Abstract
Wanshan mercury mine is the largest cinnabar deposit in Guizhou, China. Few effective methods had been achieved to remedy Hg heavily contaminated field soils. In this paper, a modified EK method with approaching cathodes (AC-EK) and an I−/I2 lixiviant was described to remedy mercury-contaminated field soils near Wanshan mercury mine. Paddy Soil I and Paddy Soil II were sampled and contained 576.73 ± 45.50 and 491.35 ± 4.73 mg/kg Hg, respectively. Although they contained 6.9 and 9.4% organic matter respectively, more than 92 and 89% Hg were removed by AC-EK within 5 days. Removal ratio increased by 0.21 and 0.68 times using EK process with ACs over that with one single cathode (SC-EK). AC-EK method saved nearly 26.4–28.1% electric power as compared to SC-EK method. As an I−/I2 lixiviant solution was used to solubilize HgS(HgO) during EK process, the bonding of Hg to organic functional S groups should be less important than the binding to inner sites of organic matter in soil. The relationship between EK remediation effect and soil organic matter content was fitted to a linear model. It turned out that when soil OM increased by 1.0%, EK removal ratio (%) of Hg would decrease by 2.63%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury is one among the most highly bioconcentrated toxic trace metals. Many national and international agencies have expended considerable effort to monitor and control mercury emissions (Lucchini et al. 2003; Mirlean et al. 2003). China is the third largest mercury producer. Total annual output was reported to be 200 tons in 2000 (Hylander and Meili 2003). Peak annual mercury emissions to the atmosphere in the process of mining and refining have reached 11 tons (Ji et al. 2006). Guizhou Province is the most important mercury mining area in China. Wanshan mercury mine, Guizhou is the largest cinnabar deposit in China, called the ‘‘mercury capital’’ of China. Exploration of mercury in Wanshan has led to serious environmental pollution and severely deteriorated local ecosystems (Ji et al. 2006; Tan et al. 1997). Although numerous studies have been carried out in Wanshan mercury mine, most of them have focused merely on the distribution, characteristics, and environmental behavior of mercury (Qu 2002; Horvat et al. 2003). To date, few effective methods have been achieved to remedy heavily contaminated field soils there.
During the past decades, electrokinetics (EK) remediation is tested to be one of the most effective methods to remove heavy metals from soil (Acar and Alshawabkeh 1993; Pierzynski et al. 2000). During EK processing, H+ ions are generated at the anode and OH− ions are generated at the cathode. Low pH in soils favors desorption of contaminants and dissolution of heavy metal contaminants, while high pH in soils facilitates sorption of contaminants and precipitates heavy metals. Nevertheless, OH− ions are inevitably generated through the electrolysis of water in a cathode compartment. To avoid precipitation of heavy metals in soils near the cathode, various enhancement techniques have been proposed and developed: (1) adding or mixing strongly complexing agents such as ammonia, citrate, oxalate, acetic acid, and EDTA into soil, which compete with soil for metal contaminants to prevent precipitation or solubilize precipitates of cationic metal contaminants near the cathode (Reddy and Chinthamreddy 2003). (2) conditioning the anode and/or the cathode reservoirs or using ion exchange membrane (IEM) to control the pH and zeta potential as well as increasing mobility of contaminants (Ottosen et al. 2005).
The difficulty of EK remediation of Hg-contaminated soils is the low solubility of Hg in most natural soils. Enhanced EK remediation has recently been developed using an I−/I2 lixiviant solution to solubilize Hg from contaminated solids (Cox et al. 1996; Reddy et al. 2003; Suer and Lifvergren 2003). Introduced near the cathode, the lixiviant migrates through the soil to the anode by electromigration. Hg, released by the oxidation of HgS compounds by I2, was complexed as HgI 2−4 . The negative complex continued to electromigrate toward the anode. Several experiments have been reported on the distribution of mercury on soils in this method. However, mobilization of mercury from field-contaminated soils has been less successful (Suer and Lifvergren 2003). The relationship between EK remediation effect and soil organic matter content had not been quantificationally studied before.
This method can still be improved to save more energy and time (Shen et al. 2007). An enhanced EK method with approaching anodes (AAs) has been proposed to accelerate electromigration effect with spiked soils. Soil remediation could be accelerated and much energy and time be saved. In this paper, a modified EK method with an I−/I2 lixiviant assisted and approaching cathodes was proposed to remedy mercury contaminated field soils, which were near Wanshan mercury mine, Guizhou, China.
Materials and methods
Soil samples used in the study
Mercury-contaminated field soil samples (Paddy Soil I and Paddy Soil II) were taken from cultivated/recultivated areas with paddy around Dashuixi Stream which flows across the Wanshan mercury smelting workshop. They were collected from the surface horizon (0–15 cm).
The pH values of soil samples in 0.01 M CaCl2 solution (1:2.5) were measured using a pHS-3TC pH meter (Shanghai Hongyi Instrument Factory, Shanghai, China). Electrical conductivity (EC) was measured with Conductmeter (DDS-11A, Shanghai Hongyi Instrument Factory, Shanghai, China). Cation exchange capacity (CEC) was obtained using ammonia acetate method (Rhoades 1982). Hydraulic conductivity was measured with constant-head permeameter (Reynolds and Elrick 2002). Mechanical analysis of the soils was carried out by gravity sedimentation after dispersion of the soils with 5% sodium hexametaphosphate. Bulk density and moisture content were tested using stainless steel Kopecky rings of 100 cm3. The samples were oven-dried at 105°C for 24 h. The results were calculated as g/cm. Organic matter (OM) was measured with a Multi N/C 3000 analyzer (Analytik Jena AG, Jena, Germany).
Electrokinetic remediation apparatus and operation
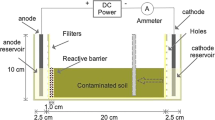
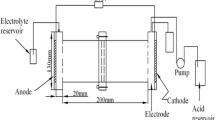
Electrokinetic remediation experiments were carried out in a rectangular translucent Plexiglas test cell (Fig. 1) that is 21-cm long, 5.5-cm wide and 11-cm high. Dimensions of the filled cell are 20.0 × 5.0 × 7.0 cm. EK remediation with one Single Cathode (SC-EK) was operated at constant voltage of 20 V(1.0 V/cm) supplied by a power source. Both the anode and the cathode are mesh Ti/Ru electrodes which are made of 2.0 mm. Titanium stick with 3.0 × 9.0 mm lozenge meshes (Xinxiang Future Hydrochemistry Co., Ltd., Xinxiang, China). Each electrode has an effective area of 40 cm2 and electrode compartments are connected to either end of the cell using screws. At the beginning, the cell was filled with a buffer solution containing 0.1 M sodium citrate, 0.1 M KI and 0.025 M I2 at pH 6. Then the tested soil was slowly filled into the cell [solid:liquid 2:1 (w:w)]. Air bubbles in the soil specimen were slowly removed to decrease the electrical resistance. Surplus buffer solution (2.0 l) was continuously pumped into the cathode compartment and out of the anode compartment through hydraulic gradient during EK process.
Electrokinetic remediation with approaching cathodes (AC-EK) was operated in the same apparatus, except that there were four mesh Ti/Ru electrodes as ACs placed in the soil bed. ACs were placed at a distance of 20, 15, 10, and 5 cm from anode. They were sequentially switched on at 0, 24, 48, and 72 h after EK process started. So, approaching cathodes worked in turn from the first one at 20 cm. Intensity of electric field was kept at 1 V/cm.
After the EK experiments were over and the cells were disassembled, the residual Hg concentrations in the soil were used to calculate the removal efficiency. No special analysis was performed to extract and analyze the mercury attached to the anode electrodes and on the cell walls or trapped.
Analysis of mercury and quality assurance
Soil samples were digested in acid according to EPA Method 3052-1996 (USEPA Method 1996). The reagents used in the acid digestion were concentrated HNO3, HCl, HF, and H2O2. The dwell time and control pressure in the microwave system followed the sequence of 5 min at 415 kPa, 10 min at 275 kPa, and finally 20 min at 205 kPa. The liquid obtained was filtered by a 0.45 μm membrane filter. Mercury was analyzed with an atomic fluorescence spectrometer (AFS-810, Beijing Titan Instruments Co. Ltd). The testing procedure and the optimized conditions were developed according to Martinez-Cortizas et al. (1999).
Every sample was analyzed three times, and the mean value was achieved. The accuracy of procedure was monitored by analysis of reference materials BCR(No. 145, certified Hg 8.82 ± 0.88 mg/kg) from Commission of the European Communities. The certified standard samples were included at every stage of analysis. Other chemical analyses were repeated for three times.
The speciation of mercury in soil was divided into five fractions (Shen et al. 2007): (1) exchangeable, (2) bound to carbonates, (3) bound to Fe–Mn oxides, (4) bound to organic matter, and (5) residual. Total Hg concentrations tested using acid digestion method of Paddy Soil I and Paddy Soil II were 576.73 ± 45.50 and 491.35 ± 4.73 mg/kg, respectively. Sum Hg concentrations of five speciations using sequential extraction were 536.73 ± 16.23 and 464.23 ± 5.88 mg/kg, respectively. Similarly, the average percent recovery was about 93.06 and 94.48% for the sequential extraction process conducted with the two soil samples. The variation of chemical analysis was thought to be acceptable.
Results and discussion
Soil physic-chemical characters
Various parameters such as pH, organic matter content, sand, silt, clay, and cation exchange capacity in soils may influence the transport of Hg during EK processes. Main physic-chemical characterization of the experimented soils was shown in Table 1. The experimented soils were attributed to the sandy loam according to the USDA classification system. As the soils contained 6.9–9.4% organic matter (OM), organic S functional groups (thiols), carboxyl groups and others in natural organic matter may bind all Hg in these tested soils. High organic matter content would decrease the transport and transformations of Hg in soil (de Oliveira et al. 2007).
Variation of electric current and electrical conductivity
Variation of electric current and electrical conductivity during SC-EK and AC-EK processes are shown in Figs. 2 and 3, respectively. Electric current is an indication of the amount of ion electromigration. Electric current decreases quickly in the first few hours, and declines slowly afterwards, even though the potential from the power supply is kept constant. It is because of the loss of ionic strength in the pore fluid and the increase of resistance polarization: Ion transport in EK process is like that in electro-dialysis process. Ions with different charges, positive or negative, move to the two ends of the electric cell, which results in a drop of ionic strength in soils (Ottosen et al. 1997; Shrestha et al. 2003). Electric current in AC-EK is higher than in SC-EK. It lied in that ACs method shortened the electrolysis distance, i.e., ion transportation distances. So, more mobile ions were maintained in the electric cell. This partly explained the possible mechanism of enhanced Hg removal in the AC-EK tests. The electrical conductivity near anode increased gradually and that near cathode decreased simultaneously during the remediation process in both series of tests. It is mainly due to that most anions in soils moved toward the anode and accumulated there.
Variation of pH and Eh (redox potential)
As soils were dunked with sodium citrate solution (pH = 6.0, 0.1 M) before EK processes, soil pH values of three samples decreased from the background values 7.18, 7.59 and 8.07–6.30, 6.51 and 6.81, respectively (Fig. 4). It lies in the soil’s buffering capacity. The soil pH close to the anode was less than 2.0 after EK (Fig. 4). It was significantly lower than that of other soil parts, for H+ ions are produced at the anode. As electrode was washed with sodium citrate solution during EK, pH values in soil bed are almost all below 7.0. It is necessary to maintain properly low pH in the soil specimen when heavy metals are to be removed by means of EK remediation (Cao et al. 2001). The soil pH values at anode during AC-EK are less than those during SC-EK, for more H+ must be produced as electrolysis distance becomes shorter.
Variations of Eh (redox potential) in the soils are shown in Fig. 5. The initial Eh values of the tested soils are 180–184 mV. Before EK treatment soils were soaked with KI/I2 solution (0.1 M KI, 0.025 M I2), and their Eh values reach 360–390 mV. During the 120-h EK treatment, Eh values near the anode increase linearly, while those near cathode decrease linearly. Generally, variation ranges of Eh during AC-EK are less than that during SC-EK. The high Eh for most of EK-treated soil near the anode indicates highly oxidizing conditions, while the low Eh near the cathode indicates reducing conditions. This is due to the electric potential of DC and its redox reactions at electrodes. The Eh greatly influences chemical association of Hg on soil particulates. According to the former work, insoluble Hg in soils at acidic and high-Eh conditions must be oxidized and released from soil particles. Oxidation of reduced insoluble Hg by I2 releases Hg(II), which is complexed as HgI 2−4 . Reactions describing the solubilization of various insoluble Hg species are given as (Cox et al. 1996):
Variation of Hg concentration and speciation
Figure 6 gives changes of Hg concentration in soil samples, with 1 V/cm DC applied for 5 days. Hg concentration in soils near the cathode decreases quickly and that near anodes slowly during EK process, because electric field causes HgI 2−4 migration from cathode toward the anode. It can be seen that migration velocity of Hg concentration is larger in soil samples near cathode than that away from cathodes. This difference of migration velocity at various places lies in electromigration direction and distance of HgI 2−4 . As it is transported from sections near the cathode to the anode, during EK remediation, the nearer the soil samples are to the cathode, the higher the HgI 2−4 removal velocities are. After 5 days of SC-EK treatment, 82 and 53% of Hg were removed from soils on the average in Paddy Soil I and Paddy Soil II, respectively. Simultaneously, after 5 days of AC-EK treatment, 92 and 89% of Hg were removed. Removal ratio reached 1.21 and 1.68 times as high as those after SC-EK process. It has been demonstrated that more than half of the removed Hg was transported to the anode compartment. As for a contaminated field soil, some 50% of the total mercury content had migrated to the anode compartment, and another 25% was recovered from the soil water in the vicinity of the anode (Suer and Lifvergren 2003). The left Hg in treated soils was less than 50 mg/kg and more than 60% of it belongs to residual speciation (Fig. 7). Although the left Hg content is still higher than the national standard level of China, 1.5 mg/kg [State Environmental Protection Administration of China (SEPAC) 1995], it still indicated that this modified AC-EK method is effective to remedy mercury-contaminated field soils. The remaining mercury was too immobile to be removed by electroremediation with iodide addition. It may be hypothesized that these compounds may remain stable for longer periods of time even under moderate changes in soil chemistry. Consequently, mercury in soils after electroremediation may pose little risk to humans and the environment.
The Hg removal ratio from the soil is greatly affected by the complicated composition, such as the presence of carbonates and organic matter, which caused Hg to adsorb to the soil and/or as an immobile chemical species. It has been testified that Hg has an affinity for organic matter (Reddy et al. 2003). However, the relationship between EK remediation effect and soil organic matter content has not been quantificationally studied. Connected with several types of soil studied by other scholars using EK method an I−/I2 lixiviant solution before, the relationship between EK remediation effect and soil organic matter content was fitted to a linear model (Fig. 8). It showed a great significance. It was calculated that when soil OM increases by 1.0%, EK removal ratio (%) of Hg will decrease by 2.63%. As most field soil was polluted by wastewater-containing soluble Hg, it could penetrate into the inner sites of OM lattices. Apparently, diffusion out of deep sites during EK process was slow. So contents of organic matters in field soil were significantly negatively correlated with EK remediation effect.
As an I−/I2 lixiviant solution was used to solubilize HgS(HgO) during EK process, the bonding to organic functional S groups (thiols) should be less important than the binding of Hg to inner sites of organic matter soils. In loam (4% OM) spiked with HgS, almost 99% of the Hg was removed after 16 days of EK treatment, to a level of less than 10 mg/kg. However, only 6% of the Hg was removed after 14 days of EK treatment from East Fork Poplar Creek (EFPC) field soil (11% OM), in Oak Ridge, TN, USA (Cox et al. 1996). Humic and fulvic acids, parts of OM, in soil have a very high affinity for Hg (Skyllberg et al. 2000). Destruction of the active sites on humic and fulvic acids, diffusion from the inner sites of OM lattices should be the most important. The destruction and diffusion reactions were accelerated when the electrolysis distance was shortened and electric current increased in the modified AC-EK. Hence, removal effect was enhanced with ACs.
For future industrial application, it might be more feasible to use cathode arrays. Besides, electrode distances and numbers should be balanced when scaling up the process. AC-EK operation with short distance would save much time and energy, but it would need many electrodes which would increase the investment.
Energy consumption and migration effect
With ACs, the distance between anodes and the cathode was gradually shortened. After working cathodes approached, cleaned soils were not yet consume energy. So, less energy was needed to provide the same migration effect of heavy metals. Energy consumption per unit volume of soil is calculated using the following equation (Shen et al. 2007):
where, V s is volume of soil processed; V is voltage difference between the electrodes; and I is electric current. E u is calculated as kWh m−3.
It is calculated that the energy consumption with ACs is much lower than that with SC, as shown in Fig. 9. Not only is treatment effect of 120-h EK with ACs better than that with SC, but also ACs method can save nearly 26.4–28.1% electric power. It shows great economic superiority to remedy field soils heavily contaminated by Hg.
Mechanism
The mechanism that AC-EK method enhances the remediation effect of mercury-contaminated field foils lies in three main aspects:
-
(1)
Enhance solubilization and transport of the contaminated Hg by adding KI/I2 solution (0.1 M KI, 0.025 M I2).
-
(2)
Electromigration of HgI 2−4 ions is expedited as electrode distance is shortened step by step. During AC-EK process, Eh variation range in soil profile is reduced. It is tested that when Eh increased by 100 mV, desorption ratio of Hg increased by 17 and 15% from Paddy Soil I and Paddy Soil II, respectively.
-
(3)
EC is enhanced and cleaned soils do not yet consume electric power with ACs. As I− ions and HgI 2−4 ions move from the cathode toward the anode, they must react with active groups in soil solution and on soil particles during its forward movement. Many types of reactions occur during the remediation, including desorption, ion exchange, decomplexation, dissolution, destruction of the active sites on the soil surface, diffusion from the inner sites of the crystal lattices, etc. The destruction and diffusion reactions were accelerated when the electrolysis distance was shortened and electric current increased in the modified AC-EK. Therefore, removal effect was enhanced with ACs.
It can be concluded as follows:
HgS(Hg2Cl2) + IK/I2 → HgI 2−4 → Electromigrating towards anode → EC increase and Eh variation decrease with ACs → HgI 2−4 removal effect improved.
References
Acar YB, Alshawabkeh AN (1993) Principles of electrokinetic remediation. Environ Sci Technol 27(13):2638–2647
Cao XD, Chen Y, Wang XR, Deng XH (2001) Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 44:655–661
Cox CD, Shoesmith MA, Ghosh MM (1996) Electrokinetic remediation of mercury-contaminated soils using iodine/iodide lixiviant. Environ Sci Technol 30:1933–1938
de Oliveira LC, Serudo RL et al (2007) Distribution of mercury in different soils of Amazonia’s mid-Negro River basin: influence of organic matter on Mercury’s biogeochemical cycle. Quimica Nova 30(2):274–280
Horvat M, Nolde N, Fajon V et al (2003) Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci Tot Environ 304:231–256
Hylander LD, Meili M (2003) 500 years of mercury production: global annual inventory by region until 2000 and associated emissions. Sci Total Environ 304(1–3):13–27
Ji XL, Hu WX, Cheng JP et al (2006) Oxidative stress on domestic ducks (Shaoxing duck) chronically exposed in a Mercury–Selenium coexisting mining area in China. Ecotoxicol Environ Saf 64(2):171–177
Lucchini R, Calza S, Camerino D et al (2003) Application of a latent variable model for a multicenter study on early effects due to mercury exposure. Neurotoxicology 24:605–616
Martinez-Cortizas A, Pontevedra-Pombal X, Garcia-Rodeja E et al (1999) Mercury in a Spanish Peat Bog: archive of climate change and atmospheric metal deposition. Science 284(7):939–942
Mirlean N, Andrus VE, Baisch P (2003) Mercury pollution sources in sediments of Patos Lagoon Estuary, southern Brazil. Mar Pollut Bull 46:331–334
Ottosen LM, Hansen HK, Laursen S, Villumsen A (1997) Electodialytic remediation of soil polluted from wood preservation industry. Environ Sci Technol 31:1711–1715
Ottosen LM, Pedersen AJ, Ribeiro AB, Hansen HK (2005) Case study on the strategy and application of enhancement solutions to improve remediation of soils contaminated with Cu, Pb and Zn by means of electrodialysis. Gen Geo 77:317–329
Pierzynski GM, Sims JT, Vance GF (2000) Soils and environmental quality, 2nd edn edn. CRC Press, Boca Raton, p 383
Qu LY (2002) The pollution and prevention of mercury in Guizhou province. J Guizhou Normal Univ (Nat Sci) 20:56–59
Reddy KR, Chinthamreddy S (2003) Sequentially enhanced electrokinetic remediation of heavy metals in low buffering clayey soils. J Environ Eng 130(4):442–455
Reddy KR, Chaparro C, Saichek RE (2003) Iodide-enhanced electrokinetic remediation of mercury-contaminated soils. J Environ Eng 129(12):1137–1148
Reynolds WD, Elrick DE (2002) Constant head well permeameter (vadose zone). In: Dane JH, Topp GC (eds) Methods of soil analysis. Part 4. Physical methods. SSSA Book Series 5, Madison, pp 844–858
Rhoades JD (1982) CEC. In: Page AL (ed) Methods of soil analysis, Part 2, Chemical and microbiological properties, 2nd edn edn. ASA, Madison, pp 149–157
Shen ZM, Chen XJ, Jia JP et al (2007) Comparison of electrokinetic soil remediation methods using one fixed anode and approaching anodes. Environ Pollut 150(2):193–199
Shrestha R, Fischer R, Rahner D (2003) Behavior of cadmium, lead and zinc at the sedimentewater interface by electrochemically initiated processes. Colloids Surf A Physicochem Eng Asp 222:261–271
Skyllberg U, Xia K et al (2000) Binding of mercury(II) to reduced sulfur in soil organic matter along upland-peat soil transects. J Environ Qual 29(3):855–865
State Environmental Protection Administration of China (SEPAC) (1995) Environment quality standard for soils, GB15618–1995. Standards Press of China, Beijing, China
Suer P, Lifvergren T (2003) Mercury-contaminated soil remediation by iodide and electroreclamation. J Environ Eng 129(5):441–446
Tan H, He J, He T (1997) Dry and wet deposition of elemental mercury by moss bag near a mercury mine. Environ Sci 18:71–72
USEPA Method 3052 (1996) Microwave assisted acid digestion of siliceous and organically based matrices
Acknowledgments
The authors acknowledge the financial support of the National Natural Science Foundation of China (NO. 28467001 and 20377028) for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, Z., Zhang, J., Qu, L. et al. A modified EK method with an I−/I2 lixiviant assisted and approaching cathodes to remedy mercury contaminated field soils. Environ Geol 57, 1399–1407 (2009). https://doi.org/10.1007/s00254-008-1418-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-008-1418-6