Abstract
Electrokinetic process has emerged as an important tool for remediating heavy metal-contaminated soil. The process can concentrate heavy metals into smaller soil volume even in the absence of hydraulic flow. This makes it an attractive soil pre-treatment method before other remediation techniques are applied such that the chemical consumption in the latter stage can be reduced. The present study evaluates the feasibility of electrokinetic process in concentrating lead (Pb) and chromium (Cr) in a co-contaminated soil using different types of wetting agents, namely 0.01 M NaNO3, 0.1 M citric acid and 0.1 M EDTA. The data obtained showed that NaNO3 and citric acid resulted in poor Pb electromigration in this study. As for Cr migration, these agents were also found to give lower electromigration rate especially at low pH region as a result of Cr(VI) adsorption and possible reduction into Cr(III). In contrast, EDTA emerged as the best wetting agent in this study as it formed water-soluble anionic complexes with both Pb and Cr. This provided effective one-way electromigration towards the anode for both ions, and they were accumulated into smaller soil volume with an enrichment ratio of 1.55–1.82. A further study on the application of approaching cathode in EDTA test showed that soil alkalisation was achieved, but this did not provide significant enhancement on electromigration for Pb and Cr. Nevertheless, the power consumption for electrokinetic process was decreased by 22.5 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrokinetic process is a potential soil remediation method. This process is carried out by the introduction of low magnitude direct current across the soil via electrodes that are in contact with the soil. During the process, electrolysis occurs in both anode and cathode chambers, and H+ and OH- are generated, respectively. The potential difference between the electrodes causes the contaminants/ions in the soil to migrate towards their respective chambers via two mechanisms, namely (i) electromigration for ions transport and (ii) electroosmosis for neutral compound transport (Acar and Alshawabkeh 1993; Acar et al. 1995). Among these mechanisms, electromigration not only transports charged ions in the soil but also transports H+ and OH− generated from electrolysis through the soil. As the migration speed of H+ is about 1.8 times faster than of OH− (Acar and Alshawabkeh 1993; Acar et al. 1995; Kim et al. 2011), soil acidification may occur, and this generally enhances heavy metals desorption from the soil. It makes electrokinetic process an effective method in remediating heavy metals polluted soil.
Electromigration is generally free from limitations of hydraulic gradient and pore flow (Acar et al. 1995). Thus, electrokinetic process can concentrate heavy metals into a smaller soil volume even in the absence of hydraulic flow. This ability provides an attractive soil pre-treatment option before other remediation methods are applied such that the reduction in waste volume and chemicals consumption can be achieved. For example, the reduction in contaminated soil volume by electrokinetic process greatly helps subsequent treatment steps such as stabilisation and solidification. It not only reduces the consumption of high cost cement (Pensaert et al. 2008) but also reduces the high solid waste generation, which is the main disadvantage for stabilisation and solidification (Wuana and Okieimen 2011).
Electrokinetic process has been successfully applied in treating both heavy metals and organic compound-contaminated soil (Acar et al. 1995; Shenbagavalli and Mahimairaja 2010). Several enhancements on the efficiency of electrokinetic process in soil remediation have been tested over the past few decades (Yeung and Gu 2011), and positive results are reported. However, it is worth noting that one of the disadvantages for electrokinetic process is the additional cost for electric power consumption (Shen et al. 2007; Zhang et al. 2014). Moreover, this process also often suffers from inefficient heavy-metal electromigration due to the accumulation/focusing effect of metals in the middle of soil region (Alcántara et al. 2012; Li et al. 2012; Probstein and Hicks 1993).
In order to improve the feasibility of electrokinetic process, approaching electrode technique has been introduced in electrokinetic process. Approaching electrode can be categorised into two types, namely approaching anode (Li et al. 2012; Shen et al. 2007; Zhang et al. 2014) and approaching cathode (Shen et al. 2009; Zhou et al. 2014). This technique involves sequential switching of either anode (approaching anode) or cathode (approaching cathode) close to the other fixed electrode during electrokinetic process. This can provide progressive soil conditioning while compressing the undesired pH region, which can further enhance the desorption of heavy metal ions while reducing the focusing effect for better electromigration (Li et al. 2012; Shen et al. 2007, 2009; Zhang et al. 2014; Zhou et al. 2014). From the cost aspect, approaching electrode is reported to provide saving in energy consumption and treatment time by 16–44 and 20–40 %, respectively (Shen et al. 2007, 2009; Zhou et al. 2014). These advantages generally improve the feasibility of electrokinetic process in soil remediation. To date, approaching electrode technique is mainly studied for remediating single-contaminated soil such as Cr (Li et al. 2012), Cd (Shen et al. 2007) and Pb (Zhang et al. 2014), Hg (Shen et al. 2009) and fluorine (Zhou et al. 2014), and the results are promising. However, it is noted that the investigation of approaching electrode-assisted electrokinetic process in treating co-contaminated soil is scarce, especially for the metals that have opposite charge. This is important as eletromigration would concentrate both metal cations and anions in cathode and anode regions, respectively, which fails the purpose of contaminated soil volume reduction. Thus, an investigation on the feasibility of approaching electrode technique in electrokinetic process in treating co-contaminated soil is necessary.
In the present study, the technical feasibility of electrokinetic process as soil pre-treatment method for concentrating heavy metals into smaller contaminated soil volume was evaluated. The performance of electrokinetic process in electromigrating and concentrating oppositely charged lead (Pb) and chromium (Cr) into smaller soil volume was investigated in a co-contaminated soil using different types of wetting agents. In addition, the practicability of approaching electrode in enhancing the migration of differently charged heavy metals was also investigated from the view of metal migration and power consumption.
Methodology
Chemicals and soil contamination
The chemicals used in the present study were supplied by R&M Chemicals, Malaysia. The soil which was taken from Hulu Langat, Malaysia, was sieved to a particle size of <0.85 mm. The soil contained 92 % sand, and the pH, oxidation-reduction potential (ORP), and electrical conductivity (EC) for the soil were of 3.97, 280 mV and 243μS/cm, respectively. The soil has 3719 mg/kg iron content, 2400 mg/kg of aluminium and 185 mg/kg of manganese. The organic matter content and cation exchange capacity for the soil were 1.4 % and 5.1 meq/100 g, respectively. The soil was spiked with solution containing Pb(NO3)2 and K2Cr2O7 to acquire a soil that was co-contaminated with Pb and Cr(VI). The slurry was then mixed thoroughly using spatula and dried in open air for 1 week before storing it in a dark place prior to use. The concentrations of Pb and Cr after spiking were detected as 402.2 and 797.9 mg/kg, respectively.
Experimental procedure
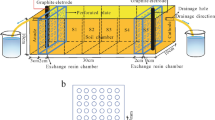
The investigation was carried out in a polypropylene sandbox with a dimension of 8.5 cm × 6.2 cm × 5 cm. In order to establish a consistent electrode environment for both fixed electrode and approaching electrode tests, the study was conducted in the absence of electrolyte chamber. A mass of 100 g of contaminated soil was compacted into the sandbox. Six graphite electrodes, each with a dimension of 0.7-cm diameter and 4-cm length, were introduced to the soil at specific positions, as shown in Fig. 1. In order to enhance the effective electric field in the soil, double anodes (S1 region) and cathodes (S4 region) were employed in this study. The electrodes were connected to a DC supply. As shown in Fig. 1, the soil is categorised into four regions, whereby S1 and S2 regions represent low pH anode region whilst S3 and S4 represent high pH cathode region. The experiment was initiated by sprinkling the wetting agent on the soil surface slowly so as to wet the soil. Three types of wetting agents were employed, namely 0.01 M NaNO3, 0.1 M citric acid and 0.1 M EDTA, and their physicochemical properties are as summarised in Table 1. After soil was saturated with the respective wetting agent, a constant voltage gradient of 1 V/cm was applied through the soil for 24 h. For ‘approaching electrodes’ study, both approaching anode (AA) and approaching cathode (AC) were considered, depending on the migration trend for both ions. For this test, the anodes (for AA) or cathodes (for AC) were switched to the middle electrodes in S2 region after the 12th hour of experiment, and the voltage gradient was maintained at 1 V/cm. The details of the experiment are as shown in Table 2.
Schematic diagram for the experimental setup and electrode positions (Ng et al. 2014)
Analytical methods
Electric current passed through the soil was measured from time to time using Multimeter Sunwa TE-832B. Soil properties such as pH as well as the concentrations of Pb and Cr in the soil were determined after the experiments. The soil was divided into four sections, denoted as S1, S2, S3 and S4 (Fig. 1), and it was dried before the analysis. Soil pH was determined using USEPA SW-846 method 9045D with a calibrated pH meter Crison MM26+. For Pb and total Cr concentrations in the soil, acid digestion USEPA 3050b was carried out, while Cr(VI) concentration was determined using USEPA 3060A method. The concentrations for Pb and Cr in the aqueous filtrates were then analysed using ICP-OES. For analysis purpose, normalised concentration was used. A normalised concentration of >1 indicated the accumulation/enrichment of metal, whilst a value of <1 represented metal migration/removal from the soil section. The normalised concentration for metal in each soil section can be calculated using Eq. (1), where C is the contaminant concentration in the soil after the experiment and C 0 is the initial concentration for the contaminant in the soil.
Results and discussion
Effect of wetting agents
The performance of electrokinetic process in treating Pb/Cr co-contaminated soil is illustrated in Fig. 3. The results were analysed from different aspects, namely (i) electric current, (ii) soil pH, (iii) Pb distribution in the soil, and (iv) Cr distribution in the soil.
Electric current and soil pH
Figure 2a, b depicts the electric current profile across the soil against time during the experiment and final soil pH at different soil sections, respectively. In general, it is found that the magnitude of electric current is in a sequence of 0.1 M EDTA > 0.1 M citric acid > 0.01 M NaNO3, as shown in Fig. 2a. This was mainly due to the fact that 0.1 M EDTA and 0.1 M citric acid had higher amount of mobile ions than 0.01 M NaNO3. EDTA could undergo multiple dissociations at solution pH of ≈8–9 in comparison to low pH citric acid. Moreover, considering the relatively high availability of Fe in the soil, EDTA, as a nonselective metal chelating agent, could also provide dissolution of Fe oxides and subsequently the formation of Fe(III)-EDTA complexes via ligand exchange reaction (Komárek et al. 2007) besides the targeted contaminants, as observed in the work of Kim et al. (2011). The multiple-stage dissociation of EDTA and extra desorption of other metal oxides into the soil solution increased the number of mobile ions in the soil system, and thus, highest electric current was observed in 0.1-M EDTA test. With respect to variation in current against time, Fig. 2a reveals that the electric current for 0.01 M NaNO3 test increases from 11.2 to 18 mA in the first 3 h of the experiment. This was mainly due to the increase in ion concentrations as a result of electrolysis on the electrodes and desorption/solubilisation of Pb and Cr. As the experiments progressed, the electric current decreased although constant voltage gradient was applied, regardless of wetting agent used. This was also reported by other researchers who attributed this phenomenon to the increase in resistance polarisation in the soil as well as loss of ionic strength in the system due to ion electromigration towards their respective electrodes (Colacicco et al. 2010; Giannis et al. 2012; Saichek and Reddy 2003; Shen et al. 2007, 2009; Shrestha et al. 2009; Zhang et al. 2014).
For soil pH, Fig. 2b illustrates that, in general, low pH of 2.45–3.05 is observed in S1, whilst high pH of 9.98–10.04 is achieved in S4 region. This was mainly due to the fact that electrolysis occurred on both electrodes where H+ and OH− were produced in anode and cathode regions, respectively, as shown in Eqs. (2) and (3) (Acar and Alshawabkeh 1993).
Nevertheless, it was found that the pH variation in the soil section was highly dependent on the type of wetting agent used. As shown in Fig. 2b, a stable pH increase from 3.05 to 10.02 is observed when NaNO3 is used as the wetting agent. This is a normal trend for electrokinetic process as electrolysis occurs at both anode and cathode, producing H+ and OH−, respectively (Acar and Alshawabkeh 1993). As the electromigration speed of H+ is about 1.8 times higher than OH− (Acar and Alshawabkeh 1993, Acar et al. 1995; Chung and Kang 1999; Gioannis et al. 2008; Kim et al. 2011; Park et al. 2009), a pH gradient was observed in S3 region. In contrast, when 0.1 M citric acid was used, lower soil pH in S1–S3 was obtained, whereas S4 showed a much higher pH value of 9.98, as shown in Fig. 2b. Citric acid served as a buffer solution for preventing pH change. Thus, the only high pH region observed after the experiment was S4 as a result of electrolysis on the cathode (Acar and Alshawabkeh 1993). For the case of 0.1 M EDTA, high overall soil pH was obtained. Figure 2b depicts that a soil pH of 6.81 was achieved in S2 region and a pH of ≈10 was obtained for S3–S4 regions, whilst the only low pH region was S1 at 2.45. This was mainly due to the pH of EDTA solution which was around 8–9. The presence of OH− in the wetting agent increased the soil pH before electrokinetic process was applied. This eventually increased soil pH and minimising soil acidification in S2–S4 regions in comparison to the tests that used 0.01 M NaNO3 and 0.1 M citric acid.
Pb distribution in the soil
Pb distribution in the soil after the experiment is illustrated in Fig. 2c. The results revealed that good Pb migration was only achieved in 0.1 M EDTA test, whilst 0.01 M NaNO3 and 0.1 M citric acid provided relatively poor electromigration. For NaNO3 and citric acid tests, minor electromigration of Pb was observed from both S1 and S4 regions. As shown in Fig. 2c, Pb was found to accumulate in the S3 region with enrichment factor of 1.04–1.12, while other regions showed normalised concentration of 0.9–1. This trend contradicts the work of Ng et al. (2014) who reported that effective Pb migration could be achieved when NaNO3 and citric acid were used as the wetting agents. Unlike the work of Ng et al. (2014), the Pb species in the present study was mainly PbCrO4, as a result of precipitation reaction between Pb(NO3)2 and K2Cr2O7 during soil spiking (Madan and Prakash 1987). PbCrO4 generally had low water solubility (Madan and Prakash 1987; Trishna Knowledge Systems 2012) and could only be dissolved in acidic and basic media, as shown in Eqs. (4) and (5), respectively (BUTE n.d.). Thus, Pb electromigration was observed from S1 to S4 regions. However, it was worth noting that overall, Pb mobility in the present study was poor when NaNO3 and citric acid were used as the wetting agents. This observation agrees with the findings of Zhang et al. (2012) who reported that the electromigration of PbCrO4 was poor when deionised water was used as the wash solution. The low overall mobility of PbCrO4 in electrokinetic process under the wetting agents used may be the main reason for poor Pb electromigration.
In contrast, Fig. 2c shows that the use of 0.1 M EDTA provides Pb electromigration from S4 to S1 region. The use of high pH EDTA solution not only provided dissolution for PbCrO4 (Koshi and Iwasaki 1983; Madan and Prakash 1987; Trishna Knowledge Systems 2012; Tuli and Madan 1999) but also served as a chelating agent for the formation of water-soluble Pb-EDTA complexes (Niinae et al. 2008; Zhang et al. 2014). As the complexes formed are anionic, Pb electromigrates towards S1 and is accumulated in the region with an enrichment factor of 1.55 when electricity is applied, while the normalised Pb concentration in S3–S4 regions is reduced to <0.4, as shown in Fig. 2c. This indicated that the soil in S3–S4 regions was pre-treated and majority of Pb was removed from these regions and was accumulated in S1–S2 regions.
Cr distribution in the soil
Figure 2d illustrates the normalised Cr concentration at different soil sections using different types of wetting agents. It was found that Cr migration was generally different from Pb, whereby Cr migrated from S4 to S1 region for all types of wetting agents used. In this study, Cr was more mobile than Pb as Cr was primarily in hexavalent form which was adsorbed on the soil before the experiment. Figure 2d shows that Cr migration follows a sequence of 0.1 M EDTA > 0.01 M NaNO3 > 0.1 M citric acid. This trend is also observed from the physical appearance of the soil after the experiment, as shown in Fig. 3. The yellow and purple textures in the figure indicate high concentration of Cr ions and Cr(III)-EDTA (Hedrick 1965), respectively, in the soil region. A stronger yellow texture in 0.01 M NaNO3 test (Fig. 3a) and purple texture in 0.1 M EDTA test (Fig. 3c) in S1 region indicates that Cr is electromigrated and concentrated in S1 region, whilst a mild yellow texture in S1–S3 regions as shown in Fig. 3b shows poor Cr migration in 0.1 M citric acid test.
Figure 2d shows that unlike the data reported for Pb, a smooth migration from S4 to S1 is observed for Cr when 0.01 M NaNO3 and 0.1 M EDTA were used as wetting agents although the former does not involve in enhancing Cr solubility in aqueous phase. This is perhaps due to the higher mobility of Cr(VI) in comparison to Pb species in this study. Among the two wetting agents, EDTA provided lower Cr concentration in S3–S4 regions, and this could be attributed to relatively high soil pH provided by 0.1 M EDTA, which favoured Cr(VI) desorption (Hu et al. 2005; Reddy 2013; Troy 2013). In addition, EDTA solution also enhanced Cr migration in S2 region due to higher Cr(VI) desorption as a result of higher soil pH in the S2 region. Besides that, it can also complex with H+ ions or be adsorbed onto soil surface for releasing Cr(VI) from the soil (Saeedi et al. 2013). Moreover, EDTA also served as chelating agent for the formation of Cr(III)-EDTA anionic complexes (Cao et al. 2011; Jung et al. 1997; Saeedi et al. 2013), which ensured one-way electromigration of Cr towards S1. This migration trend was also in line with the work of Reddy and Chinthamreddy (2004) as well as Saeedi et al. (2013). In contrast, as shown in Fig. 2d, Cr migration slowed down at lower pH S1–S2 regions when 0.01 M NaNO3 was applied. Low soil pH condition provided more positively charged binding sites for the adsorption of negatively charged Cr(VI) compounds such as Cr2O7 2− and CrO4 2− (Hawley et al. 2005). This was also in line with the works of Hu et al. (2005) and Troy (2013) which reported that acidic condition favoured Cr(VI) adsorption on the soil surface’s iron oxides. This in turn reduced the desorption process and Cr(VI) mobility in S1–S2 regions, and thus, the electromigration was slowed down. In addition, low pH condition may also cause Cr(VI) reduction into Cr(III), especially in the presence of iron species as electron donor (Barrera-Díaz et al. 2012; Hawley et al. 2005; Huang et al. 1995; Weng and Tsai 2009). These conditions were achieved in S1–S2 regions in this test, as the soil had significant amount of iron content (3719 mg/kg). The formation of positively charged Cr(III) species and the inability of NaNO3 to form anionic complexes with Cr(III) may lead to transport of Cr(III) towards the cathode region (S4) via electromigration. Consequently, the net electromigration rate for Cr towards anode in S1–S2 regions decreased.
However, as shown in Figs. 2d and 3b, when 0.1 M citric acid was used as the wetting agent, significant Cr migration was observed in S4 region, whilst S1–S3 regions showed relatively weak migration. The migration in S4 was mainly attributed to high pH condition in S4 for Cr(VI) desorption (Hu et al. 2005; Troy 2013). In addition, citric acid may also cause reduction of Cr(VI) into Cr(III) (Meichtry et al. 2007), which could form anionic complexes with citrate ion at a pH of >9 (Cao et al. 2011). Consequently, Cr migrated away from S4. However, as S1–S3 were low in pH, the migration rate declined. Low soil pH condition not only favoured Cr(VI) adsorption (Hu et al. 2005; Troy 2013) but also leads to possible Cr(VI) reduction (Barrera-Díaz et al. 2012), especially in the presence of citric acid (Meichtry et al. 2007). This is observed in the results shown in Fig. 2d whereby most of the Cr detected in the soil after the experiment is Cr(III). Moreover, the formation of Cr(III)-citrate complex in the presence of citric acid could be another reason for poor Cr electromigration in S1–S3 as majority of Cr(III)-citrate complexes formed are in either neutral or positively charged (Cao et al. 2011) at the given pH condition in S1–S3 regions. This may cease Cr electromigration towards S1. Furthermore, Cr(III)-citrate is also reported to have high affinity towards soil at low pH (Cao et al. 2011), and this could reduce the migration in S1–S3 regions.
Effect of approaching electrode
The results, as shown in ‘Effect of Wetting Agents’ section, reveal that Pb mobility was poor in the Pb/Cr co-contaminated soil when 0.01 M NaNO3 and 0.1 M citric acid are applied as the wetting agent. On the other hand, 0.1 M EDTA provided one-way electromigration for both Pb and Cr from S4 to S1 region. Thus, the feasibility of approaching electrode in enhancing Pb and Cr migration was evaluated for 0.1 M EDTA only. Since the direction for electromigration was headed to the anode, approaching cathode was investigated. The results are as shown in Fig. 4.
Electric current and soil pH
Unlike the works of Shen et al. (2009) and Zhou et al. (2014) which reported that approaching cathode enhanced electric current in the remediation process, Fig. 4a shows that the electric current decreases from 17.1 to 8.7 mA when the cathodes are switched to the middle electrodes (S2) at 12th hours. The difference in observation could be due to the present experimental setup, which was operated in a closed system in which the wash solution chamber was absent for continuous supply of wetting agent to the system. In addition, the switching of cathode to the middle soil section (S2) after 12th hour may also reduce the amount of mobile ions that could be available under the influence of the electric field. This may happen as significant amount of ions present in S3–S4 regions such as H+, OH− and EDTA complexes was no longer involved in the electrokinetic process. Hence, the electric current was reduced.
Nevertheless, despite electric current reduction in approaching cathode tests, Fig. 4b reveals that soil pH in S2 increases from 6.81 to 8.18, indicating progressive soil alkalisation. Similar observation was also reported by Shen et al. (2009) and Zhou et al. (2014), as a result of OH− generation in S2 region in nullifying local H+ as well as reduction in OH− migration distance for better base front.
Distribution of Pb and Cr in the soil
Figure 4c, d illustrates the distribution of Pb and Cr, respectively, at different soil sections for both fixed electrode and approaching cathode tests. Figure 4c shows that the improvement in Pb migration from S2 to S1 is insignificant for the present experimental conditions. Unlike the work of Shen et al. (2009), the increase in soil pH in S2 region and the reduction in migration distance did not enhance Pb accumulation in S1 significantly. This could be due to the fact that Pb had already electromigrated close to the S1 region. The reduction in ions mobility in S1–S2 via possible adsorption of negatively charged Pb-EDTA complexes (Reddy et al. 2010), especially at low pH S1 region, may be the reason for poor ion mobility and electromigration enhancements. Furthermore, the switching process also results in higher Pb concentration remaining in S3–S4 regions than that for fixed electrode test. This was perhaps caused by incomplete Pb-EDTA migration due to the absence of electric field in these regions after cathode switching. Similar observation is also found for Cr, as shown in Fig. 4d. The use of approaching anode did not enhance Cr accumulation in S1 region significantly. Instead, it showed a slightly higher Cr concentration in S3–S4 regions, as a result of incomplete Cr migration due to cathode switching.
Removal efficiency and power consumption
The experimental results showed that electrokinetic process concentrated both Pb and Cr in S1–S2 regions via electromgiration when 0.1 M EDTA was applied. This generally provided soil pre-treatment and soil volume reduction indirectly in S3–S4 regions as the concentration for Pb and Cr was reduced. Therefore, Pb and Cr removal was achieved in these regions. The removal efficiency for the contaminants in these pre-treated regions and power consumed during the electrokinetic process was determined using Eqs. (6) and (7), respectively, where V is the voltage (V), I is the electric current (A) and t is time (h).
Figure 5 illustrates that electrokinetic process provides high removal efficiency for both Pb and Cr at 64–70 % in S3–S4 regions when 0.1 M EDTA is applied as the wetting agent in comparison with the other two. In contrast, 0.01 M NaNO3 and 0.1 M citric acid show poor Pb removal efficiency in these regions, as a result of their inability to provide high PbCrO4 mobility and the nature of Pb(II) to electromigrate towards S3–S4. Moreover, the removal efficiency for Cr in S3–S4 regions using these agents is also found to be lower than 0.1 M EDTA, as shown in Fig. 5. However, it is worth noting that despite 0.1 M EDTA showing higher removal efficiency for Pb and Cr, Fig. 5 shows that 0.1 M EDTA causes an electric power consumption of ≈2.8 and ≈2.4 times higher than that for 0.01 M NaNO3 and 0.1 M citric acid, respectively. The use of approaching cathode is found to save the power consumption by of 22.5 % from 3.65 × 10−3 to 2.83 × 10−3 kWh. However, this change also slightly decreased the removal efficiency for both Pb and Cr, by 4 to 8 %, as a result of incomplete electromigration in S3–S4 regions.
Comparison with other studies
Table 3 presents a comparison of the present and past studies for approaching electrode-assisted electrokinetic process. It is worth noting that a direct comparison among these works is difficult as each work uses different approach in their experimental methodology, such as equipment size, experimental duration, number of electrode switching, chemical agents used and types of contaminants.
Unlike the works of Shen et al. (2009), Li et al. (2012) and Zhang et al. (2014) which showed that significant enhancement in heavy metal removal from the soil system when approaching electrode was applied, the enhancement in removal for Pb and Cr from the overall soil system was negligible in this study. Instead, the removal efficiency of Pb and Cr out of the soil system was maintained at ≈22 and ≈11 %, respectively, via electrodepositing on the electrode. This was mainly due to the difference in the experimental setup whereby the present study was conducted in a closed sandbox system which had no wetting agent/electrolyte chamber for removing Pb and Cr out of the soil system. Moreover, the use of electrolyte replenishment, as reported in the work of Li et al. (2012) may also cause significantly higher metal removal in comparison to the present study.
In addition, other parameters such as soil characteristics, number of switching, size of soil treatment equipment as well as treatment time also contributed to the effectiveness of the system on metal migration. For example, Shen et al. (2007) reported that high Cd enrichment ratio of ≈4.5 was achieved in the cathode region in approaching anode test, whilst the present study only showed an enrichment of 1.64–1.86 in anode region. This could be due to the difference in operating parameters used in the work of Shen et al. (2007), such as larger soil capacity and multiple electrode switching. Besides that, the use of relatively low contaminant concentration in their works (≈100 mg/kg of Cd) in comparison to the present study (≈400 mg/kg for Pb and ≈ 800 mg/kg for Cr) may be another reason for the difference in enrichment ratio. This makes the direct comparison of performances quite difficult. Hence, in order to evaluate the feasibility of approaching electrode-assisted electrokinetic process as soil pre-treatment method, further study is necessary, especially from the view of scaling up, electrode arrangement and different heavy metals in other types of soils.
Conclusions
The study investigated the effects of wetting agents and approaching electrode on electrokinetic process for the electromigration of Pb and Cr in a co-contaminated soil. Electrokinetic process was suitable for application as a soil pre-treatment method as it can reduce contaminated soil volume by concentrating the heavy metals into smaller soil portion even in the absence of hydraulic flow. Based on the experimental results in this study, several conclusions can be made.
-
1.
NaNO3 and citric acid failed to provide high mobility and electromigration for PbCrO4, the Pb species used in this study. In contrast, EDTA emerged as a better wetting agent by dissolving PbCrO4 and form higher mobility anionic Pb-EDTA complexes.
-
2.
The effectiveness of wetting agent on Cr migration followed a trend of 0.1 M EDTA > 0.01 M NaNO3 > 0.1 M citric acid. A 0.1 M EDTA solution emerged as the best wetting agent in this study as it not only provided high pH condition for Cr(VI) desorption from the soil surface but also supported the formation of anionic water-soluble complexes with Cr(III) and ensuring one-way electromigration towards anode.
-
3.
The use of approaching cathode in 0.1 M EDTA test provided progressive soil alkalisation. However, this did not improve electromigration for Pb and Cr from S2 to S1 significantly. Instead, the removal efficiency for Pb and Cr in S3–S4 regions was slightly reduced by 4 to 8 %. Nevertheless, the power consumption in electrokinetic process was reduced by ≈22.5 % when approaching cathode was used, suggesting that power wastage could be minimised.
References
Acar YB, Alshawabkeh AN (1993) Principles of electrokinetic remediation. Environ Sci Technol 27:2638–2647
Acar YB, Gale RJ, Alshawabkeh AN, Marks RE, Puppala S, Bricka M, Parker R (1995) Electrokinetic remediation: basics and technology status. J Hazard Mater 40:117–137
Alcántara MT, Gómez J, Pazos M, Sanromán MA (2012) Electrokinetic remediation of lead and phenanthrene polluted soils. Geoderma 173–174:128–133
Barrera-Díaz CE, Lugo-Lugo V, Bilyeu B (2012) A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J Hazard Mater 223–224:1–12
BUTE (n.d.) The Group Ia Elements (Li, Na, K, Rb, Cs) and Their Principle Ions (Me+) http://www.inc.bme.hu/en/subjects/inchem/sillabus/7-35.pdf. Accessed 3 March 2015
Cao X, Guo J, Mao J, Lan Y (2011) Adsorption and mobility of Cr(III)-organic acid complexes in soils. J Hazard Mater 192:1533–1538
Chung HI, Kang BH (1999) Lead removal from contaminated marine clay by electrokinetic soil decontamination. Eng Geol 53:139–150
Colacicco A, De Gioannis G, Muntoni A, Pettinao E, Polettini A, Pomi R (2010) Enhanced electrokinetic treatment of marine sediments contaminated by heavy metals and PAHs. Chemosphere 81:46–56
Giannis A, Tay E, Kao J, Wang J-Y (2012) Impact of vertical electrokinetic-flushing technology to remove heavy metals and polycyclic aromatic hydrocarbons from contaminated soil. Electrochim Acta 86:72–79
Gioannis GD, Muntoni A, Polettini A, Pomi R (2008) Enhanced electrokinetic treatment of different marine sediments contaminated by heavy metals. J Environ Sci Health A Tox Hazard Subst Environ Eng 43:852
Hawley EL, Deeb RA, Kavanaugh MC, Jacobs J (2005) Treatment Technologies for Chromium(VI). In: Guertin J, Avakian CP, Jacobs JA (Editors), Chromium(VI) Handbook. CRC Press, Florida, 273-308
Hedrick CE (1965) Formation of the chromium-EDTA complex: an undergraduate kinetics experiment. J Chem Edu 42:479–480
Hu J, Chen G, Lo IMC (2005) Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res 39:4528–4536
Huang CP, Shin HM, Allen HE, Cheng AHD (1995) Effect of specific chemical reactions on the transformation and the transport of chromium in the soil-water system. http://www.state.nj.us/dep/dsr/chromium/Cr-year3-part2.pdf. Accessed 2 July 2015
Jung GY, Kim YS, Lim HB (1997) Simultaneous determination of chromium(III) and chromium(VI) in aqueous solution by capillary electrophoresis with on-column UV-VIS detection. Anal Sci 13:463–467
Kim K-J, Kim D-H, Yoo J-C, Baek K (2011) Electrokinetic extraction of heavy metals from dredged marine sediment. Sep Purif Technol 79:164–169
Komárek M, Tlustoš P, Száková J, Chrastný V, Balík J (2007) The role of Fe- and Mn-oxides during EDTA-enhanced phytoextraction of heavy metals. Plant Soil Environ 53:216–224
Koshi K, Iwasaki K (1983) Solubility of low-solubility chromates and their clastogenic activity in cultured cells. Ind Health 21:57–65
Li G, Guo S, Li S, Zhang L, Wang S (2012) Comparison of approaching and fixed anodes for avoiding the ‘focusing’ effect during electrokinetic remediation of chromium-contaminated soil. Chem Eng J 203:231–238
Madan RD, Prakash S (1987) Modern inorganic chemistry. S. Chand & Company Ltd, New Delhi
Meichtry JM, Brusa M, Mailhot G, Grela MA, Litter MI (2007) Heterogeneous photocatalysis of Cr(VI) in the presence of citric acid over TiO2 particles: relevance of Cr(V)–citrate complexes. Appl Catal B 71:101–107
Ng YS, Gupta BS, Hashim MA (2014) Effects of wetting agents and approaching anode on lead migration in electrokinetic soil remediation. In: Gong T (Ed) International Proceedings of Chemical, Biological & Environmental Engineering: Chemical Engineering and Applications V. Paper presented at 5th International Conference on Chemical Engineering and Applications. Taipei, IACSIT Press, Singapore, pp. 44-47
Niinae M, Nishigaki K, Aoki K (2008) Removal of lead from contaminated soils with chelating agents. Mater Trans 49:2377–2382
Park S-W, Lee J-Y, Yang J-S, Kim K-J, Baek K (2009) Electrokinetic remediation of contaminated soil with waste-lubricant oils and zinc. J Hazard Mater 169:1168–1172
Pensaert S, De Groeve S, Staveley C, Menge P, De Puydt S (2008) Immobilisation, stabilisation, solidification: a new approach for the treatment of contaminated soils. Case studies: London Olympics & Total Ertvelde Paper presented at 15th Innovatieforum Geotechniek, Antwerp
Probstein RF, Hicks RE (1993) Removal of contaminants from soils by electric fields. Science 260:498–503
Reddy KR (2013) Electrokinetic remediation of soils at complex contaminated sites. In: Manassero M, Dominijanni A, Foti S, Musso G (eds) Coupled phenomena in environmental geotechnics. CRC Press, London, pp 131–147
Reddy K, Chinthamreddy S (2004) Enhanced Electrokinetic Remediation of Heavy Metals in Glacial Till Soils Using Different Electrolyte Solutions. J Environ Eng 130:442–455
Reddy KR, Cameselle C, Ala P (2010) Integrated electrokinetic-soil flushing to remove mixed organic and metal contaminants. J Appl Electrochem 40:1269–1279
Saeedi M, Li LY, Gharehtapeh AM (2013) Effect of alternative electrolytes on enhanced electrokinetic remediation of hexavalent chromium in clayey soil. Int J Environ Res 7:39–50
Saichek RE, Reddy KR (2003) Effect of pH control at the anode for the electrokinetic removal of phenanthrene from kaolin soil. Chemosphere 51:273–287
Shen Z, Chen X, Jia J, Qu L, Wang W (2007) Comparison of electrokinetic soil remediation methods using one fixed anode and approaching anodes. Environ Pollut 150:193–199
Shen Z, Zhang J, Qu L, Dong Z, Zheng S, Wang W (2009) A modified EK method with an I−/I2 lixiviant assisted and approaching cathodes to remedy mercury contaminated field soils. Environ Geol 57:1399–1407
Shenbagavalli S, Mahimairaja S (2010) Electro kinetic remediation of contaminated habitats. Afr J Environ Sci Technol 4:930–935
Shrestha RA, TD P, Sillanpää M (2009) Remediation of chrysene from contaminated soil by enhanced electrokinetics. Int J Electrochem Sci 4:1387-1394
Trishna Knowledge Systems (2012) Super course in chemistry for the IIT-JEE: inorganic chemistry. Dorling Kindersley Pvt. Ltd., New Delhi
Troy A (2013) Co and Cr adsorption on maghemite, quartz, and maghemite-quartz mixtures. http://scholarworks.wmich.edu/cgi/viewcontent.cgi?article = 3314&context = honors_theses Accessed 2 July 2015
Tuli GD, Madan RL (1999) S. Chand success guide inorganic chemistry. S. Chand & Company Ltd, New Delhi
Weng CH, Tsai HW (2009) A pilot-scale test of electrokinetic remediation of Cr(VI) contaminated kaolinite incorporated with zero-valen iron. J Environ Eng Manag 19:379–387
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Scholarly Res Network Ecol 2011:1–20
Yeung AT, Gu Y-Y (2011) A review on techniques to enhance electrochemical remediation of contaminated soils. J Hazard Mater 195:11–29
Zhang T, Zou H, Ji M, Li X, Li L, Tang T (2014) Enhanced electrokinetic remediation of lead-contaminated soil by complexing agents and approaching anodes. Environ Sci Pollut Res 21:3126–3133
Zhang W, Zhuang L, Tong L, Lo IMC, Qiu R (2012) Electro-migration of heavy metals in an aged electroplating contaminated soil affected by the coexisting hexavalent chromium. Chemosphere 86:809–816
Zhou M, Zhu S, Liu Y, Wang H (2014) Electrokinetic remediation of fluorine-contaminated soil using approaching cathodes. Clean Soil Air water 42:1771–1775
Acknowledgments
This work was a part of a collaborative project between Queen’s University Belfast and University of Malaya and is financially supported by grant UM-QUB6A-2011 and PPP grant PG143-2012B, University of Malaya.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Ng, YS., Sen Gupta, B. & Hashim, M.A. Remediation of Pb/Cr co-contaminated soil using electrokinetic process and approaching electrode technique. Environ Sci Pollut Res 23, 546–555 (2016). https://doi.org/10.1007/s11356-015-5290-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5290-0