Abstract
Purpose
The aim of the present study was to assess vitamin D levels in a large cohort of OSA patients and to investigate possible correlations with clinical and polysomnographic parameters.
Methods
In this cross-sectional study, 685 consecutive patients underwent type 1 polysomnography (PSG) for OSA diagnosis. They were grouped according to apnea–hypopnea index (AHI) as mild, moderate, and severe. Patients with AHI < 5 served as controls. Demographic, PSG data, and serum levels of vitamin D were measured and compared between groups.
Results
OSA was diagnosed in 617 of the patients (90%). Of those, 94 (15%) had mild OSA, 150 (24%) moderate OSA, and 373 (61%) severe OSA. The risk of vitamin D deficiency (< 20 ng/mL) was observed in 38% of the cohort. OSA patients had lower vitamin D levels compared to controls (23 ng/mL vs 26 ng/mL, p = 0.006). The lowest levels of vitamin D [mean 21] (p < 0.001 among all groups) and the higher prevalence for vitamin D deficiency (45%) were observed in severe OSA patients. After multiparametric adjustments for age, gender, obesity, and comorbidities, severe OSA showed significant independent associations with the risk of vitamin D deficiency [OR (95% CI) 2.002 (1.049–3.819), p = 0.035].
Conclusions
A large proportion of patients referred for OSA evaluation had vitamin D deficiency, which was independently associated with severe OSA. However, further research is needed in order to determine the role of vitamin D in OSA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a frequent sleep-related breathing disorder that involves obstructive apneas and hypopneas caused by repetitive collapse of the upper airway during sleep. Several adverse medical outcomes are associated with OSA, such as poor neurocognitive performance, cardio-metabolic diseases, and increased all-cause mortality [1]. Despite the fact that intermittent hypoxia and sleep fragmentation have been suggested as potential factors, the underlying mechanisms by which OSA may promote unfavorable medical sequelae are still not fully understood [2].
Recent evidence has identified a link between OSA and vitamin D serum concentration [3, 4], which seems to be decreasing with increasing severity of sleep apnea. Still, it is not known if low serum levels of vitamin D may contribute to OSA development or if OSA is a risk factor for vitamin D deficiency. OSA shares similar risk factors with vitamin D deficiency, such as age, obesity, and comorbidities [5,6,7]. In particular, daytime sleepiness and sleep fragmentation attributed to OSA may increase the risk of vitamin D deficiency [3, 8, 9]. Furthermore, it is worth noting that vitamin D homeostasis restoration is reported after OSA treatment with continuous positive airway pressure (CPAP) [10].
Vitamin D deficiency is frequent worldwide, with rates ranging up to 40% in European countries [11]. Sunny Mediterranean countries like Greece demonstrate relative low prevalence of vitamin D deficiency compared to other European countries, with a considerable variability according to gender, seasonality, and age [12]. Vitamin D coordinates calcium and phosphorus homeostasis and thus plays an important role in bone metabolism and several metabolic processes [13]. Moreover, a lot of cells in the body have vitamin D receptors as well as the ability to convert vitamin D into its active form, 1,25-dihydroxyvitamin D3 [14, 15]. Circulating 25-hydroxy vitamin D [25(OH)D] levels are considered indicative of vitamin D status [16]. It is worth noting that vitamin D is implicated in several non-skeletal conditions, including cardiovascular diseases and diabetes, all of which are overrepresented in patients with OSA [17, 18].
To date, few data exist regarding vitamin D status in OSA patients in Greece, but especially in Crete, which has abundant sunshine and high temperatures throughout the year as compared to other regions of Greece. Therefore, the aim of our study was to assess vitamin D levels in a large cohort of OSA patients in Southern Greece and assess potential association with clinical and polysomnographic parameters.
Methods
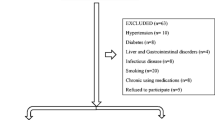
In this single-center, cross-sectional study, consecutive patients aged ≥18 years were evaluated in our Sleep Disorders Center for suspected sleep-disordered breathing during a 3-year period (2015–2018). Exclusion criteria included history of osteoporosis, vitamin D and calcium supplementation, conditions or intake of medications known to affect vitamin D metabolism and absorption, inflammatory diseases, cancer, and chronic liver or renal diseases. Ethical approval was provided by the University Hospital Ethics Committee (protocol number 23542) and the patients gave written informed consent.
Demographic characteristics
The collected data included anthropometric parameters, such as age, gender, height, weight, body mass index (BMI), neck circumference, waist/hip ratio, details of comorbidities, smoking history, and alcohol intake. The Epworth Sleepiness Scale (ESS) was used to estimate subjective daytime sleepiness [19]. Insomnia was assessed with Athens Insomnia Scale (AIS), an 8-item self-assessment psychometric instrument, which has been used as a tool to evaluate the severity of insomnia [20, 21]. Beck Depression Inventory (BDI) consists of 21 items used to evaluate characteristic attitudes and symptoms of depression [22,23,24]. Scores below 10 is considered normal, and higher scores indicate greater depressive severity (range 0–63).
Sleep study
All patients underwent a single-night full diagnostic polysomnography (PSG) study (Alice 5, Diagnostics System, Respironics, USA). PSG studies were performed and analyzed according to the American Academy of Sleep Medicine (AASM) standard criteria [25]. The apnea–hypopnea index (AHI), the average number of apneas plus hypopneas expressed per hour of sleep, was used for OSA diagnosis and assessment of its severity. OSA was classified as mild if 5 ≤ AHI < 15, as moderate if 15 ≤ AHI < 30, and as severe if AHI was ≥30 per hour.
Blood samples and measurements
Serum samples for vitamin D levels assessed as 25(OH)D were obtained the day following the PSG. Blood samples were taken after at least 8 h of fasting, they were immediately centrifuged (3000 rpm for 10 min), and the serum obtained had been frozen at −80 °C until processing. Serum concentration of 25(OH)D was measured utilizing a commercial radioimmunoassay kit based on manufacturer’s specifications (DiaSorin, Stillwater, MN). The 25(OH)D levels were assessed in agreement with the Endocrine Society parameters, which classify deficiency, insufficiency, and sufficiency as levels <20 ng/mL, 20–29.9 ng/mL, and > 30 ng/mL, respectively [26].
Statistical analysis
Continuous variables if normally distributed are presented as mean ± standard deviation (SD) and as median (25th–75th percentile) if they are not. Qualitative values are given as absolute number (percentage). Variables that were normally distributed were compared among the three groups using ANOVA. If ANOVA was significant, we used the Tukey-Kramer post hoc test to compare each pair. For variables that were not normally distributed, we used the Kruskal-Wallis test to compare the three groups. If the Kruskal-Wallis test was significant, Dunn’s pairwise tests were carried out for the three pairs of groups with adjustments for multiple testing using the Bonferroni correction. For categorical variables, we applied χ2 to test the differences between observed frequencies of the three groups. Correlation coefficients were calculated utilizing Pearson or Spearman’s (for non-normally distributed data) correlation test. We analyzed the association of OSA severity with vitamin D levels after adjustments for various potential explanatory variables, including age, BMI, smoking status, and comorbidities. Moreover, logistic regression analysis was applied to examine the effect of OSA on the risk of vitamin D deficiency, after adjusting for the previous confounding factors. Age was evaluated continuously and categorically, as age groups of 18–59 and > 60 years; BMI was also considered continuously and categorically, as BMI groups of <30 and ≥ 30 kg/m2. Statistical significance was confirmed at a p value of <0.05. Analysis was performed using SPSS software (version 25, SPSS Inc., Chicago, IL).
Results
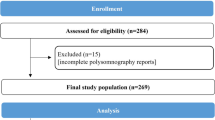
A total of 685 patients (mean age 54 years, 74% men) was included (Fig. 1). The baseline characteristics of study population, according to AHI, are shown in Table 1. OSA was diagnosed in 617 of the patients (90%). Of those, 94 (15%) had mild OSA, 150 (24%) moderate OSA, 373 (61%) severe OSA. Severe OSA was overrepresented compared to mild and moderate OSA, reflecting the typical demographics of our clinic population. Important confounding factors, such as percentage of males, age, obesity (as reflected by body mass index, neck circumference, and waist-to-hip ratio), and comorbidities significantly increased with increasing AHI.
OSA patients had lower vitamin D levels compared to controls (23 ng/mL vs 26 ng/mL, p = 0.02). The risk of vitamin D deficiency (< 20 ng/mL) was observed in 38% of the cohort. There was a significantly higher proportion of patients with female gender (34% versus 22%%, p = 0.001), severe OSA (64 vs 49%, p = 0.001), and obesity (72 vs 58%, p < 0.001) in the vitamin D deficiency group compared to the group without deficiency. Differences in other evaluated characteristics remained relative insignificant between those patients with vitamin D deficiency and those without, including age, diabetes, hypertension, cardiovascular disease, and smoking (all p > 0.05). Vitamin D deficiency was more prevalent during winter months (January–March 46%, April–June 43%, July–September 32% and October–December 30%; p = 0.03). The lowest levels (mean 21.4) (Fig. 2) and the highest prevalence for vitamin D deficiency (45%) were observed in severe OSA patients (Table 2).
Vitamin D correlations with several parameters
Levels of vitamin D were correlated with gender (r = −0.102, p = 0.008), BMI (r = −0.274, p < 0.001), neck circumference (r = −0.153, p < 0.001), presence of COPD (r = −0.119, p = 0.002), diabetes (r = −0.085, p = 0.026), indices of OSA severity including AHI (r = −0.221, p < 0.001), ODI (r = −0.197, p < 0.001), mean (r = 0.190, p < 0.001) and lowest nocturnal SpO2 (r = 0.186, p < 0.001), percentage of time with oxyhemoglobin saturation < 90% (TST90) (r = −0.215, p < 0.001), and sleep architecture parameters including SE(%) (r = 0.128, p = 0.001), WASO (r = −0.099, p = 0.01), SWS (%TST) (r = 0.129, p = 0.01), and AI (r = −0.185, p < 0.001). There was no association between vitamin D and age, waist-to-hip ratio, ESS score, other comorbidities, or PSG variables.
In stepwise multiple linear regression models, vitamin D levels were still associated with AHI [β = −0.054 (95% CI, −0.086, −0.022); p = 0.001], ODI [β = −0.035 (95% CI, −0.065, −0.004); p = 0.001], mean SaO2 [β = 0.303 (95% CI, 0.025, 0.582); p = 0.034], lowest SaO2 [β = 0.120 (95% CI, 0.009, 0.231); p = 0.034], TST90 [β = −0.017 (95% CI, −0.029, −0.005); p = 0.004], AI [β = −0.065 (95% CI, −0.120, −0.010); p = 0.021], SWS (%TST) [β = 0.159 (95% CI, 0.042, −0.275); p = 0.008], SE(%) [β = 0.066 (95% CI, 0.002, 0.130); p = 0.043], gender [β = −2.361 (95 CI%, −4.159, −0.564); p = 0.010], and BMI [β = −0.270 (95 CI%, −0.398, −0.143); p < 0.001] after adjusting for the potential confounders that were found to be significant.
Table 3 illustrates multiple stepwise logistic regression analysis of the relationship between vitamin D deficiency and various independent variables. After multiparametric adjustments for age, gender, obesity comorbidities, and seasonality, severe OSA showed significant independent associations with the risk of vitamin D deficiency [OR (95% CI) 2.002 (1.049–3.819), p = 0.035] (fig. 3). Analysis also showed that female gender was significantly associated with higher odds for vitamin D deficiency.
We also explored other measures of OSA activity as predictors for vitamin D deficiency. In the previous adjusted models, ODI (OR = 1.011, 95% CI = 1.005–1.018, p < 0.001), mean SaO2 (OR = 0.891, 95% CI = 0.837–0.950, p = <0.001) and lowest SaO2 (OR = 0.954, 95% CI = 0.932–0.977, p < 0.001), TST90 (OR = 1.004, 95% CI = 1.002–1.007, p < 0.001), and AI (OR = 1.016, 95% CI = 1.005–1.028, p < 0.001) also predicted vitamin D deficiency.
Vitamin D correlations with questionnaire scores
OSA patients had higher ESS score compared to controls (10.5 ± 5.4 vs 7.3 ± 5.1, p < 0.001) and higher despite non-significant levels of vitamin D deficiency (39 vs 29%, p = 0.11). ESS score was not correlated with vitamin D levels (−0.061, p = 0.112). Furthermore, daytime sleepiness was not significant between efficient and deficient vitamin D groups (63 vs 59%, p = 0.39).
BDI score was not different between OSA patients and controls (10 vs 11, p = 0.29). BDI > 10 was also insignificant between groups (42 vs 48, p = 0.41). BDI score was correlated with vitamin D levels (r = −0.097, p = 0.025). Nevertheless, this correlation no longer existed after adjustment for confounding factors.
Athens Insomnia Scale score was not correlated with vitamin D levels (r = −0.043, p = 0.357) and did not differ between OSA and controls (8.03 vs 8.59, p = 0.453).
Subgroup analysis by age, gender, BMI, and sleepiness
Further analysis in subgroups stratified by age or BMI demonstrated that severe OSA predicted vitamin D deficiency only in the age group <60 years (OR = 2.367, 95% CI = 1.110–5.047, p = 0.026) and in the BMI group <30 (OR = 2.403, 95% CI = 1.011–5.714, p = 0.04). For above 60 years (OR = 1.311, 95% CI = 0.305–5.635, p = 0.716) and in obese patients (BMI ≥ 30) (OR = 1.344, 95% CI = 0.422–4.277, p = 0.617), severe OSA was not a significant predictor of vitamin D deficiency. Additionally, the odds for vitamin D deficiency of severe OSA was marginally significant in males (OR = 2.389, 95% CI = 0.962–5.929, p = 0.06) and not in females OR = 1.956, 95% CI = 0.680–5.624, p = 0.213). Notably, the association of severe OSA and vitamin D deficiency was stronger in sleepy patients (ESS ≥ 10) (OR = 3.665, 95% CI = 1.220–11.013, p = 0.021) compared to non-sleepy patients (ESS < 10) (OR = 0.794, 95% CI = 0.323–1.950, p = 0.615) (Fig. 4).
Discussion
Our study has shown that a notable proportion of patients referred for OSA evaluation was found to have vitamin D deficiency. The presence of severe OSA was associated with a 2 times risk increase of vitamin D deficiency and the association was significantly stronger in the younger, non-obese, and sleepy OSA patients. Most importantly, although patients in severe OSA group were significantly different from control population and other OSA subgroups in demographics and comorbidities, the severe OSA-mediated risk for vitamin D deficiency was not explained by age, gender, obesity, comorbidities, or season.
Several studies have attempted to describe the relationship between vitamin D levels and OSA; however, these results should be interpreted with caution, as different cut-offs (<20 ng/mL, <30 ng/mL) have been employed for defining vitamin D deficiency. Even with different cut-offs, most of them have shown decreasing levels with increasing severity of OSA [3,4,5,6,7,8,9, 15, 27,28,29,30,31,32,33], while others did not replicate a causal link between serum vitamin D levels and OSA severity [34,35,36,37]. Similar to the results reported in most of the studies, we observed that vitamin D levels did not differ among controls and mild and moderate OSA patients; however, vitamin D deficiency became more notable in severe OSA patients. The mechanisms between OSA and vitamin D level and the direction of effect are not yet known. One likely hypothesis for this association might be that vitamin D levels decrease by a hypoxia-induced mechanism, which is a fundamental feature of OSA. The correlation analysis in our study indicated that vitamin D was independently associated with several measures of intermittent hypoxia, in agreement with previous studies [9, 31, 33], reinforcing this theory. Consequently, as intermittent hypoxia is more notable in severe OSA patients, the lack of difference in vitamin D levels between controls and other OSA categories could be explained. Furthermore, treatment of severe OSA with positive airway pressure therapy was able to increase vitamin D levels, mainly in patients with mean residual AHI < 5/h during CPAP therapy [10, 38], suggesting that normalizing nocturnal oxygen saturation might affect positively the level of vitamin D in these patients.
OSA and vitamin D deficiency are two separate disorders both associated with obesity, inflammation, and many comorbidities, such as diabetes and cardiovascular diseases [39]. Considering the association of OSA with vitamin D deficiency and the association of both these conditions with the comorbidities developing, routine assessment of vitamin D serum levels, and vitamin D supplementation in severe OSA patients might be proven of clinical benefit. However, further studies are needed to determine the clinical relevance of the vitamin D insufficiency observed in OSA patients and the utility of vitamin D measurement and possibly the use of supplements in OSA patients.
Furthermore, obesity, another common comorbidity in people with OSA, has been closely associated with vitamin D, probably due to increased deposition of 25-hydroxyvitamin D into adipose tissues [40, 41]. In our study, an association between vitamin D deficiency and severe OSA was still observed after adjusting for obesity, suggesting that OSA is a major predictor of vitamin D deficiency, and coupled with obesity, it may further increase the risk of vitamin D deficiency.
Another potential explanation of the relation between OSA and vitamin D is that OSA associates with excessive daytime sleepiness. As might be expected, OSA patients reporting excessive daytime sleepiness are more likely to have limited access to outdoor activities and hence reduced exposure to sunlight resulting in lower vitamin D [42]. On the other hand, persistently low vitamin D levels are associated with myopathy, tonsillar hypertrophy, and rhinitis, potentially predisposing to OSA development and daytime sleepiness [36]. Despite that, our results do not provide evidence of an association between vitamin D levels and sleepiness, in this Greek population with untreated OSA, contrary to previous studies in ethnically diverse populations [43, 44]. However, the evidence on the potential cause-effect relationship between vitamin D and excessive daytime sleepiness remains inconclusive, and so further studies are necessary to ascertain the mechanisms involved in this relationship.
In our study, all patients were Caucasian and permanent residents in Crete, in Southern Greece. So far, few studies have been conducted in Greece examining vitamin D serum concentrations in OSA and this is the first study that provides relevant data in southern Greece. In northern Greece, the limited data available showed that vitamin D concentration of OSA patients was lower compared to non-apneic subjects [31, 45, 46]. Although our results are consistent with these studies, the mean vitamin D level in our study population was higher, probably due to the abundant sunshine of Crete (southern part of Greece). Nevertheless, vitamin D deficiency was a prevalent disorder in our population (38%), with females exhibiting the highest odds ratio for predicting vitamin D deficiency.
It is plausible that several limitations might have affected our results. First given that subjects were recruited from a sleep center, we cannot generalize our findings to other populations. Secondly, given the cross-sectional design of our study, we cannot attribute directionality to the associations between OSA and vitamin D. Another potential limitation is the lack of data on skin pigmentation, sunscreen use, dietary habits, daily activities (indoor, outdoor), and occupation which are linked to sunlight exposure that could potentially affect vitamin D levels. However, no significant differences are anticipated with respect to skin pigmentation and dietary habits, given that our study included only Cretan subjects, living in the same area, with similar dietary habits. In addition, vitamin D and calcium supplementation, conditions or intake of medications known to affect vitamin D metabolism were excluded. Furthermore, other confounding factors such as renal function and parathyroid hormone were not included in our analysis which may have been influenced the outcome of our study. Lastly, although we adjusted for seasonality in our regression analysis, we had no measures of within-participant seasonal variation in vitamin D concentrations.
In conclusion, we found a notable proportion of patients evaluated for OSA to have vitamin D deficiency, which was independently associated with severe OSA. Additional work will resolve the role of vitamin D in OSA; however, by that time, vitamin D deficiency should be suspected in patients with severe OSA. Further research is also needed in order to assess the levels of vitamin D in severe OSA after CPAP treatment, as wells as the examination of the effect of vitamin D supplementation on OSA severity.
References
Parish JM (2012) Metabolic syndrome, obstructive sleep apnea, and risk of cardiovascular disease. Sleep Breath 16:595–597
Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS (2002) Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 165:670–676
Neighbors CLP, Noller MW, Song SA, Zaghi S, Neighbors J, Feldman D, Kushida CA, Camacho M (2018) Vitamin D and obstructive sleep apnea: a systematic review and metaanalysis. Sleep Med 43:100–108
Upala S, Sanguankeo A (2015) Association between 25-hydroxyvitamin D and obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med 11:1347–1347
Puckrin R, Iqbal S, Zidulka A, Vasilevsky M, Barre P (2015) Renoprotective effects of continuous positive airway pressure in chronic kidney disease patients with sleep apnea. Int Urol Nephrol 47:1839–1845
Nakashima A, Yokoyama K, Yokoo T, Urashima M (2016) Role of vitamin D in diabetes mellitus and chronic kidney disease. World J Diabetes 7:89–100
Evatt ML (2015) Vitamin D associations and sleep physiology—promising rays of information. Sleep 38:171–172
Bozkurt NC, Cakal E, Sahin M, Ozkaya EC, Firat H, Delibasi T (2012) The relation of serum 25-hydroxyvitamin-D levels with severity of obstructive sleep apnea and glucose metabolism abnormalities. Endocrine 41:518–525
Kerley CP, Hutchinson K, Bolger K, McGowan A, Faul J, Cormican L (2016) Serum vitamin D is significantly inversely associated with disease severity in Caucasian adults with obstructive sleep apnea syndrome. Sleep 39:293–300
Liguori C, Izzi F, Mercuri NB, Romigi A, Cordella A, Tarantino U, Placidi F (2017) Vitamin D status of male OSAS patients improved after long-term CPAP treatment mainly in obese subjects. Sleep Med 29:81–85
Cashman KD, Dowling KG, Skrabakova Z, Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Mølgaard C, Jorde R, Grimnes G, Moschonis G, Mavrogianni C, Manios Y, Thamm M, Mensink GB, Rabenberg M, Busch MA, Cox L, Meadows S, Goldberg G, Prentice A, Dekker JM, Nijpels G, Pilz S, Swart KM, van Schoor NM, Lips P, Eiriksdottir G, Gudnason V, Cotch MF, Koskinen S, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Kiely M (2016) Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr 103:1033–1044
Manios Y, Moschonis G, Lambrinou CP, Mavrogianni C, Tsirigoti L, Hoeller U, Roos FF, Bendik I, Eggersdorfer M, Celis-Morales C, Livingstone KM, Marsaux CFM, Macready AL, Fallaize R, O'Donovan CB, Woolhead C, Forster H, Walsh MC, Navas-Carretero S, San-Cristobal R, Kolossa S, Hallmann J, Jarosz M, Surwiłło A, Traczyk I, Drevon CA, van Ommen B, Grimaldi K, Matthews JNS, Daniel H, Martinez JA, Lovegrove JA, Gibney ER, Brennan L, Saris WHM, Gibney M, Mathers JC, Food4Me Study (2018) Associations of vitamin D status with dietary intakes and physical activity levels among adults from seven European countries: the Food4Me study. Eur J Nutr 57:1357–1368
Takiishi T, Gysemans C, Bouillon R, Mathieu C (2010) Vitamin D and diabetes. Endocrinol Metab Clin North Am 39:419–446
Holick MF (2007) Vitamin D deficiency. N Engl J Med 35:266–281
McCarty DE, Chesson AL Jr, Jain SK, Marino AA (2014) The link between vitamin D metabolism and sleep medicine. Sleep Med Rev 18:311–319
Lips P (2010) Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol 121:297–300
Herrmann M, Sullivan DR, Veillard AS, McCorquodale T, Straub IR, Scott R, Laakso M, Topliss D, Jenkins AJ, Blankenberg S, Burton A, Keech AC, FIELD Study Investigators (2015) Serum 25- hydroxyvitamin D: a predictor of macrovascular and microvascular complications in patients with type 2 diabetes. Diabetes Care 38:521–528
Du W, Liu J, Zhou J, Ye D, OuYang Y, Deng Q (2018) Obstructive sleep apnea, COPD, the overlap syndrome, and mortality: results from the 2005-2008 National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis 13:665–674
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
Soldatos CR, Dikeos DG, Paparrigopoulos TJ (2000) Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res 48:555–560
Soldatos CR, Dikeos DG, Paparrigopoulos TJ (2003) The diagnostic validity of the Athens Insomnia Scale. J Psychosom Res 55:263–267
Beck AT, Beamesderfer A (1974) Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry 7:151–169
Beck AT, Steer RA, Garbin MG (1988) Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev 8:77–100
Richter P, Werner J, Heerlein A, Kraus A, Sauer H (1998) On the validity of the Beck Depression Inventory. A review. Psychopathology 31:160–168
Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV, for the American Academy of Sleep Medicine (2016) The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.3. American Academy of Sleep Medicine, Darien
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930
Mete T, Yalcin Y, Berker D, Ciftci B, Guven SF, Topaloglu O, Yavuz HC, Guler S (2013) Obstructive sleep apnea syndrome and its association with vitamin D deficiency. J Endocrinol Investig 36:681–685
Gronewold J, Haensel R, Kleinschnitz C, Frohnhofen H, Hermann DM (2019) Sleep-disordered breathing in hospitalized geriatric patients with mild dementia and its association with cognition, emotion and mobility. Int J Environ Res Public Health 16(5):E863
Barceló A, Esquinas C, Pierola J, De la Peña M, Sánchez-de-la-Torre M, Montserrat JM, Marín JM, Duran J, Arqué M, Bauça JM, Barbé F (2013) Vitamin D status and parathyroid hormone levels in patients with obstructive sleep apnea. Respiration 86:295–301
Piovezan RD, Hirotsu C, Feres MC, Cintra FD, Andersen ML, Tufik S, Poyares D (2017) Obstructive sleep apnea and objective short sleep duration are independently associated with the risk of serum vitamin D deficiency. PLoS One 12:e0180901
Archontogeorgis K, Nena E, Papanas N, Zissimopoulos A, Voulgaris A, Xanthoudaki M, Manolopoulos V, Froudarakis M, Steiropoulos P (2018) Vitamin D levels in middle-aged patients with obstructive sleep apnoea syndrome. Curr Vasc Pharmacol 16:289–297
Goswami U, Ensrud KE, Paudel ML, Redline S, Schernhammer ES, Shikany JM, Stone KL, Kunisaki KM, Osteoporotic Fractures in Men Study Research Group (2016) Vitamin D concentrations and obstructive sleep apnea in a multicenter cohort of older males. Ann Am Thorac Soc 13:712–718
Toujani S, Kaabachi W, Mjid M, Hamzaoui K, Cherif J, Beji M (2017) Vitamin D deficiency and interleukin-17 relationship in severe obstructive sleep apnea–hypopnea syndrome. Ann Thorac Med 12:107–113
Klobucnikova K, Siarnik P, Sivakova M, Wágnerová H, Mucska I, Kollár B, Turčáni P (2017) Carotid intima-media thickness is not associated with homocysteine and vitamin D levels in obstructive sleep apnea. Scand J Clin Lab Invest 77:263–266
Erden ES, Genc S, Motor S, Ustun I, Ulutas KT, Bilgic HK, Oktar S, Sungur S, Erem C, Gokce (2014) Investigation of serum bisphenol a, vitamin D, and parathyroid hormone levels in patients with obstructive sleep apnea syndrome. Endocrine 45:311–318
Salepci B, Caglayan B, Nahid P, Parmaksiz ET, Kiral N, Fidan A, Comert SS, Dogan C, Gungor GA (2017) Vitamin D deficiency in patients referred for evaluation of obstructive sleep apnea. J Clin Sleep Med 13:607–612
Yassa OY, Domac SF, Kenangil G (2019) Serum vitamin D status does not correlate with the severity of obstructive sleep apnea in male adults: a controlled study design with minimized factors influencing serum vitamin D levels. Int J Vitam Nutr Res 20:1–7
Liguori C, Romigi A, Izzi F, Mercuri NB, Cordella A, Tarquini E, Giambrone MP, Marciani MG, Placidi F (2015) Continuous positive airway pressure treatment increases serum vitamin D levels in male patients with obstructive sleep apnea. J Clin Sleep Med 11:603–607
Beveridge LA, Witham MD (2013) Vitamin D and the cardiovascular system. Osteoporos Int 24:2167–2180
Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P (2005) Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab 90:4119–4123
Young T (2009) Rationale, design and findings from the Wisconsin Sleep Cohort Study: toward understanding the total societal burden of sleep disordered breathing. Sleep Med Clin 4:37–46
Igelstrom H, Emtner M, Lindberg E, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P (2013) Physical activity and sedentary time in persons with obstructive sleep apnea and overweight enrolled in a randomized controlled trial for enhanced physical activity and healthy eating. Sleep Breath 17:1257–1266
McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA (2012) Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med 8:693–697
Bertisch SM, Sillau S, de Boer IH, Szklo M, Redline S (2015) 25-hydroxyvitamin D concentration and sleep duration and continuity: multi-ethnic study of atherosclerosis. Sleep 38:1305–1311
Ragia G, Archontogeorgis K, Simmaco M, Gentile G, Borro M, Zissimopoulos A, Froudarakis M, Manolopoulos VG, Steiropoulos P (2019) Genetics of obstructive sleep apnea: vitamin D receptor gene variation affects both vitamin D serum concentration and disease susceptibility. OMICS 23:45–53
Archontogeorgis K, Nena E, Papanas N, Rizzo M, Voulgaris A, Xanthoudaki M, Kouratzi M, Ragia G, Manolopoulos V, Zissimopoulos A, Froudarakis M, Steiropoulos P (2018) Metabolic syndrome and vitamin D levels in patients with obstructive sleep apnea syndrome. Metab Syndr Relat Disord 16:190–196
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouloukaki, I., Tsiligianni, I., Mermigkis, C. et al. Vitamin D deficiency in patients evaluated for obstructive sleep apnea: is it associated with disease severity?. Sleep Breath 25, 1109–1117 (2021). https://doi.org/10.1007/s11325-020-02142-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-020-02142-w