dimethylacetophenone), musk xylene (MX, 1-tert-butyl-3,5-dimethyl-2,4,6-trinitrobenzene), musk ambrette (MA, 1-tert-butyl-2-methoxy-4-methyl-3,5-dinitrobenzene), and musk moskene (MM, 1,1,3,3,5-pentamethyl-4,6-dinitroindane); (2) polycyclic musk compounds, including HHCB (Galaxolide®, 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-(γ)-2-benzopyran), AHTN (Tonalide®, 7-acetyl-1,1,3,4,4,6-hexamethyl-1,2,3,4-tetrahydronaphthalene), ADBI (Celestolide®, 4-acetyl-1,1-dimethyl-6-tert-butylindane), AHMI (Phantolide®, 6-acetyl-1,1,2,3,3,5-hexamethylindane), ATII (Traseolide®, 5-acetyl-1,1,2,6-tetramethyl-3-isopropylindane), DPMI (Cashmeran®, 6,7-dihydro-1,1,2,3,3-pentamethyl-4(5H)-indanon); and (3) macrocyclic musks such as MT (Musk T®, 1,4-dioxacycloheptadecane-5,17-dione). These compounds, along with OTNE (marketed as Iso E Super®, (1,2,3,4,5,6,7,8-octahydro-2,3,8,8-tetramethylnaphthalen-2yl)ethan-1-one), are widely used in various consumer products such as perfumes, body lotions, soaps, shampoos, shower gels, bubble bath, facial creams, and other cosmetics, air fresheners, detergents, fabric softeners, household cleaners; they can also be used in food additives, cigarettes and fish bait [1, 2]. As a result, synthetic musk compounds are ubiquitous in the environment and have been found in water [3], sediments [4], indoor and outdoor air [5, 6], and house dust [5, 7–9]. They have also been detected in biota [10] and in human biological samples, including adipose tissue [11], breast milk [12], human blood [13], maternal serum and umbilical cord blood [14].
Synthetic musks are structurally and chemically different from the natural musk compounds they are designed to replace. Their physical and chemical properties, such as vapour pressures (V p) and log K ow (Table 1), are more similar to those of man-made chemicals such as polychlorinated biphenyls and organochlorine pesticides, which are known to biomagnify through the food chain [15]. Several musks (e.g., MX, MK, HHCB, ADBI, AHTN, ATII) were able to inhibit efflux (drug) transporters in fish and such effect persisted up to 24–48 h after removal of the musk compounds [16]. HHCB and AHTN have been shown to be weakly oestrogenic in experiments with human cell lines [17] and are weakly anti-oestrogenic in fish [18]. Results from in vitro tests indicated that MX, MK and AHTN could increase the proliferation rate of human MCF-7 breast cancer cells, demonstrating their potential oestrogenic activity [19]. Recent studies demonstrated some adverse effects of synthetic polycyclic musks on the early life stage of male medaka (Oryzias latipes) and potential estrogenic effects upon the addition of AHTN and HHCB, indicative of the induction of hepatic vitellogenin protein synthesis in the livers of male medaka [20].
Although the levels and fates of some synthetic musk compounds in different environmental matrices have been extensively studied, knowledge of their distribution in the indoor environment is very limited. Several musk compounds including AHTN are susceptible to photo-degradation [21], but such a process could be slow in the indoor environment. Consumer products containing synthetic musks are mostly used in the home and thus musk compounds may accumulate in the indoor residential environment. One study found that the concentrations of HHCB and AHTN in house dust were up to 77 and 94 μg/g, respectively, suggesting that house dust could be an indicator of substantial human exposure to these compounds [7].
A few methods for the analysis of synthetic musk compounds in the outdoor environment and aquatic systems have been reported; however, analytical methods for indoor dust are rather limited. Butte reported a method for the analysis of several nitromusks in house dust based on solvent extraction [7]. Without further cleanup, the extracts were analysed by GC coupled with an electron capture detector (ECD) [7]. The ECD detector proved to be selective and sensitive for MX and MK, but it was not suitable for other musks. Fromme et al. [5] reported a similar method based on accelerated solvent extraction and detection by GC/MS. Without sample cleanup, significant interferences could be present during the GC analysis, compromising the analytical results and potentially contaminating the GC and detection systems. Lu et al. [9] developed a method to analyse a few nitromusks and polycyclic musks in house dust based on solvent extraction and sample cleanup by silica gel solid-phase extraction (SPE) prior to GC/MS analysis. Two studies have reported the analysis of selected polycyclic musks in NIST SRM 2781 (domestic sludge) and SRM 2585 (organic contaminants in house dust) using two-stage cleanup by alumina SPE and size exclusion chromatography prior to the detection by GC/MS [22, 23]. Although such intensive sample cleanup could remove most of the matrix interferences, co-eluting fragment ions from other non-targeted compounds can also occur and could compromise the analysis if using a single quadrupole MS detector. The current study was undertaken to develop a method for the simultaneous determination of 11 synthetic musk compounds in indoor house dust, to support the on-going Canadian House Dust Study [24], which would involve analysis of several hundred dust samples. The fragrance compound Iso E Super® and a metabolite of HHCB known as HHCB-lactone, were also analysed. As sample mass is limited for each analysis, a sensitive and robust analytical method was required for this study. The results of this study will contribute to a better understanding of the accumulation of these compounds in indoor house dust and can be used to inform human exposure estimates.
Experimental
Reagents and standards
A standard solution of synthetic musks mixture in cyclohexane (10 mg/L) was purchased from Dr. Ehrenstorfer-Schafers Laboratory (Augsburg, Germany), which included HHCB, AHTN, DPMI, ADBI, AHDI, ATII, MA, MK, MX and MT. OTNE (purity >98 %) was obtained from Toronto Research Chemicals Inc. (Toronto, ON, Canada). Musk moskene (100 mg/L in acetonitrile) and phenanthrene-d10 (100 mg/L in methylene chloride) was purchased from Sigma-Aldrich (Oakville, ON, Canada). HHCB-lactone (purity >97 %) was purchased from Dr. Berset’s group (Water and Soil Protection Laboratory, Bern, Switzerland). Working standard solutions were prepared by mixing individual standard and diluting in hexane to the appropriate concentrations (2 to 300 pg/μL). The internal standard (MX-D15, 100 mg/L in cyclohexane, purity >99 %) was purchased from Dr. Ehrenstorfer-Schafers Laboratory (Augsburg, Germany). Hexane and acetone (GC-grade) were purchased from EMD Chemicals Inc. (Gibbstown, NJ, USA).
Sample collection and processing
Two types of house dust samples were collected from randomly selected, urban, Canadian single family dwellings under the Canadian House Dust Study according to the procedures described previously [25, 26] and briefly summarized here. Household vacuum dust (HD) samples were obtained from the vacuum systems used by the study participants as part of their regular house cleaning routine. Fresh dust (FD) sampling was based on the German standard VDI 4300 [27]. FD samples were collected by Health Canada’s contractor from living areas (bedrooms, living rooms, hallways, offices) using a Pullman Holt (model 102 ASB-12PD) vacuum sampler, in which dust particles follow a direct pathway from the floor to the vacuum bag, without passing through internal mechanical parts, thus avoiding potential contamination. The areas sampled to collect the FD samples consisted of “active” dust and minimized the inclusion of old house dust found in joints and cracks in flooring or in areas where the householder did not vacuum on a regular basis. Wet areas in the home (kitchens, bathrooms, laundry rooms) were avoided to protect the integrity of the FD sample. The home owner was asked not to vacuum the sampling areas for a period of 1 week before the scheduled FD sampling.
Powder-free nitrile gloves were worn at every stage of sample collection and preparation to avoid inadvertent sample contamination. In each home, the vacuum samples were folded and secured with masking tape and placed inside double ZipLoc® bags, for shipment to the laboratory. The collected vacuum samples were air-dried in their bags, which were opened and placed on stainless steel shelving for 24 h in an access-restricted laboratory environment dedicated to drying house dust samples. Pet and human hair and large particles were manually removed by technicians wearing gloves, face masks and protective clothing. The dust was fractionated using stainless steel sieves, and the fraction with particle sizes less than 80 μm was collected for analysis. Sieving was conducted inside a laminar flow hood which was vacuumed thoroughly after each sample. Sieves were cleaned in an ultrasonic bath between samples to eliminate cross-contamination. Sieved dust samples were kept frozen in gas tight amber glass jars (Fisher Scientific, Ottawa, ON) to prevent potential photolysis. The samples were collected in the winter season.
Sonication extraction
Sieved dust (0.05 ± 0.001 g; <80 μm) was accurately weighed and transferred to 10-mL glass centrifuge tubes. Fifty microliters of the internal standard MX-D15 (0.4 ng/μL in hexane) was spiked into the sample and then vortexed for 10 s. Hexane (1.5 mL) was added and the tube was vortexed for about 10 s. The tube was then sonicated for 10 min and vortexed again to re-suspend the dust pellets. This process was repeated three times and then the tube was centrifuged for 5 min at 1,500 rpm. The clear supernatant was transferred to a 5-mL amber vial. The extraction cycle was repeated two more times and the supernatant was combined with the extract from the first extraction cycle. The combined extract was concentrated to ca. 2 mL by a gentle stream of nitrogen at room temperature.
Sample cleanup
An Oasis HLB polypropylene cartridge (6 cc, 500 mg, Waters Inc, Milford, MA) was used for sample cleanup. It was preconditioned with 5 mL of acetone and 2 × 5 mL of hexane. One milliliter of the extract was loaded onto the cartridge. Target analytes were then eluted with 3 × 3.5 mL of hexane at a rate of approximately one drop per second. The eluant was evaporated to just dryness under a gentle stream of nitrogen at room temperature and then reconstituted in 180 μL of hexane. After adding 20 μL of phenanthrene-d10 (0.4 ng/μL in hexane), the solution was mixed well prior to GC/MS analysis. Phenanthrene-d10 was added to check the GC injection variations and the absolute recovery of the internal standard (i.e., MX-D15) spiked in dust samples.
GC/MS analysis
Sample extracts were analysed using a Varian GC (CP-3800) coupled with a Varian Saturn 2200 ion trap mass spectrometer (Palo Alto, CA). The GC column was a DB-5 ms (30 m × 0.25 mm, 0.25 μm film thickness) from J&W Scientific (Folsom, CA). The carrier gas was helium with a constant flow of 1 mL/min. The oven temperature was initially held at 80 °C for 1.5 min, increased to 170 °C at 30 °C/min, held at 170 °C for 20 min, increased to 300 °C at 30 °C/min and then held at 300 °C for 5 min. The injection port temperature was set at 250 °C and the splitless injection volume was 1 μL.
Results and discussion
Method performance and validation
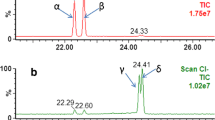
Since synthetic musks are used in many consumer products, the analysts took great care not to use hand lotions, perfumes, or any other products possibly containing synthetic musks during the sample preparation and analysis. Solvents (i.e., acetone and hexane), extraction equipment, and method blanks were routinely checked for the presence of target analytes. Sample cleanup coupled with GC/MS/MS detection provided clean chromatograms for target analytes in dust samples, and all target analytes were well separated (Fig. 1a and b). All the compounds were monitored in multiple reaction mode (MRM) except for MA, MX and MM. These three musk compounds were monitored in μSIS (single ion storage) mode, since MRM did not provide fragment ions of sufficient intensity. The parent ion and collision-induced dissociation (CID) voltage for a given analyte were carefully selected to optimize CID efficiency to produce daughter ion(s) and minimize interference during the analysis. The parent ion for each analyte, the fragment corresponding to the loss of either one methyl group [M-15]+ or one propyl group [M-43]+ from its molecular ion was isolated from other ions and subjected to CID. The MS detection conditions for each target compound are listed in Table 2. In order to maximize recovery of the target analytes and internal standard, four extraction cycles were initially investigated. Results of these trials demonstrated that less than 5 % of the target analytes remained in the third extraction cycle and no analyte was detected in the fourth cycle. Two extraction cycles showed to be adequate for the complete extraction of most of the analytes; however, as a precautionary measure, three extraction cycles were used to increase the extraction efficiency. Target analytes in the samples were identified by the retention time, parent ions, and confirmation ions (Table 2). The calibration curve was linear over a concentration range from 2 to 300 pg/μL for each target analyte in hexane (R 2 > 0.996). The method detection limit (MDL) was determined according to the EPA Regulation 40 CFR part 136 (Appendix B) method, Revision 1.11 [28], whereby the standard deviation associated with seven replicate analyses of solvent-washed dust samples spiked with 3 ng of each target analyte and processed through the entire analytical procedure was multiplied by the Student’s t value of 3.143 (appropriate for a 99 % confidence level). The MDL ranged from 12 ng/g for MX to 48 ng/g for HHCB-lactone (Table 1).
One challenge in the analysis of synthetic musks is the lack of isotope-labelled internal standards. D3-AHTN, which was commercially available, could be used as surrogate recovery standard, but D-H exchange was observed during GC/MS analysis and/or sample preparation process in this study. Such D-H exchange was also reported in other studies [29, 30]. Therefore, MX-D15 was selected as surrogate recovery standard. Another challenge is the lack of a good surrogate for household dust; therefore, the method of standard addition was used to investigate the recovery of target analytes from one pooled dust sample, as previously described by Rudel et al. [10]. Preliminary results showed that the pooled sample contained high levels of OTNE, HHCB, AHTN and HHCB-lactone, but much lower levels of the other analytes. This pooled sample was then extracted with hexane to remove most of these compounds (i.e., OTNE, HHCB, AHTN and HHCB-lactone) prior to spiking with different levels of native target compounds. This pre-extracted sample was divided into seven aliquots. Six aliquots were each spiked with increasing concentrations of the target analytes. All aliquots were processed and analysed according to the described method. The measured amounts were plotted against the spiked values for each target analyte. The resulting recovery functions were linear for all compounds (R 2 > 0.99). The slopes, corresponding to the average recoveries, ranged from 54.0 % for DPMI to 117 % for MK. All reported results for each analyte were recovery-corrected. Relatively low recoveries were obtained for compounds with high volatility such as DPMI and OTNE (Table 1). Therefore, extreme care was taken during sample preparation, particularly during extract concentration.
Precision of the method was evaluated with replicate analysis of a spiked pooled dust sample at three different days. As discussed earlier, the pooled sample was pre-extracted with hexane prior to the spiking with native musk compounds. Five replicates were processed on the same day. Reproducibility was investigated with 15 replicates of the same spiked pooled sample in three different days and ranged from 9.1 % for HHCB to 19.7 % for MA (Table 1). For the analysis of samples, dust samples were processed batch by batch; each batch consisted of one set of calibration standards, one matrix blank and 14 samples, among which two duplicates were also included. Twenty dust samples were analysed in duplicate and the results agreed well for each analyte with an average RSD of 6.1 %, ranging from 0.1 to 29 %.
NIST SRM analysis
The selected synthetic musk compounds were also analysed in NIST SRM 2781 (domestic sludge) and SRM 2585 (organic contaminants in house dust). There are no certified values for synthetic musk compounds in SRM 2781 or SRM 2585. Therefore, the need for certified values to facilitate method development and validation for the determination of synthetic musks in similar matrices becomes imperative. To date, only three studies had reported data on a few synthetic musks in these two SRMs [8, 22, 23]. Based on IUPAC guidelines [31], the z score (Eq. 1) might be useful to evaluate the closeness to a “true” or “assigned” value.
where x is the average of the measurements; X is the “true” value or “assigned” value; and σ is the target standard deviation value. As described in the IUPAC guidelines [31], a fixed performance criterion (e.g., 10%X) is suggested for many environmental measurements. Although the IUPAC does not recommend the classification of z scores, it allows the possibility of classifying scores as follows: satisfactory (|z| ≤ 2), questionable (2 < |z| < 3), and unsatisfactory (|z| ≥ 3). Since there are no certified values for musk compounds in the two SRMs, the “assigned” value in Table 3 for each musk compound detected is the average from four studies including the present one. Most measurements in this study are close to the “assigned” values except ADBI and ATII in SRM 2585 with z scores of −2.1 (Table 3). The deviation of ADBI and ATII from the “assigned” values could be attributed to their low concentrations in SRM 2585. Very low concentration of MM in SRM 2585 was reported by Regueiro et al. [8], but MM was not detected in this study.
Synthetic musks in indoor house dust
Synthetic musks were found in all of the samples analysed. The concentrations levels of the synthetic musks and HHCB-lactone detected in this study varied widely (Table 4). No correlation was found amongst target analytes, suggesting the use of consumer products could be different from home to home. The concentrations for each target compound were not normally distributed according to the Shapiro–Wilk test of normality, and are therefore summarized using median values. The general trend of median concentrations was: HHCB > AHTN > HHCB-lactone > OTNE >> MK ≈ MX (Table 4).
Polycyclic musk fragrances are most commonly used in the fragrance industry. In this study, the predominant polycyclic musks were HHCB and AHTN. They were detected in every sample with median concentrations (in nanograms per gram of dust, range in parentheses) of 676 (39-9000) and 552 (208–1990) for FD samples, and 992 (36-31100) and 405 (91-2360) for HD samples. HHCB and AHTN are on the 2007 OECD (Organisation for Economic Co-operation and Development) list of high production volume chemicals (HPV), indicating these two chemicals were produced or imported at levels greater than 1,000 tonnes per year in at least one member country/region [32]. HHCB was also listed by the USEPA as a high production volume chemical, meaning it was produced or imported in the US in quantities of 450,000 kg or more per year [33]. The categorization of HHCB and AHTN as HPV chemicals correlates well with their use in many consumer products. Recent studies reported that HHCB was added in some consumer products at levels up to 4.99 mg/g and AHTN up to 4.51 mg/g [1, 34]. These usage statistics are consistent with the relatively high concentrations of HHCB and AHTN that were detected in house dust in this study. Three polycyclic musks, ADBI, ATII, and AHMI, were detected at low frequencies and with much lower concentrations, while DPMI were not detected in any samples in this study (Table 4).
OTNE (marketed as Iso E Super®), a fragrance compound which is often used to impart fullness and subtle strength to fragrances, has become one of the most popular fragrance compounds during the last decade. In this study, OTNE was the third most predominant compound detected in most dust samples with median values of 252 (<MDL—12,500 ng/g) and 212 (<MDL—5,620 ng/g) in FD and HD samples, respectively. The GC separation of technical OTNE exhibits a pattern of several isomer peaks (peaks a, b, c, d in Fig. 2a) with similar mass spectra as previously reported by Bester et al. [35, 36]. A comparison of retention times and peak patterns for OTNE from a standard solution and a dust sample is shown in Fig. 2a. The peak pattern in the sample and in the standard is identical. Therefore, only the largest peak (Peak b in Fig. 2a) was selected as a representative for quantitation. To the best of the authors’ knowledge, this study is the first to report OTNE levels in indoor dust.
Nitro-musk compounds MX and MK were detected at high frequencies with 100 and 78 % in FD samples and 98 and 69 % in HD samples, although their median concentrations were on the order of 40–50 ng/g, significantly lower than those of HHCB, AHTN, or OTNE (Table 4), indicating their use is still common, probably in relatively smaller quantity. MA and MM were not detected in any sample in this study. The use of these compounds has been significantly reduced during the past decades due to their potential toxic health effects, persistence in the environment, and accumulation in biota [2]. For example, MA has been shown to be photo-allergenic and neurotoxic [37] and was included in 1995 on the list of “products cosmetics must not contain” [2].
Macrocyclic musk compounds, owing to their outstanding properties (stability to light and alkaline conditions, fixation, and high quality odours), are of high value for the fragrance industry and their use was expected to increase [38]. Musk T (MT, or ethylene brassylate) is one of the most important compounds of this group. Most of the macrocyclic musk compounds have natural origins but their toxicological data are rather limited. Only one study has reported that MT was not estrogenically active in the proliferation of human MCF-7 breast cancer cells [19]. In this study, MT was detected in 8 % of FD samples and 43 % in HD samples. Such a difference could be attributed to the low concentrations in both samples. MT concentrations in the majority of the samples were close to or below the MDL (21 ng/g), with median concentrations below MDL in both FD and HD samples. To the best of the authors’ knowledge, this study is the first to report MT levels in indoor dust. Its retention time and MRM mass spectrum matched well with those of the pure standard (Fig. 2b).
HHCB-lactone is believed to be a degradation product of HHCB and has been detected in different environmental media. For example, high levels of HHCB-lactone in comparison to HHCB were detected in river water [39]. Horri et al. found that more than 70 % of HHCB in two waste water treatment plants could be removed, but HHCB-lactone concentrations were increased following the treatment, suggesting formation of a lactone through the oxidation of HHCB [40]. In addition, HHCB-lactone could also be released directly from the use of household products containing musk compounds, since it has been detected in some household products with concentrations up to 217 μg/g [1]. In this study, HHCB-lactone was detected in every sample with median concentrations of 453 (157-2060) ng/g and 492 (76-2190) ng/g for FD and HD samples, respectively.
Synthetic musks are ubiquitous in the environment; however, data describing their presence in the indoor environment are scarce. A few studies have reported the occurrence of synthetic musks in indoor house dust from European countries and China [5, 7–9]. The wide variations of musk concentrations from these studies suggest different uses of consumer products containing musk compounds. Major indoor sources of synthetic musks could be the use of liquid consumer products and spray of aerosols (e.g., air freshener). Direct leaching from fabric and textiles could also be another minor contributor to indoor musk residues. Once released in the indoor air, synthetic musks may partition from the gas phase to airborne particulates and eventually settle down to indoor dust, since several major musk compounds (e.g., HHCB, AHTN, MX, MK) have been detected in indoor air [41, 42].
Strong positive correlations are observed between the two sampling methods (i.e., HD vs FD) for target musk compounds; correlations for HHCB, AHTN, OTNE, MX, MK, ATII and HHCB-Lactone are significant based on Spearman’s rank correlation coefficients (Table 4). Differences between the HD and the FD results may be attributed to the longer sampling period represented by the household vacuum sample (HD) and the larger area within the home represented by the HD sample. Since the FD sample is known to be collected 1 week after normal cleaning, the HD sample is anticipated to represent a longer accumulation period. The HD sample, which represents regular house cleaning activity, and generally includes all rooms in the house, appears to be advantageous as residues from personal care products being used in the bathrooms, kitchens and laundry rooms are more likely to be captured. In contrast, the FD protocol, which avoids all damp rooms in the home, excludes these areas. In addition, FD sample represents one vacuuming episode only. However, lack of uniformity in the household vacuum sampling device might introduce greater variability compared to the FD method, as the HD dust was collected from various models of canister vacuums, central vacuum systems and traditional bag vacuum cleaners. In addition, scented vacuum bags may be used, which could then overestimate the concentrations of some musk compounds in dust samples.
Conclusion
A simple and robust method was developed for the simultaneous measurement of 11 synthetic musk compounds, OTNE and HHCB-lactone in indoor house dust. The method was sensitive, with good recovery and precision for each analyte. High detection frequencies of HHCB (Galaxolide®), AHTN (Tonalide®) and OTNE (Iso E Super®) with higher concentrations confirmed their wide applications in consumer products. Musk xylene and musk ketone were also detected at high frequencies but at much lower concentrations, suggesting they may still be in use but in much smaller quantities or at lower frequency. These data support the fact that indoor dust may represent an important source to be considered in the assessment of human exposure to synthetic musk compounds.
References
Reiner JL, Kannan K (2006) Chemosphere 62:867–873
OSPAR (2004) Background document on musk xylene and other musks, Oslo and Paris Convention for the Protection of the Marine Environment of the North-East Atlantic. www.ospar.org. Accessed 10 Feb 2012
Reiner JL, Berset JD, Kannan K (2007) Arch Environ Contam Toxicol 52:451–457
Peck AM, Linebaugh EK, Hornbuckle KC (2006) Environ Sci Technol 40:5629–5635
Fromme H, Lahrz T, Piloty M, Gebhart H, Oddoy A, Ruden H (2004) Indoor Air 14:188–195
Peck AM (2006) Anal Bioanal Chem 386:907–939
Butte W (2004) In: Rimkus GG (ed) The handbook of environmental chemistry, vol 3. Springer, Berlin
Regueiro J, Llompart M, Garcia-Jares C, Cela R (2007) J Chromatogr A 1174:112–124
Lu Y, Yuan T, Yun SH, Wang W, Kannan K (2011) Arch Environ Contam Toxicol 60:182–189
Rudel H, Bohmer W, Schroter-Kermani C (2006) J Environ Monit 8:812–823
Wan Y, Wei Q, Hu J, Jin X, Zhang Z, Zhen H, Liu J (2007) Environ Sci Technol 41:424–430
Reiner JL, Wong CM, Arcaro KF, Kannan K (2007) Environ Sci Technol 41:3815–3820
Hutter HP, Wallner P, Hartl W, Uhl M, Lorbeer G, Gminski R, Mersch-Sundermann V, Kundi M (2010) Int J Hyg Environ Health 213:124–130
Kang CS, Lee JH, Kim SK, Lee KT, Lee JS, Park PS, Yun SH, Kannan K, Yoo YW, Ha JY, Lee SW (2010) Chemosphere 80:116–122
Peck AM, Hornbuckle KC (2004) Environ Sci Technol 38:367–372
Luckenbach T, Epel D (2005) Environ Health Perspect 113:17–24
Seinen W, Lemmen JG, Pieters RH, Verbruggen EM, van der Burg B (1999) Toxicol Lett 111:161–168
Schreurs RH, Legler J, Artola-Garicano E, Sinnige TL, Lanser PH, Seinen W, Van der Burg B (2004) Environ Sci Technol 38:997–1002
Bitsch N, Dudas C, Körner W, Failing K, Biselli S, Rimkus G, Brunn H (2002) Arch Environ Contam Toxicol 43:0257–0264
Yamauchi R, Ishibashi H, Hirano M, Mori T, Kim JW, Arizono K (2008) Aquat Toxicol 90:261–268
Buerge IJ, Buser HR, Muller MD, Poiger T (2003) Environ Sci Technol 37:5636–5644
Reiner JL, Schantz MM (2010) In: Proceedings of Dioxin 2010, San Antonio, TX
Peck AM, Kucklick JR, Schantz MM (2007) Anal Bioanal Chem 387:2381–2388
Health Canada (2012) The Canadian House Dust Study. www.hc-sc.gc.ca/ewh-semt/contaminants/dust-poussiere-eng.php. Accessed 2 Mar 2012
Rasmussen PE, Beauchemin S, Chénier M, Levesque C, MacLean LCW, Marro L, Jones-Otazo H, Petrovic S, McDonald LT, Gardner HD (2011) Environ Sci Technol 45:4959–4965
Fan X, Kubwabo C, Rasmussen P, Jones-Otazo H (2010) J Environ Monit 12:1891–1897
Verein deutscher Ingenieure (VDI) (2001) German Protocol VDI 4300, Part 8 Measurement of indoor air - sampling of house dust
USEPA (1986) Regulation 40 CFR Part 136 (Appendix B)—Definition and procedure for the determination of the method detection limit—Revision 1.11. www.nmenv.state.nm.us/swqb/Projects/NPDES/40cfr136.pdf. Accessed 15 Feb 2012
Bester K (2009) J Chromatogr A 1216:470–480
Osemwengie LI (2006) J Environ Monit 8:897–903
Thompson M, Ellison SR, Roger W (2006) Pure Appl Chem 78:145–196
OECD (2009) The 2007 OECD list of high production volume chemicals by Organisation for Economic Co-operation and Developmen. www.oecd.org/dataoecd/32/9/43947965.pdf. Accessed 25 Feb 2012
USEPA (2007) High production volume (HPV) chemical data. www.epa.gov/hpv/pubs/general/hpvchemdata.htm. Accessed 5 Mar 2012
Roosens L, Covaci A, Neels H (2007) Chemosphere 69:1540–1547
Bester K, Huffmeyer N, Schaub E, Klasmeier J (2008) Chemosphere 73:1366–1372
Bester K, Klasmeier J, Kupper T (2008) Chemosphere 71:2003–2010
Kanchan PA, Shenoi SD, Balachandran C (2002) Indian J Dermatol Venereol Leprol 68:86–87
Sommer C (2004) In: Rimkus GG (ed) The handbook of environmental chemistry, vol. 3. Springer, Berlin
Bester K (2004) Chemosphere 57:863–870
Horii Y, Reiner JL, Loganathan BG, Senthil Kumar K, Sajwan K, Kannan K (2007) Chemosphere 68:2011–2020
Chen D, Zeng X, Sheng Y, Bi X, Gui H, Sheng G, Fu J (2007) Chemosphere 66:252–258
Regueiro J, Garcia-Jares C, Llompart M, Lamas JP, Cela R (2009) J Chromatogr A 1216:2805–2815
Chen X, Pauly U, Rehfus S, Bester K (2009) Chemosphere 76:1094–1101
USEPA and Syracuse Research Corporation (SRC) (2009) Estimation Program Interface (EPI) Suite. www.epa.gov/oppt/exposure/pubs/episuite.htm. Accessed 18 Nov 2011
Acknowledgements
We would like to thank Ivana Kosarac, Kaela Lalonde and Genevieve Grenier for technical advice and discussion. We also want to acknowledge Water and Earth Science Associates Ltd for participant recruitment and vacuum sampling; Christine Levesque, Tessa Roselli, and Monique Lanouette for sample preparation and inventory; and Sanya Petrovic and Lauren McDonald for valuable assistance. The study protocol was approved by Health Canada’s Research Ethics Board.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubwabo, C., Fan, X., Rasmussen, P.E. et al. Determination of synthetic musk compounds in indoor house dust by gas chromatography–ion trap mass spectrometry. Anal Bioanal Chem 404, 467–477 (2012). https://doi.org/10.1007/s00216-012-6124-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6124-2