Abstract
Optimizing process parameters that affect the remediation time and power consumption can improve the treatment efficiency of the electrokinetic remediation as well as determine the cost of a remediation action. Lab-scale electrokinetic remediation of Pb-contaminated soils was investigated for the effect of complexant ethylenediaminetetraacetic acid (EDTA) and acetic acid and approaching anode on the removal efficiency of Pb. When EDTA was added to the catholyte, EDTA dissolved insoluble Pb in soils to form soluble Pb–EDTA complexes, increasing Pb mobility and accordingly removal efficiency. The removal efficiency was enhanced from 47.8 to 61.5 % when the EDTA concentration was increased from 0.1 to 0.2 M, showing that EDTA played an important role in remediation. And the migration rate of Pb was increased to 72.3 % when both EDTA and acetic acid were used in the catholyte. The “approaching anode electrokinetic remediation” process in the presence of both EDTA and acetic acid had a higher Pb-removal efficiency with an average efficiency of 83.8 %. The efficiency of electrokinetic remediation was closely related to Pb speciation. Exchangeable and carbonate-bounded Pb were likely the forms which could be removed. All results indicate that the approaching anode method in the presence of EDTA and acetic acid is an advisable choice for electrokinetic remediation of Pb-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution has been one of the important environmental issues of worldwide concern. Because of its persistence and irreversibility, heavy metal contamination soil has reached a very serious level. Lead (Pb) in soil has a low solubility and a stable and long residence time. Accumulated Pb in soil threatens the environment and human health.
Electrokinetic remediation is a technique for removal of contaminants by inserting the electrodes into the contaminated soils (Acar and Alshawabkeh 1993; Lageman 1993; Hansen et al. 1997) and applying low DC voltage gradient or DC current to the electrodes. During electrokinetic (EK) remediation, the applied current causes water electrolysis at electrodes and leads to a series of electric effects (electromigration, electroosmosis, and electrophoresis), and accordingly, the pollutants are enriched near the electrodes and removed from the soil (Reddy and Chinthamreddy 2003; Suer and Lifvergren 2003). With the advantages of a wide range of application, no secondary pollution and short remediation time, EK remediation has been extensively investigated (Acar and Alshawabkeh 1993; Lageman et al. 1989; Probstein and Hicks 1993).
Many studies have found that anions, such as carbonate and sulfate in soils, could form precipitates with heavy metals, thereby reducing the mobility of the metals and decreasing the remediation efficiency (Hicks and Tondorf 1994). Complexing agents can solubilize heavy metals by forming complexes, thereby enhancing migration of heavy metals in soils and accordingly removal efficiency. As a ligand with six coordination atoms, ethylenediaminetetraacetic acid (EDTA) could form very stable and soluble metal–EDTA complexes that can be removed efficiently in the EK process (Lo and Yang 1999; Kedziorek and Bourg 2000; Kim et al. 2003). EDTA is usually added to the catholyte (Wong et al. 1997; Zhou et al. 2004; Giannis and Gidarakos 2005) and soils (Virkutyte et al. 2005; Nogueira et al. 2007; Giannis et al. 2008; Kimura et al. 2007). Yeung et al. (1996) studied the effect of EDTA on removal of Pb from kaolinite-spiked samples characterized by a high acid/base buffer capacity. Results of the study showed that approximately 90 % of the Pb was migrated toward the anode and accumulated within 15 % of soils. Amrate et al. (2005) studied the effect of EDTA at various concentrations (0.05–0.20 M) on the enhancement of Pb transport by applying a constant voltage corresponding to a nominal electric field strength of 1 V/cm−1. Results of Pb distribution across the experimental cell showed efficient transport of Pb toward the anode despite the presence of calcite (25 %) and the high acid/base buffer capacity of the soil.
However, EK remediation is time- and energy-consuming. EK remediation takes several days even a few years to remediate polluted soils, and it requires approximately 500 kWh m−3 of energy (Virkutyte et al. 2002). To improve the removal efficiency of the EK remediation, some researchers have experimented with the “approaching anode electrokinetic remediation” (AA-EK) method. The AA-EK method involves sequential approaching the anode close to the fixed cathode after the EK process has started (Li et al. 2012). During AA-EK remediation, the pH near the anode can be continuously decreased, while the zone of heavy metal precipitates can be compressed. It is more time-effective and energy-effective than the EK method. Shen et al. (2007) have reported that the AA-EK method saves nearly 44 % of energy and 40 % of time.
The aim of this work is to examine the Pb-removal efficiency of the electrokinetic process from Pb-contaminated soils in the present of EDTA and acetic acid. The removal efficiency of the AA-EK method was also examined.
Materials and methods
Sample preparation
The contaminated soil used in this study was collected from an abandoned industrial site in Jiangsu Province, China. Weeds, leaves, and rocks were removed before sample collection. The top soils (5–20 cm) were collected using plastic spade. The sample was put into a black plastic bag and transported to our lab. The soil sample was air-dried, and the portion passing through a no.10 mesh sieve (≤2 mm) was used in the experiment.
Pb(NO3)2 solutions were added to 5 kg of the above sieved soil to achieve a Pb-contamination level of about 1,000 mg/kg. The soil-contaminant mixture was manually mixed. To achieve a homogeneous contaminant concentration and moisture content, the mixture was put aside for 240 h. The as-prepared slurry was transferred into a rectangle electrokinetic cell and compacted by the consolidation method described in Reddy and Shirani (1997)). Finally, excess portions found at the edges of the cell were shaved away.
Experimental setup
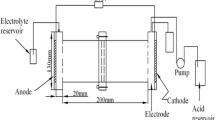
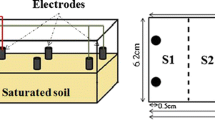
Figure 1 shows the setup of the electrokinetic experiments. The electrokinetic remediation apparatus adapted from the Technical University of Denmark primarily consists of an electrokinetic remediation cell, a DC electrokinetic power supply, a multimeter, and two peristaltic pumps. The electrokinetic remediation cell includes a rectangle electrokinetic cell (20 × 15 × 10 cm, L × W × H) made of polymethylmethacrylate (PMMA), two electrode chambers (5 × 15 × 10 cm, L × W × H), and graphite electrodes. The graphite electrode with a 30-cm2 size covered the whole soil cross-section to provide a uniform electric current. Electrode chambers were connected to the soil cell with screws. Glass filter paper was installed between electrode chambers and the soil cell to avoid leakage. The rectangle electrokinetic cell was filled with the Pb-spiked soils. Five sample collection ports were located on the top of the soil cell and named as S1–S5 from the anode to the cathode. Two electrode chambers were placed at each end of the soil cell to simulate one-dimensional transport of contaminants under an introduced electric potential.
The electrolyte reservoirs were covered to avoid excessive water evaporation but permit the release of gasses (i.e., oxygen and hydrogen) produced at the electrodes. The electrolyte reservoirs were kept level to avoid any difference in static hydraulic pressure along the sample.
A DC power supply (WYJ-2A60V, ShangHai QuanLi Instruments Ltd. Co.) was used. The voltage and current ranges used were 0.1–60.0 V and 0.1–2.0 A, respectively.
Electrokinetic experiments
After placing 1,000 mg/kg Pb-contaminated soils in the electrokinetic cell, the sample was subjected to electrokinetic remediation for different remediation periods and different conditions, which was carried out in triplicate to ensure the accuracy of the results. A summary of the experiments is shown in Table 1. EK remediation with the fixed anode was operated at constant voltage of 20 V (1 V/cm), supplied by a DC power supply, and changed the voltage based on the distance between two electrodes during operating AA-EK. All the experiments were carried out at room temperature (25 ± 3 °C). Readings of current intensity and pH in the electrode compartments were recorded periodically. At the end of electrokinetic remediation tests, soil samples were disassembled from the cell and sliced into five sections (S1–S5), each of which was 4-cm long. The final soil pH in each section was then measured using a spear type pH electrode. Additionally, the overall treatment efficacies were calculated as follows:
where C0 is the initial amount of Pb in the soils (milligram) and C1 is amount of Pb that remains in the soils after treatment. And Tessier sequential extraction was used to analyze the fractionation of lead (Tessier et al. 1979).
Analytical methods
These soils have been characterized in detail and used in previous investigations. All reagents used were of analytical grade. The pH value of the solid sample was measured in KCl solution (1 M) at a liquid/solid ratio of 2.5:1 using a Mettler Toledo EL20. The moisture content of the sediment was calculated with the loss of weight of the sample after heating at 105 °C for 24 h. The cation exchange capacity (CEC) is determined by the ammonium acetate method, and the organic matter is calculated using potassium dichromate oxidation procedure. The soil conductivity was measured by adding distilled water with a ratio of 2.5 mL/g dry soil. The suspension was agitated for 30 min, and the conductivity was measured using a conductivimeter after 20 min of response period. An atomic absorption spectrophotometer (AAS) was used to determine the concentration of total Pb according to USEPA methods 7190 and 7130, using a SHIMADZU AA7000 (Ashraf and Krishna 2008). All the experiments are triplicate, and duplicate measures were carried out with an experimental error lower than 3 %. Physicochemical properties of the unreacted soil samples are reported in Table 2.
To determine metal fractionation in the soil, a five-step sequential extraction analysis was carried out. The sequential chemical extraction procedures can help in assessing the potential mobility and solubility of metals in contaminated soils. The adopted procedure closely followed the scheme proposed by Tessier et al. (1979). However, these fraction designations may not apply to freshly and/or heavily contaminated soils that contain small amounts of organic matter (Reddy et al. 2001). Therefore, the five fractions could be categorized as: (1) exchangeable and soluble forms, (2) carbonates and easily soluble oxides/hydroxides (weakly adsorbed) under slightly acidic conditions, (3) Fe–Mn oxides and additional soluble oxides/hydroxides, (4) organic matter and metals associated with easily oxidizable solids or compounds, and (5) residual and strongly held complexes. The detailed extraction procedure for the five fractions is described by Chen et al. (2006).
Results and discussion
Distribution of pH and current after EK treatment
Under an electrical field, the electrolysis of water occurs in the electrode chamber accompanying the electrokinetic process. The H+ produced decreased the pH near the anode. On the other hand, an increase in the OH− concentration increases the pH near the cathode. H+ and OH− migrated through the soil toward the opposite electrode, forming an acidic and a basic front. When both fronts met, soil was divided into two zones: a high- and a low-pH zone with a sharp pH peak in between (Li et al. 1997). The pH peak was next to the cathode because the mobility of H+ is 1.8 times greater than that of OH− (Acar and Alshawabkeh 1993).
Figure 2 shows the change of pH in the different soil sections at the completion of experiments. The soil pH varied to different extent compared to the initial conditions because electrode reactions and pH control the electrolytes. A general trend of low pH near the anode and high pH near the cathode was found in test-1–test-3, which was due to the formation and transport of H+ and OH− ions at the anode and cathode, respectively. But in test-2 and test-3, the soil pH decreased to a less extent compared to that in test-1, suggesting a successful pH control of the electrolytes with adding EDTA. And the pH of the soil samples with both EDTA and acetic acid (test-4) after the application of EK ranged from 2.5 to 4.1 (below the initial pH = 6.86). It is ascribed to the high buffer capacity of the acidic soil for OH− ions and limited ions introduced into the soil column, which was affirmed by the low current. In addition, it suggested that soil pH and metal compound solubility are of crucial importance for the successful removal of Pb from contaminated soil (Suer et al. 2003).
Electric current is an indication of the amount of ion electromigration (Shenbagavalli and Mahimairaja 2010). The time course of electric current during an electrokinetic experiment is shown in Fig. 3, indicating that the electric current in the process firstly increased and then decreased, finally remaining stable. Initial electric current was 40, 45, 50, and 60 mA for test-1, test-2, test-3, and test-4, respectively. When the reservoir pH was not conditioned with EDTA (test-1), the electric current was relatively small. As water electrolyzes, the generated large amounts of H+ moved toward the cathode, and the soil was acidified, which in turn promoted the desorption of heavy metal ions and other ions and consequently increased the electrolyte concentration. As a result, the electric current increased to 140 mA rapidly at 96 h, then decreased to 60 mA, and reached to 52 mA. Similar phenomenon was observed in other three EK systems; this may be caused by two reasons: (1) resistance at the interface between electrodes and electrolyte might increase because of concentration polarization and water dissociation and (2) ions with positive or negative charges move to the two ends of the electric cell as in electrodialysis, which results in the drop of ionic strength in soils and the current as well (Acar and Alshawabkeh 1996).
With the concentration of EDTA increased, electric current gradually decreased because EDTA is nonconductive organic molecules, decreasing the conductivity of soil solution and consequently diminishing the electric current. Additionally, because of the effect of acetic acid buffer solution, the electric current in test-4 was higher than the others. The acidic solution enhanced desorption of ions from the surface of the soil into pore water, and higher concentration of ions in pore water increased the electric current under a constant voltage condition. However, a higher current led to higher power consumption.
A similar trend of the gradual decrease in electric current to a stable value among test-1–test-4 suggests that the soil itself contained free ions, which were removed from soil matrix at early stages (Zhou et al. 2004). The time for obtaining stable current in different treatments varied depending on the migration rates of ions from solution to soil columns.
Removal efficiency after electrokinetic treatment
Generally, the principal mechanisms leading to the removal of metals from the soil are electromigration and electroosmotic flow in EK remediation (Acar and Alshawabkeh 1993; Sparks et al. 1996). Electromigration refers to that the charged ions in the water flow move to the counter-electrode by the application of electrical current. The electroosmosis flow is a water flow caused by potential difference of the electrode and contaminants carried toward the electrodes.
After remediation, the Pb remaining in the soil was determined, and the average value of Pb in the five sections was calculated as the content of lead in the whole soil cell. Figure 4 shows the distribution of Pb in the soil sections at the end of the EK remediation. In test-1, the distribution of soil Pb showed accumulation and a peak in the sections closed to the cathode. Compared with test-2, the results indicated that the remediation efficiencies of lead were significantly improved by adding EDTA in the catholyte. And with the concentration of EDTA increased, the removal efficiency was also improved gradually, which was enhanced from 47.8 % (0.1 M, test-2) to 61.5 % (0.2 M, test-3). As a kind of chelating agent, EDTA can attach to a metal ion up to six sites and make heavy metals desorb from the surface of soil particle and increases the rates of migration of heavy metal ions in the soil. In the electrokinetic remediation, EDTA forms negatively charged complexes, which migrate toward the anode, at high pH values.
Figure 4 also shows that the effective migration of EDTA from the catholyte into the soils as well as the effective formation of Pb–EDTA complexes are required to improve the remediation efficiencies, which weakens the deposition of Pb2+ near the cathode and improves the removal of Pb in the soil. Additionally, adding the buffer solution can prevent the cathode polarization, and then improve the remediation efficiency of EK. In test-4, after adding the acetic acid to catholyte, the removal efficiency increased to 72.3 %.
An analysis of variance (ANOVA) was conducted in order to compare and analyze the average values of the variable. The difference significance between different treatments was analyzed by the one-way ANOVA using SPSS 16.0. Significant difference at p < 0.05 was shown with different letters. The significant differences among the four tests (test1–test4) are shown in Table 3. Data were presented as mean ± standard deviation (SD).
Approaching anodes
The above methods greatly increased the removal efficiency but were time- and energy-consuming. To increase efficiency, the EK method with approaching anodes was tested.
Figure 5 shows the operation voltage and electric current in electrokinetic remediation with approaching anodes. The voltage was maintained at 1 V/cm during the course of the operation. At the beginning of the electrifying mode, the electrical current started at 60 mA and increased up to its highest (120 mA) at 48 h, then decreased after that until less than 40 mA after 240-h treatment. As shown in the fixed anode experiments (Fig. 3), the electrical current exhibited higher values than that. These results suggested that approaching anodes electrokinetic remediation could maintain more mobile ions in the system, and the phenomenon partially explained the possible mechanism of enhanced Pb removal in the tests.
AA-EK is a process of progressive acidification in a soil system. Figure 6 shows pH distribution of soil sections during an enhanced electrokinetic remediation. The initial pH of soil was 6.86, as shown in the basic remediation, and the pH of soil samples after 48 h was 4.73, which was the same as that in the fixed anode experiments. However, when the electrode was approaching toward the cathode, the pH in the area near the cathode (mainly in S5) increased gradually, and the pH in S4, S3, and S2 decreased.
At the end, soil pH in the cell was 5.05, indicating that AA-EK had controlled the pH focusing effect. It can also be seen that the soil pH value dropped evidently faster with AA-EK than with fixed anode experiments. Similar pH variation was reported in a previous study (Shen et al. 2007). With the electrodes’ distances shortened, the migration distances of H+ ions decrease, and then H+ ions can quickly approach the cathode.
Metal fractionations in the soil provide important information related to the electrokinetic phenomena (Kim et al. 2002). The distribution and fractionation of Pb in the soil section after electrokinetic experiment were shown in Fig. 7. The average removal efficiency in AA-EK process was higher than others obviously, and it reached to 83.3 %. Five forms of Pb in contaminated soil account for a certain proportion. The initial concentrations of exchangeable, carbonate-bound, Fe–Mn oxides-bound, organic-bound, and residual forms in soils were 140.14, 413.56, 297.92, 104.86, and 23.52 mg/kg, respectively. And the content of residual forms had small changes in soil sections after EK experiments. In test-1, the removal efficiency of Pb in the soils was decreased from anode to cathode, and the exchangeable was the mainly fractionations until S4 because there was a peak of pH in the section near the cathode and the current decreased gradually. The acidification ceased in the peak soils, and then the concentration of OH− was increased gradually, causing that partial exchangeable fractionations were changed to Fe–Mn oxides bound. In the soil near the cathode, Pb mainly exists in the state of carbonate bound and Fe–Mn oxides bound and less exchangeable Pb. It can clearly be seen that the increase of pH seriously impedes the removal of Pb, and the pH must be controlled. When both EDTA and acetic acid solution were used in the catholyte (test-4), a high concentration of Pb as exchangeable fraction was retained in the contaminated soil after a 360-h electrokinetic treatment.
After remediation in the AA-EK experiment (test-5), the average content of Pb in the soluble, the percent of exchangeable and carbonate fractions was higher than those in the fixed anode electrokinetic experiments; whereas, the average content of Pb in the residue fraction was not only lower in the AA-EK experiment than that in the fixed anode electrokinetic experiments but also lower than the content prior to remediation. The low soil pH in the AA-EK experiment favored desorption and dissolution of lead from the soil. Therefore, the transformation of lead in the Fe–Mn oxides-bound, organic-bound fraction to exchangeable fractions was more significant. Based on above results, through the AA-EK remediation, the Pb form that is more difficult to extract can be transformed to the more easier-extracted form (i.e., the exchangeable fraction).
Conclusion
Conclusions derived from the experimental studies have been summarized as follows:
-
(1)
Lead was not effectively removed from contaminated soils without the presence of EDTA due to its poor water solubility. The remediation efficiency was significantly enhanced by adding EDTA, indicating that the formation of the Pb–EDTA complexes increased the apparent lead water solubility. And the migration rate of Pb was also further improved when both EDTA acetic acid were used in the experiment.
-
(2)
The AA-EK process not only improved the removal efficiency of Pb but also decreased the lead accumulation phenomenon in soil. The average removal efficiency of lead was 83.7 % for AA-EK. In addition, the total operating time and the energy consumed were accordingly saved.
-
(3)
The efficiency of electrokinetic remediation was closely related to Pb speciation, and the exchangeable and carbonate-bounded Pb were the dominant forms which could be removed.
References
Acar YB, Alshawabkeh AN (1993) Principles of electrokinetic remediation. Environ Sci Technol 27:2638–2647
Acar YB, Alshawabkeh AN (1996) Electrokinetic remediation. I: pilot-scale tests with lead spiked kaolinite. J Geotech Geoenviron Eng ASCE 122:173–185
Amrate S, Akretche DE, Innocent C (2005) Removal of Pb from a calcareous soil during EDTA-enhanced electrokinetic extraction. Sci Total Environ 349:56–66
Ashraf Z, Krishna R (2008) Transient behavior of heavy metals in soils during electrokinetic remediation. Chemosphere 71:860–871
Chen XJ, Shen ZM, Lei YM, Ju BX, Wang WH (2006) Enhanced electrokinetic remediation of Cd and Pb spiked soil coupled with cation exchange membrane. Aust J Soil Res 44:523–529
Giannis A, Gidarakos E (2005) Washing enhanced electrokinetic remediation for removal cadmium from real contaminated soil. J Hazard Mater 123:165–175
Giannis A, Gidarakos E, Skouta A (2008) Transport of cadmium and assessment of phytotoxicity after electrokinetic remediation. J Environ Manag 86:535–544
Hansen HK, Ottosen LM, Kliem BK, Villumsen A (1997) Electrodialytic remediation of soils polluted with Cu, Cr, Hg, Pb and Zn. J Chem Technol Biotechnol 70:67–73
Hicks PE, Tondorf S (1994) Electrorestoration of metal contaminated soils. Environ Sci Technol 28:2203–2210
Kedziorek MAM, Bourg ACM (2000) Solubilization of lead and cadmium during the percolation of EDTA through a soil polluted by smelting activities. J Contam Hydrol 40:381–392
Kim SO, Kim KW, Stuben D (2002) Evaluation of electrokinetic removal of heavy metals from tailing soils. J Environ Eng 128:705–715
Kim C, Lee Y, Ong SK (2003) Factors affecting EDTA extraction of lead from lead-contaminated soils. Chemosphere 51:845–853
Kimura T, Takase K, Tanaka S (2007) Concentration of copper and a copper-EDTA complex at the pH junction formed in soil by an electrokinetic remediation process. J Hazard Mater 143:668–672
Lageman R (1993) Electroreclamation: application in the Netherlands. Environ Sci Technol 27:2648–2650
Lageman R, Pool W, Seffinga G (1989) Electro-reclamation: theory and practice. Chem Ind 18:585–590
Li G, Guo SH, Li SC, Zhang LY, Wang SS (2012) Comparison of approaching and fixed anodes for avoiding the ‘focusing’ effect during electrokinetic remediation of chromium-contaminated soil. Chem Eng J 203:231–238
Li Z, Neretnieks I, Yu JW (1997) Removal of Cu(II) and Cr(III) from naturally contaminated loam by electromigration. Journal of Environmental Science and Health. Part A 32: 1293
Lo IMC, Yang XY (1999) EDTA extraction of heavy metals from different soil fractions and synthetic soils. Water Air Soil Pollut 109:219–236
Nogueira MG, Pazos M, Sanroman MA, Cameselle C (2007) Improving on electrokinetic remediation in spiked Mn kaolinite by addition of complexing agents. Electrochim Acta 52:3349–3354
Probstein RF, Hicks RE (1993) Removal of contaminants from soils by electric fields. Science 260:498–503
Reddy KR, Shirani AB (1997) Electrokinetic remediation of metal contaminated glacial tills. Geotech Geol Eng 15:3–29
Reddy KR, Xu CY, Chinthamreddy S (2001) Assessment of electrokinetic removal of heavy metals from soils by sequential extraction analysis. J Hazard Mater 84:279–296
Reddy KR, Chinthamreddy S (2003) Sequentially enhanced electrokinetic remediation of heavy metals in low buffering clayey soils. J Geotech Geoenviron 129:263–277
Shen ZM, Chen XJ, Jia JP, Qu LY, Wang WH (2007) Comparison of electrokinetic soil remediation methods using one fixed anode and approaching anodes. Environ Pollut 150:193–199
Shenbagavalli S, Mahimairaja S (2010) Electrokinetic kinetic remediation of contaminated habitats. Afr J Environ Sci Technol 4:930–935
Sparks DL, Page AL, Helmke PA (1996) Methods of Soil Analysis. Part 3: Chemical Methods (Soil Science Society of America Book Series, No. 5). American Society of Agronomy-Soil Science Society of America.
Suer P, Gitye K, Allard B (2003) Speciation and transport of heavy metals and macroelements during electroremediation. Environ Sci Technol 37:177–181
Suer P, Lifvergren T (2003) Mercury-contaminated soil remediation by iodide and electroreclamation. J Environ Eng 129:441–446
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Virkutyte J, Sillanpaa M, Latostenmaa P (2002) Electrokinetic soil remediation—critical overview. Sci Total Environ 289:97–121
Virkutyte J, Hullebusch E, Sillanpaa M, Lens P (2005) Copper and trace element fractionation in electrokinetically treated methanogenic anaerobic granular sludge. Environ Pollut 138:517–528
Wong JS, Hicks RE, Probsteini RF (1997) EDTA-enhanced electroremediation of metal contaminated soils. J Hazard Mater 55:61–80
Yeung AT, Hsu C, Menon RM (1996) EDTA-enhanced electrokinetic extraction of lead. J Geotech Geoenviron 122:666–673
Zhou DM, Deng CF, Cang L (2004) Electrokinetic remediation of a Cu contaminated red soil by conditioning catholyte pH with different enhancing chemical reagents. Chemosphere 56:265–273
Acknowledgments
This study was supported by grants from the National Special Project on Water Pollution Control and Management (no. 2012ZX07503-002). Prof. Mengqiang Zhu from the University of Wyoming (USA) is appreciated for his comments during the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Zhang, T., Zou, H., Ji, M. et al. Enhanced electrokinetic remediation of lead-contaminated soil by complexing agents and approaching anodes. Environ Sci Pollut Res 21, 3126–3133 (2014). https://doi.org/10.1007/s11356-013-2274-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2274-9