Abstract
In the present study, an analytical method has been developed and validated for the simultaneous detection and quantification of 19 PFRs (14 legacy organophosphorus flame retardants (PFRs) and 5 emerging PFRs (ePFRs)) and 20 plasticizers (7 legacy plasticizers (LPs) and 13 alternative plasticizers (APs)). Sample preparation was based on the combination of previously validated analytical protocols including ultrasonic extraction and Florisil fractionation/cleanup. The analysis was performed by using liquid chromatography–tandem mass spectrometry (LC-MS/MS) for all targeted compounds, except for bis (2-ethylhexyl) phthalate (DEHP) and bis (2-ethylhexyl) terephthalate (DEHT), for which the separation of the isomers resulted in more favorable gas chromatography–electron ionization–mass spectrometry (GC-EI-MS). The new method was in-house validated by applying two levels of fortification in dust. The achieved linearity (R2) ranged between 0.993 and 0.999. Limits of detection and quantification (LODs and LOQs) ranged between 1 and 265 ng/g and between 1 and 870 ng/g for all analytes, respectively, except for DEHP and DEHT, for which relatively higher LODs (665 and 1100 ng/g, respectively) and LOQs (2100 and 3500 ng/g, respectively) were observed. Accuracy ranged between 75 and 125% for most of the targeted analytes, and repeatability was good with relative standard deviation (RSD) < 15% for most compounds. Finally, the method was applied for the determination and quantification of the targeted chemicals in house dust samples (n = 10) from the megacity of Guangzhou (China). Median values ranged from 3 to 210 ng/g for PFRs, from 4 to 165 ng/g for ePFRs, from 30 to 100,000 ng/g for LPs, and from 6 to 34,000 ng/g for APs. Main contributors to the total contamination were LPs 63% and APs 37% in total plasticizers, whereas PFRs and ePFRs contributed 90% and 10% in total flame retardants.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, the quality of indoor environment has been acknowledged as a hot issue due to the time humans spend indoors, leading to a fast increase to the number of research studies focused on this topic [1, 2]. Indoor dust is a contributor to indoor pollution as it acts as a repository material for different groups of compounds, including semi-volatile organic compounds (SVOCs) [3, 4]. Humans, and especially sensitive groups such as children, are exposed to these compounds via inhalation, accidental ingestion of dust, and dermal absorption [3, 5]. Since there are strong indications of adverse health effects due to the human exposure to SVOCs, the importance of monitoring indoor dust contamination has been highlighted [6, 7].
Major groups of SVOCs are flame retardants and plasticizers and their presence in indoor dust is constant [3, 8].
Organophosphorus flame retardants (PFRs), legacy and emerging (ePFRs), are chemical compounds that have been incorporated to products such as furniture foams, textiles, construction materials, and different kinds of electronic devices to reduce the risk of fire [9]. They are major replacements for certain brominated flame retardants (BFRs), such as polybrominated diphenyl ethers (PBDEs), after bans and restrictions of the latter [10]. They are not chemically bonded to the materials and they can easily be released from products into the indoor environment, accumulating in dust and contributing to human exposure. Recent human biomonitoring studies linked the intake of contaminants to the adverse health effects (allergies, neuro- and reproductive toxicity, potential human carcinogenicity etc.) [11,12,13,14].
Plasticizers are a category of compounds applied as additives into polymers to provide special features of durability, elasticity, and flexibility to the products [13, 14]. Phthalic esters, referred in the present study as legacy phthalates (LPs), are a major group of plasticizers. Their replacements are alternative plasticizers (APs) that have been introduced lately into the market due to suspected adverse effects of LPs to human and direct links to asthma events in children [15, 16]. Similarly to PFRs, plasticizers can easily be transferred from the consumer products to the indoor environment [17].
Consequently, there is a need to develop appropriate analytical methods that will allow a rapid, simultaneous, sensitive, and selective determination and quantification of these compounds in dust, combined with a low cost and time consumption. Therefore, the present study aims at the simultaneous analysis of flame retardants and plasticizers, two major groups of indoor contaminants in dust. Several studies have already reported on the analysis of these compounds separately using gas chromatography (GC) coupled to MS or liquid chromatography (LC) coupled to MS/MS [18,19,20,21,22]. Specifically, ePFRs are suitable only to be analyzed using LC method and TBOEP showed also ambiguous analytical behavior during GC-MS analysis [21]. Recently study has reported the simultaneous analysis of PFR and ePFRs using LC-MS/MS [23,24,25,26,27,28]. However, to the best of our knowledge, this is the first attempt to combine flame retardants (i.e., PFRs and ePFRs) and plasticizers (i.e., LPs and APs) in a single method, by applying a single sample treatment protocol and using LC-MS/MS for the analysis. The aims of our study were (i) to develop and validate a method for the simultaneous quantification of the targeted analytes by LC-MS/MS, and (ii) to test its applicability to the analysis of PFRs and plasticizers in household dust (n = 10) sampled in Guangzhou, China.
Materials and methods
Chemicals and reagents
Labeled triphenyl phosphate (TPHP-d15), tris(1,3-dichloro-2-propyl)phosphate (TDCIPP-d15), tris(2-butoxyethyl) phosphate (TBOEP-d6), and tris(2-chloroethyl) phosphate (TCEP-d12) with isotopic purity of 98% (custom synthesized) were purchased from Dr. Vladimir Belov, Max Planck Institute for Biophysical Chemistry, Göttingen, Germany. Labeled dibenzyl phthalate (DBzP-d4) was purchased from AccuStandard (New Heaven, CT, USA); labeled bis (2-ethylhexyl) phthalate (DEHP-d4) and labeled di-N-butyl phthalate (DNBP-d4) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were used as internal standards (ISs) for LP and AP analysis. Triamyl phosphate (TAP) was used as recovery standard (RS) and was purchased from TCI Europe (Zwijndrecht, Belgium). Standards of TIBP, TNBP, TDCIPP, TCIPP, TEHP, TCEP, TOTP, TPTP, TMTP, EHDPHP, TEP, TPHP, TBOEP, and TBuPHP were purchased from Chiron AS (Trondheim, Norway). Standards of TDBPP, V6, RDP, BDP, iDDPHP, DMP, DEP, DNBP, DIBP, BBP, DEHP, DPP, DBA, ATEC, DBS, ATBC, DEHA, DCPC, BTHC, DEHT, THTM, TOTM, DINCH, DINP, and DIDP were purchased from AccuStandard (New Heaven, CT, USA). Indoor dust standard reference material SRM 2585 was purchased from the US National Institute of Standards and Technology (NIST; Gaithersburg, MD, USA). Florisil® ENVI (500 mg, 3 mL) cartridges were purchased from Supelco (Bellefonte, PA, USA). Centrifugal filters (modified Nylon membrane) of 0.45 μm were purchased from VWR™ (North America). All solvents were of chromatography grade. n-Hexane (n-Hex) was purchased from Acros Organics (Belgium); ethyl acetate (EtAc), dichloromethane (DCM), acetone, and toluene were purchased from Merck (Germany); and methanol (MeOH) was purchased from Fisher Scientific (UK). Detailed information regarding the analytes is provided in Table S1 in the Electronic Supplementary Material (ESM).
Sample collection
Two house dust samples, one collected from the USA (New England, 2015) and the other from an e-waste recycling site, South China, in August 2017, were chosen as representative samples for the method development and validation [19]. Once validated, the method was used for the determination and quantification of 10 indoor dust samples collected from the interior of 10 homes in the city of Guangzhou (China) between July and September 2017. Dust sampling was conducted using clean brushes onto bare floors of the living rooms, according to a protocol previously described [7, 21]. The brushes were thoroughly pre-cleaned with ethanol between each sampling in order to eliminate the possibility of cross-contamination. After collection, the samples were stored in aluminum foil, sealed in ziplock plastic bags, and transported to the lab. They were stored at − 20 °C pending analysis and then sieved (500 μm) in room temperature prior to extraction.
Extraction and cleanup
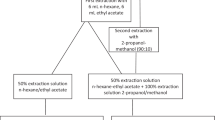
For all targeted analytes, the applied analytical method was based on a combination of previously published protocols [22, 23]. Dust aliquots of 20 mg were weighted in pre-cleaned glass tubes (solvent washed and dried at 400 °C oven) and spiked with TCEP-d12, TDCIPP-d15, TPhP-d15, and TBOEP-d6 (each at 100 ng), and DBzP-d4, DEHP-d4, and DNBP-d4 (each at 500 ng). Samples were extracted using 2.5 mL of n-Hex/acetone mixture (3:1 v/v) and 0.5 mL toluene by a combination of vortexing (1 min) and ultrasonication (5 min) repeated for two cycles by adding fresh solvents. Extracts were centrifuged for 3 min at 3000 rpm. Supernatants were pooled into pre-clean glass tubes and evaporated to near dryness under a gentle nitrogen stream. They were redissolved in 1 mL of n-Hex/toluene (1:1 v/v) and vortexed (1 min). Florisil® ENVI cartridges (500 mg, 3 mL) were washed with 4 mL acetone, 6 mL EtAc, and 6 mL n-Hex. The extracts were quantitatively transferred onto the cartridges, and fractionation was achieved by eluting the first fraction (F1) with 12 mL n-hexane/DCM (4:1 v/v) and the second fraction (F2) with 10 mL EtAc. F1 was discarded and F2, containing the targeted compounds, was evaporated until 4 mL under a gentle nitrogen stream. Then, elution with 8 mL acetone was followed up for extracting V6 from the Florisil cartridges. F2 was evaporated near dryness under a gentle nitrogen stream. After evaporation, the extract of F2 was dissolved in 50 μL of MeOH and 50 μL of RS and vortexed (30 s). A volume of 15 μL of the final aliquot was transferred to an amber injection vial with the addition of 135 μL of EtAc for the separation and quantitative analysis of DEHP and DEHT by GC-EI-MS. The rest of the aliquot was filtered in 0.45-μm centrifugal filters (9000 rpm, 3 min), previously tested for their suitability, and transferred to a vial for LC-MS/MS analysis.
Instrumental analysis
LC-MS/MS analysis
Agilent 1200 Infinity liquid chromatography (LC) system (Agilent Technologies, Santa Clara, CA, USA) coupled to an Agilent 6410 Triple Quadrupole mass spectrometer (MS) was used. The mobile phases were as follows: A, ultrapure water 5 mM ammonium formate; B, MeOH 5 mM ammonium formate. The gradient of separation was 30–70% B in 5 min, 70–100% B in 20 min, hold for 5 min, and returning to the initial conditions from 25.10 min until 35 min. The total duration of each injection was 35 min and the flow rate 0.25 mL/min. Kinetex Biphenyl column (100 × 2.1 mm, 2.6 μm) was used at 40 °C working temperature. Source parameters were set as follows; gas temperature at 350 °C, gas flow at 10 mL/min, nebulizer gas at 40 psi, and capillary voltage at 4000 V. Positive electrospray ionization was applied for all target analytes. The Agilent Mass Hunter software B.06.00 was used for the data analysis. Detailed chromatographic information is reported in ESM Table S1.
GC-EI-MS analysis
DEHP and DEHT were analyzed using Agilent GC coupled to an Agilent 5973 MS operated in electron ionization mode (EI). A GC HT-8 column (25 m × 0.22 mm, 0.25 μm), electronic pressure control, and a programmable-temperature vaporizer (PTV) inlet were used. The injection temperature was 90 °C, hold time 0.04 min, ramped at 700 °C/min to 300 °C, hold time 25 min. Injection volume was 1 μL and was performed under a pressure of 14.36 psi until 1.25 min and purge flow to split vent of 50 mL/min after 1.25 min, ramped at 30 °C/min to 250 °C, hold time 1.5 min, ramped at 10 °C/min to 310 °C, hold time 7 min. Carrier gas was helium with a flow rate of 1.0 mL/min until 28 min, and then increased to 1.5 mL/min. The mass spectrometer was run in selected ion monitoring (SIM) mode with 2 characteristic ions acquired for each analyte and for the IS DEHP-d4 (details are reported in ESM Table S1).
Uncertainty
The uncertainty (U) of the method was calculated based the measurements during the method validation. The applied equations were derived from Poma et al. [22] and were the following (Eq. (1)):
where Uc is the combined standard uncertainty and k is the coverage factor equal to 2 for level of confidence 95%.
The combined uncertainty Uc is the combination of all the contributing uncertainties and in this study were involving accuracy and repeatability (Eq. (2)):
where Ur is the uncertainty of the repeatability and expressed as the standard deviation of the measurements and Ut is the uncertainty of accuracy. In cases that SRM is used, Ut is calculated using Eq. (3):
where St is the standard deviation of the analyzed replicates, nt the square root of the number of the analyzed replicates, and the USRM the uncertainty of SRM analysis.
Results and discussion
Method development
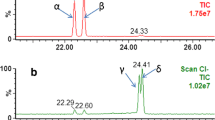
Standards of individual native compounds were used to set optimal values of multiple reaction monitoring (MRM), SIM m/z, and quantitative and qualitative ions/transitions with the corresponding collision energy and fragmentor voltage (ESM Table S1). The source parameters of gas temperature, gas flow, nebulizer, and capillary voltage were also optimized pursuing the maximum response per each compound. For the optimization of the chromatographic separation, different mobile phases were tested such as MeOH, acetonitrile, and water with ammonium formate. Various analytical columns were also tested, such as Kinetex Biphenyl (100 × 2.1 mm, 2.6 μm), Luna C18 (150 × 2 mm, 3 μm), and Alltima HP C18 (100 × 2.1 mm, 3 μm) at temperatures of 25 °C and 40 °C. The most efficient separation was obtained using MeOH 5 mM ammonium formate (B) and H2O 5 mM ammonium formate (A), and the best gradient in terms of separation and time duration was applied (ESM Table S2). The optimal column was Kinetex Biphenyl (100 × 2.1 mm, 2.6 μm) at 40 °C, generating a higher signal for each analyte. The separation of the isomers DEHP and DEHT was not achieved by LC-MS/MS, and the sample preparation protocol was adapted accordingly in order to inject a sub-aliquot into GC-EI-MS for further analysis (Fig. 1). Figure 2 shows chromatograms of the targeted analyte standard mix in LC-MS/MS and GC-EI-MS.
Six various protocols were tested for sample treatment. The parameters of extraction and elution solvent were optimized based on different proportions in mixes n-Hex/acetone and n-Hex/DCM, respectively. Procedural blanks and dust samples were spiked with the internal standards (ISs), DBzP-d4 for LPs and APs and a mixture of TCEP-d12, TDCIPP-d15, TPHP-d15, and TBOEP-d6 for PFRs and ePFRs. The purpose was to test the maximum extraction efficiency, combined with removal of interferences by discarding F1 (ESM Table S3). The optimal sample protocol is described in “Extraction and cleanup,” and the selection was based on best IS recoveries (%) and lowest RSDs of the tested protocols (ESM Fig. S1).
Method in-house validation
Linearity
Four calibration curves were prepared for the targeted analytes based on standard mixture divisions: PFR/ePFR standard mix (TCEP, TEHP, TBOEP, TPHP, EHDPHP, TCIPP, TDCIPP, TIBP, TNBP, TOTP, TPTP, TMTP, TEP, TBuPHP, V6, iDDPHP, BDP, RDP, TDBPP); LP standard mix (DMP, DEP, DNBP, DIBP, BBP, DPP); and AP standard mix (DIBA, ATEC, DBS, ATBC, DEHA, DCPC, BTHC, THTM, TOTM). A separate standard mix of DIDP, DINP, and DINCH was created due to the special chromatographic behavior of these compounds (broader peaks) (ESM Fig. S2) and a standard mix of DEHP and DEHT for use in GC-EI-MS. The ranges of the calibration curves were adapted accordingly to the expected concentration in dust sample (Table 1). Calibration curves were formed by plotting the area ratio analyte divided by IS against the concentration ratio of each analyte to the corresponding IS. Calibration curves were best fitted to a quadratic model for all mixtures, except for PFRs/ePFRs for which the best model was linear. The linearity was estimated by the R2. All targeted analytes showed a good correlation within the tested intervals with coefficients of determination higher or equal to 0.993 (Table 1).
Limits of detection–quantification
Three procedural blanks were analyzed for the limits of quantification (LOQ) estimation (Table 1). Limits of detection (LODs) were estimated from the lowest calibration point, giving a signal/noise ratio of 3 (S/N = 3). When the targeted analyte concentrations in the blanks were negligible, LOQs were calculated by the instrument performance (S/N = 10). In this case, the lowest calibration point was used as LOQ. For the analytes detected in the blanks, the LOQs were based on the standard deviation of the blanks, and a value equal to 3*SD of the blank concentration was used as a cutoff value. Determined LOQ range was 4–98 ng/g for PFRs, 4.5–59 ng/g for ePFRs, 24–873 ng/g for LPs except for DEHP, for which it was 2190 ng/g, and 15–764 ng/g for APs except for DEHT, for which it was 3600 ng/g (Table 1). The expected relatively higher LOQs for DEHP and DEHT were most probably due to the lower instrumental sensitivity for these compounds (GC-EI-MS) and their ubiquitous presence in the procedural blanks.
Accuracy
The certified reference material SRM 2585 was analyzed in triplicate to evaluate the accuracy of our method for the quantification of the targeted PFRs. This SRM has indicative values for certain PFRs, but LPs and APs have been analyzed and reported by other studies in the literature [20, 25, 26] (ESM Table S5). Due to the lack of information for the rest of the compounds, a real dust sample collected from an e-waste recycling site (South China) was used after fortification (test sample). This sample was divided in 9 aliquots; three of them were spiked with low-level (LL) mass with the targeted analytes, three with high-level (HL) mass, and three non-spiked aliquots were used as controls. Three solvent blanks were included to control the laboratory background contamination. Eventually, the measured concentrations in solvent blanks and controls were subtracted from the concentrations of the spiked replicates. Accuracy was calculated and reported per each analyte in Table 1, ranging from 70 to 140% for all compounds except for TPHP (61%), TEP (33%), iDDPHP (52%), TDBPP (48%), DMP (59%), and THTM (143%) (ESM Figs. S3 and S4).
Intra-day repeatability
The intra-day repeatability was calculated as the relative standard deviation (RSD) of three replicate analyses within 1 day and under repeatable conditions. Three aliquots of the same dust sample (test sample) and three solvent blanks were spiked with HL and LL mass of the targeted analytes and used for the validation. RSD for LL mass was < 15% in 92% of the total analytes and 90% for the HL. RSD values were 21% and 24% for TEP and BDP in LL mass and 23%, 22%, 49%, and 22% for TIBP, TEP, DMP, and TOTM in HL mass.
Method uncertainty
The expanded uncertainty, U, was calculated for all the targeted analytes, and the mean expanded uncertainty (Umean) of the two levels of fortification was reported (Table 1). The Umean values were in the range between 4 and 40% for PFRs, between 6 and 114% for ePFRs, between 6 and 14% for LPs, and between 5 and 34% for APs. For the GC-analyzed compounds DEHP and DEHT, the Umean was 16% and 28%, respectively.
Method applicability
The validated analytical method was applied for the quantification of the targeted compounds in 10 indoor household dust samples collected from Guangzhou (South China). The quality assurance and quality control were performed by analyzing SRM 2585 for the targeted compounds, estimating the recoveries of ISs and analyzing 3 procedural blank samples in the same batch. Mean recoveries for the ISs were 86 ± 14% (TCEP-d12), 86 ± 13% (TDCIPP-d15), 94 ± 11% (TPhP-d15), 106 ± 9% (TBOEP-d6), 98 ± 10% (DBzP-d4), 90 ± 31% (DNBP-d4), and 89 ± 20% (DEHP-d4). Concentration values below the LOQs were treated as LOQ*f during descriptive statistics, where f is the detection frequency of the compound above the LOQ in the samples [25]. For the analyzed dust samples, 26 out of 39 target analytes were found in concentrations above LOQ (ESM Table S4), while DEP, DPP, DIBA, DBS, TEP, TOTP, TMTP, iDDPHP, and TDBPP were not detected in any sample.
Table 2 reports the descriptive statistics related to 10 dust samples from Guangzhou, and Table S4 and Fig. S6 in the ESM present the concentration levels and contribution patterns of target analytes detected in samples, respectively. DEHP (48%), DINP (18%), DIDP (13%), and DNBP (12%) were the main contributors in dust samples, whereas the rest of the compounds are much lower (5%) (Fig. 3).
Among the 14 PFRs tested, 10 compounds were detected in the indoor dust samples from Guangzhou (Table 2), and the mean concentrations of each compound were in the range between 6.4 ng/g (TNBP) and 797 ng/g (TCIPP), which were lower than those recently reported in indoor home dust from other locations such as Belgium, Canada, China, Japan, and the USA [27–31]. Among the 10 detected PFRs, TCIPP (31.9 ± 11.7%) was found as the most abundant compound, followed by TCEP (17.2 ± 9.4%) and TEHP (14.1 ± 8.0%) (ESM Fig. S5). Additionally, three ePFRs (i.e., V6, RDP, and BDP) were also frequently detected (detection frequency = 40–80%) in the indoor dust samples from Guangzhou, with mean concentrations as 31, 23, and 142 ng/g for V6, RDP, and BDP, respectively (Table 2). BDP dominated over the other two ePFRs in these dust samples (ESM Fig. S5), whereas its concentrations were generally 1–2 orders of magnitude lower than those in a previous study [28].
DEHP (78.0 ± 13.1%) for LPs and DINP (53.6 ± 17.6%) for APs were the dominant plasticizers found in the dust samples (ESM Fig. S5); with determined mean concentrations ranged from 32 ng/g (DMP) to 100,112 ng/g (DEHP) for LPs and from 43 ng/g (CDPHP) to 36,460 ng/g (DINP) for APs, respectively (Table 2). The concentrations for plasticizers (LPs and APs) found in this study were lower than those in home dust from Belgium, Netherlands, and Ireland [22].
The contribution of the main groups was estimated based on means of sum (i) only among plasticizers, (ii) only among PFRs, and (iii) for the total presence (Fig. 3). LPs were the dominant group of plasticizers contributing 63% in dust, followed by APs at 37% whereas for the flame retardants, contribution of PFRs and ePFRs was 90% and 10%, respectively. One possible explanation for the extremely low percentage of PFRs could be the basic level of indoor equipment in the sampled homes lacking of commodities that usually contain PFRs. Figure 4 represents the contribution per individual analyte in the two groups of compounds. For plasticizers, dominant compounds were DEHP (49%), followed by DINP (18%) > DIDP (13%) > DNBP (12%), whereas the rest of the compounds were found in percentages less than 10%. On the other hand, PFRs were more equally distributed, with main co-main contributors to be TCIPP (23%)> TCEP = BDP (14%) > EHDPHP (11%) > TEHP (10%), and the rest of the compounds were less than 10%. These results suggest that the concentrations of PFRs/ePFRs in indoor dust tend to be lower than the concentrations of plasticizers. Similar results were found in previous studies where the measured plasticizers were more abundant than PFRs and typically showed tenfold higher total concentrations [13, 25]. Statistical correlations between concentration levels and indoor equipment (furniture and electronics) were not applicable due to the low sample size and the eliminated indoor equipment, and further study is needed for a comprehensive evaluation on the human exposure of these chemicals in indoor environments.
Conclusions
A reliable analytical method was developed for the simultaneous analysis and quantification of 39 PFRs, ePFRs, LPs, and APs in indoor dust. LC-MS/MS was used for the quantification of all targeted analytes except for DEHP and DEHT for which GC-EI-MS was proved to be more suitable. The application of liquid chromatography enhanced the sensitivity of the analysis and solved certain analytical problems (e.g., ambiguous analytical behavior of TBOEP during GC-EI-MS analysis). Method validation proved that accuracy, repeatability, LODs, and LOQs were in the acceptable range for most analytes. The application of the method to indoor dust samples confirmed the feasibility and robustness of the method. The results based on these 10 samples showed that DEHP, DINP, DIDP, and DNBP were the dominant compounds in indoor dust.
References
Mujan I, Munćan V, Ružić D, Kljajić M, Anđelković AS. Influence of indoor environmental quality on human health and productivity - a review. J Clean Prod. 2019;217:646–57. https://doi.org/10.1016/j.jclepro.2019.01.307.
Bu Z, Mmereki D, Wang J, Dong C. Exposure to commonly-used phthalates and the associated health risks in indoor environment of urban China. Sci Total Environ. 2019;658:843–53. https://doi.org/10.1016/j.scitotenv.2018.12.260.
Harrad S, Goosey E, Desborough J, Abdallah MAE, Roosens L, Covaci A. Dust from U.K. primary school classrooms and daycare centers: the significance of dust as a pathway of exposure of young U.K. children to brominated flame retardants and polychlorinated biphenyls. Environ Sci Technol. 2010;44:4198–202.
Little JC, Weschler CJ, Nazaro WW, Liu Z, Hubal EAC. Rapid methods to estimate potential exposure to semivolatile organic compounds in the indoor environment. Environ Sci Technol. 2012:11171–8. https://doi.org/10.1021/es301088a.
Mercier F, Glorennec P, Thomas O, Le Bot B. Organic contamination of settled house dust, a review for exposure assessment purposes. Environ Sci Technol. 2011;45:6716–27. https://doi.org/10.1021/es200925h.
Calafat AM, Ye X, Valentin-blasini L, Li Z, Mortensen ME. Pre-school aged children: a pilot study. Int J Hyg Environ Health. 2017;220:55–63. https://doi.org/10.1016/j.ijheh.2016.10.008.
Zheng X, Xu F, Chen K, Zeng Y, Luo X, Chen S, et al. Flame retardants and organochlorines in indoor dust from several e-waste recycling sites in South China: composition variations and implications for human exposure. Environ Int. 2015;78:1–7. https://doi.org/10.1016/j.envint.2015.02.006.
Bui TT, Giovanoulis G, Palm A, Magnér J, Cousins IT, Wit CA De. Science of the Total Environment Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci Total Environ 2016;541:451–467. doi:https://doi.org/10.1016/j.scitotenv.2015.09.036.
Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Chemosphere Flame retardant associations between children’s handwipes and house dust. Chemosphere 2014;116:54–60. doi:https://doi.org/10.1016/j.chemosphere.2013.12.100.
Abdallah MA, Covaci A. Organophosphate flame retardants in indoor dust from Egypt: implications for human exposure. Environ Sci Technol. 2014;48:4782–9. https://doi.org/10.1021/es501078s.
Tokumura M, Hatayama R, Tatsu K, Naito T. Organophosphate flame retardants in the indoor air and dust in cars in Japan. Environ Monit Assess. 2017;189:1–11. https://doi.org/10.1007/s10661-016-5725-1.
Naito H, Cho K, Araki A, Mitsui T, Nakajima T, Ito S, et al. Association between maternal exposure to di(2-ethylhexyl) phthalate and reproductive hormone levels in fetal blood: the Hokkaido Study on Environment and Children’s Health. PLoS One. 2014;9:1–10. https://doi.org/10.1371/journal.pone.0109039.
Bergh C, Torgrip G, Emenius C, Östman C. Organophosphate and phthalate esters in air and settled dust – a multi-location indoor study. Indoor Air. 2011;21:67–76. https://doi.org/10.1111/j.1600-0668.2010.00684.x.
Liu L, Bao H, Liu F, Zhang J, Shen H. Phthalates exposure of Chinese reproductive age couples and its effect on male semen quality, a primary study. Environ Int. 2012;42:78–83. https://doi.org/10.1016/j.envint.2011.04.005.
Rudell RA, Camann D, Spengler JD, Korn L, Brody J. Other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol. 2003;20:4543–53. https://doi.org/10.1021/es0264596.
Bi X, Yuan S, Pan X, Winstead C, Wang Q. Comparison, association, and risk assessment of phthalates in floor dust at different indoor environments in Delaware, USA. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2015. https://doi.org/10.1080/10934529.2015.1074482.
Larsson K, Lindh CH, Ag B, Giovanoulis G, Bibi M, Bottai M, et al. Phthalates, non-phthalate plasticizers and bisphenols in Swedish preschool dust in relation to children’s exposure. Environ Int. 2017;102:114–24. https://doi.org/10.1016/j.envint.2017.02.006.
Bergh C, Luongo G, Wise S. Organophosphate and phthalate esters in standard reference material 2585 organic contaminants in house dust. Anal Bioanal Chem. 2012;402:51–9. https://doi.org/10.1007/s00216-011-5440-2.
Dodson RE, Rodgers KM, Carey G, Cedeno Laurent JG, Covaci A, Poma G, et al. Flame retardant chemicals in college dormitories: flammability standards influence dust concentrations. Environ Sci Technol. 2017;51:4860–9. https://doi.org/10.1021/acs.est.7b00429.
Wang J, Ma Y, Chen S, Tian M, Luo X, Mai B. Brominated flame retardants in house dust from e-waste recycling and urban areas in South China: implications on human exposure. Environ Int. 2010;36:535–41. https://doi.org/10.1016/j.envint.2010.04.005.
Christia C, Poma G, Besis A, Samara C, Covaci A. Legacy and emerging organophosphorus flame retardants in car dust from Greece: implications for human exposure. Chemosphere. 2018;196:231–9. https://doi.org/10.1016/j.chemosphere.2017.12.132.
Christia C, Poma G, Harrad S, de Wit CA, Sjostrom Y, Leonards P, et al. Occurrence of legacy and alternative plasticizers in indoor dust from various EU countries and implications for human exposure via dust ingestion and dermal absorption. Environ Res. 2019;171:204–12. https://doi.org/10.1016/j.envres.2018.11.034.
Poma G, Malarvannan G, Voorspoels S, Symons N, Malysheva SV, Van Loco J, et al. Determination of halogenated flame retardants in food: optimization and validation of a method based on a two-step clean-up and gas chromatography e mass spectrometry. Food Control. 2016;65:168–76. https://doi.org/10.1016/j.foodcont.2016.01.027.
Luongo G, Ostman C. Organophosphate and phthalate esters in settled dust from apartment buildings in Stockholm. Indoor Air. 2016;26:414–25. https://doi.org/10.1111/ina.12217.
James RA, Hertz-Picciotto I, Willman E, Keller JA, Judith CM. Determinants of serum polychlorinated biphenyls and organochlorine pesticides measured in women from the Child Health and Development Study cohort, 1963-1967. Environ Health Perspect. 2002;110:617–24. https://doi.org/10.1289/ehp.02110617.
Dodson RE, Perovich LJ, Covaci A, Eede N Van Den, Ionas AC, Dirtu AC, et al. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California 2012.
Cao Z, Xu F, Covaci A, Wu M, Wang H, Yu G, et al. Distribution patterns of brominated, chlorinated, and phosphorus flame retardants with particle size in indoor and outdoor dust and implications for human exposure. Environ Sci Technol. 2014;48:8839–46. https://doi.org/10.1021/es501224b.
Tan H, Chen D, Peng C, Liu X, Wu Y, Li X, et al. Novel and traditional organophosphate esters in house dust from South China: association with hand wipes and exposure estimation. Environ Sci Technol. 2018;52:11017–26. https://doi.org/10.1021/acs.est.8b02933.
Acknowledgments
The authors would like to thank Dr. Maarten Degreef for offering valuable guidance to the method optimization steps, Dr. McGrath for contributing in language revision and all dust sample donors from China. Dr. Christina Christia acknowledges a doctoral fellowship BOF DOCPRO 3 from the University of Antwerp. Dr. Giulia Poma acknowledges a post-doctoral fellowship from the University of Antwerp. Dr. Bin Tang and Dr. Shan-Shan Yin acknowledge research scholarship (201704910738 and 201706320119, respectively) provided by the China Scholarship Council for their research stays at the University of Antwerp.
Funding
The present work was supported by the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (No. 2017BT01Z134).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1103 kb)
Rights and permissions
About this article
Cite this article
Christia, C., Tang, B., Yin, SS. et al. Simultaneous determination of legacy and emerging organophosphorus flame retardants and plasticizers in indoor dust using liquid and gas chromatography–tandem mass spectrometry: method development, validation, and application. Anal Bioanal Chem 411, 7015–7025 (2019). https://doi.org/10.1007/s00216-019-02078-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02078-5