Abstract
Purpose
Chronic kidney disease (CKD) is associated with a high incidence of obstructive sleep apnea (OSA). We assessed the effect of continuous positive airway pressure (CPAP) on renal function in patients with CKD and OSA.
Methods
In this retrospective cohort study, 42 patients with Stage 3–5 CKD and OSA were stratified into two groups: patients who use CPAP more (average >4 h/night on >70 % of nights) and patients who use CPAP less (average ≤4 h/night on ≤70 % of nights). Median follow-up time was 2.3 (1.6–2.9) years for greater and 2.0 (0.6–3.5) years for lesser CPAP users. Chart reviews were carried out to record clinical characteristics, proteinuria measurements by urine dipstick, and eGFR values calculated by CKD–EPI equations. Univariate analyses were performed using Wilcoxon rank-sum and Kruskal–Wallis tests. Multivariate logistic regression models were applied to assess eGFR decline after CPAP prescription.
Results
Twelve (29 %) of the 42 subjects used CPAP more. Groups were similar with respect to age, body mass index, blood pressure, Charlson Comorbidity Index, and baseline eGFR and proteinuria. The median rate of decline of eGFR was significantly slower at −0.07 mL/min/1.73 m2/year (range −30 to 13) in those who used more CPAP compared to those who used it less at −3.15 mL/min/1.73 m2/year (range −27 to 7) (p = 0.027).Greater use of CPAP was also associated with a significantly reduced level of proteinuria at 0.15 (range 0.0–3.0) versus 0.70 g/L (range 0.0–3.0) (p = 0.046). Less compliant CPAP users were more likely to have progressive decline of eGFR (decline >3 mL/min/1.73 m2/year), with unadjusted OR 5.0 (95 % CI 0.93–26.8) and adjusted OR 8.9 (95 % CI 1.1–72.8), adjusting for CCI and baseline eGFR.
Conclusions
Compliance to CPAP therapy is associated with a slower rate of progression of CKD in patients with CKD and OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is growing consensus that obstructive sleep apnea (OSA) may constitute a novel independent risk factor for the development and progression of chronic kidney disease (CKD) [1–4]. The prevalence of sleep apnea has been found to be as high as 54–94 % in non-dialysis dependent CKD patients, and 30–93 % in end-stage renal disease (ESRD) populations [1, 5]. Sleep apnea has been shown to affect renal function through its association with hypoxemia, renin-angiotensin-aldosterone system dysregulation, and the metabolic syndrome [1–3].
Obstructive sleep apnea can be effectively treated by maintaining upper airway patency during sleep through the administration of continuous positive airway pressure (CPAP) via a nasal or full face mask. This has been shown to improve sleep quality, hypertension, and nocturnal hypoxemia [6]. Several recent trials conducted mainly in non-CKD populations suggest that short-term use of CPAP may improve renal function in patients with sleep apnea [7–12]. However, the long-term therapeutic effect of CPAP on renal function in patients with CKD has not been well studied.
This study was designed to determine if the administration of CPAP therapy in patients with Stage 3–5 CKD and OSA alters the long-term progression of renal disease [13].
Materials and methods
Study population
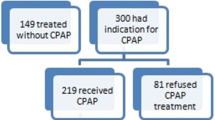
This retrospective cohort study included patients over age 18 followed at the CKD and sleep disorder clinics of the McGill University Health Centre in Montreal, Quebec, Canada between January 1, 2002 and June 30, 2014. We identified 220 patients with Stage 3–5 CKD who had been diagnosed with OSA. Of these, we excluded 178 patients who had no documented diagnostic sleep study, <6 months of follow-up time after CPAP prescription, or no available CPAP compliance reports. Other exclusion criteria included patients with a malignancy that would result in mortality within 6 months and those under palliative care management. After these exclusions, 42 patients were enrolled in this study. The research protocol was approved by the institutional Research Ethics Board and designed in accordance with the Declaration of Helsinki.
Sleep study and CPAP use
Sleep apnea is defined by the presence of signs and symptoms of inadequate sleep in conjunction with overnight monitoring revealing five or more episodes of apnea or hypopnea per hour of sleep (ie. apnea-hypopnea index [AHI] ≥5) [14]. All patients were assessed by a respirologist and underwent a diagnostic sleep study with standard overnight sleep laboratory polysomnography or a portable Embletta® home sleep testing device. All subjects were prescribed a home CPAP device, which delivered either fixed-pressure or auto-titrating CPAP therapy. Individual CPAP requirements were determined by an overnight laboratory CPAP titration study, or by starting patients on a home auto-titrating CPAP device of 5–15 or 5–20 cm H2O with subsequent readjustments of pressures made in accordance with device downloads. Use of CPAP was determined with downloaded compliance reports obtained from the patients’ home CPAP device, which recorded the number of nights and duration of nightly CPAP use.
Study protocol
Subjects were stratified into two groups: patients who used CPAP more (average >4 h per night on >70 % of nights) and patients who used CPAP less (average ≤4 h per night on ≤70 % of nights). These are standard definitions of CPAP compliance commonly employed in the literature [9, 15, 16]. Chart reviews were carried out to record potential confounding factors such as age, sex, body mass index (BMI), cigarette use, blood pressure, medications at baseline and end of follow-up, lipid profile, Charlson Comorbidity Index (CCI), cause of CKD, and AHI and sleep oxygen saturation nadir at the time of diagnosis of sleep apnea. Clinical endpoints included all available serum creatinine values, estimated glomerular filtration rate (eGFR) values calculated by CKD-EPI equations, all available proteinuria measurements by urine dipstick, all available blood pressure measurements, and the number of all-cause hospitalizations [17]. Data were collected during the 12 months preceding initiation of CPAP therapy and for up to 42 months afterward. The median duration of follow-up time after CPAP prescription was 2.3 years (range 1.6–2.9) for regular CPAP users and 2.0 years (range 0.6–3.5) for less compliant users. The primary objective was to compare the rate of decline in eGFR after 2 years of CPAP therapy between patients who used CPAP more with those who used it less. The secondary objectives were to assess the difference in the development or change of proteinuria, blood pressure, and the frequency of hospitalization in the two groups after 2 years of CPAP use.
Statistical analysis
Patient characteristics at baseline were summarized using proportion (number) or median (range) as appropriate. Multivariate logistic regression models were applied to assess eGFR decline during the 12 months preceding the initiation of CPAP therapy and during the 2 years after CPAP prescription. Statistical analyses were performed using the SAS System, version 9.3 (SAS Institute, Cary, NC). Paired analyses with Wilcoxon rank-sum and Kruskal–Wallis tests were used to compare clinical variables between patients who had been more compliant with CPAP therapy and those who were less compliant for non-normally distributed data. Multivariate logistic regression models with repeated measurements were conducted to assess the association between CPAP therapy and rapid decline in renal function (defined as eGFR decline >3 mL/min/1.73 m2/year), adjusting for known risk factors for progressive renal disease. For this analysis, the Odds Ratio (OR) and 95 % confidence interval (CI) for each variable are reported.
Results
Clinical characteristics of study subjects
Clinical characteristics of patients who used CPAP more (n = 12) and less (n = 30) are presented in Table 1. There were no significant differences between the two groups with respect to age, sex, body mass index, Charlson Comorbidity Index, baseline eGFR and proteinuria, Stage of CKD, serum LDL, blood pressure, cigarette smoking status, medications, cause of CKD, and apnea-hypopnea index or nocturnal oxygen saturation nadir at the time of diagnosis of sleep apnea.
Severity of OSA and compliance to CPAP therapy
As illustrated in Fig. 1, the majority of patients enrolled in this study had severe sleep apnea (AHI > 30): 69 % of Stage 3, 54 % of Stage 4, and 100 % of Stage 5 CKD patients. There was no significant relationship between baseline eGFR and apnea-hypopnea index at the time of diagnosis of OSA (p = 0.358). Twelve (29 %) of the 42 subjects in this study were more compliant with CPAP. By Stage of CKD, no patients with Stage 5, 2 patients with Stage 4 (15 %), and 9 patients with Stage 3 (35 %) CKD were using CPAP more. Greater users of CPAP therapy used CPAP for a median 6.2 h per night (4.0–10.4) on a median 87 % of nights (71–100), compared to 2.2 h per night (0–3.9) on 32 % of nights (0–50) among lesser users.
Effect of CPAP therapy on progression of renal disease
During the 12 months preceding initiation of CPAP therapy, there was no significant difference in median rate of decline of eGFR at −3.4 mL/min/1.73 m2/year (−24 to 2.0) in patients who went on to use CPAP more compared to −2.9 mL/min/1.73 m2/year (−24 to 13) in patients who would use CPAP less (p = 0.666). The change in eGFR during the median 2.3 years following initiation of CPAP therapy is presented in Table 2. After the prescription of CPAP, the median rate of decline of eGFR was significantly slower among more compliant CPAP users at −0.07 mL/min/1.73 m2/year (−30 to 13) compared to those who did not use CPAP regularly at −3.15 mL/min/1.73 m2/year (−27 to 7.0) (p = 0.027). Greater use of CPAP was associated with a significantly reduced level of proteinuria at 0.15 g/L (0.0–3.0) versus the lesser users at 0.70 g/L (0.0–3.0) after the median 2.3 years of CPAP use (p = 0.046). The multivariate logistic regression models in Table 3 demonstrate that less compliant CPAP users were more likely to have progressive decline of eGFR (decline >3 mL/min/1.73 m2/year), with unadjusted OR 5.0 (95 % CI 0.93–26.8) and adjusted OR 8.9 (95 % CI 1.1–72.8), adjusting for CCI and baseline eGFR.
Effect of CPAP therapy on blood pressure and hospitalization rate
Table 2 demonstrates there was no significant difference in median systolic blood pressure (127 mmHg vs. 131 mmHg, p = 0.455), diastolic blood pressure (77 mmHg vs. 72 mmHg, p = 0.987), and occurrences of all-cause hospitalization per patient (0.5 vs. 1, p = 0.569) between subjects who used CPAP more versus less after a median 2.3 years of CPAP therapy.
Discussion
This retrospective cohort study demonstrated that compliance to CPAP therapy is associated with a slower longterm rate of progression of renal dysfunction in patients with Stage 3–5 CKD and OSA. Patients who used CPAP regularly had a significantly slower rate of decline in eGFR and a significantly reduced level of proteinuria after a median 2.3 years of CPAP therapy. This relationship was found to be independent of sex, age, body mass index, blood pressure, baseline eGFR and proteinuria, and comorbid medical conditions.
To the best our knowledge, the present study is the first to investigate the longterm effect of CPAP therapy on the progression of eGFR and proteinuria in patients with CKD. Our findings corroborate the results of two recent studies which evaluated renal function in mostly non-CKD patients undergoing treatment for sleep apnea. Koga et al. [7] reported that 3 months of CPAP therapy led to increased eGFR in 27 men with sleep apnea (from baseline eGFR 72.9 ± 12.0 [mean ± S.D.] to 79.3 ± 17.9 mL/min/1.73 m2, p = 0.014). Tahrani et al. [9] found that compliant use of CPAP slowed the decline of eGFR in 16 diabetic patients with sleep apnea (−7.7 %) in comparison with 31 diabetic patients who did not use CPAP (−10.0 %) after an average 2.5 years of follow-up (p = 0.01). Our finding that compliance to CPAP is associated with a slower rate of decline of eGFR confirms these results and, furthermore, extends their validity to the CKD population.
Two other recent trials studied the effect of adaptive servo-ventilation (ASV), a type of positive airway pressure used in the treatment of central sleep apnea, on renal function in patients with sleep apnea and CKD. Owada et al. [10] reported that 6 months of ASV use increased eGFR in 36 CKD patients with congestive heart failure and sleep-disordered breathing, from baseline eGFR 46.6 ± 13.4 to 53.8 ± 22.6 mL/min/1.73 m2 (p = 0.031). Similarly, Koyama et al. [11] reported that 12 months of ASV use improved eGFR in 27 CKD patients with heart failure and sleep-disordered breathing, from baseline eGFR 44.2 ± 12.3 to 48.2 ± 12.9 ml/min/1.73 m2 (p = 0.0095). However, these studies did not evaluate the change in proteinuria and included a disproportionate number of patients with central sleep apnea, which is less common in CKD populations than obstructive sleep apnea [18]. Nevertheless, their findings are consistent with those of the present study. The difference in effect size between our study and others is likely attributable to a small study population and variation in baseline eGFR.
Several other trials have evaluated the effect of CPAP therapy on urinary protein excretion in patients without CKD. Most recently, Yasar et al. [12] found that 1 month of CPAP therapy reduced albuminuria from 50 to 22.7 mg/day in 18 patients with sleep apnea (p = 0.001). This is in keeping with our finding that regular use of CPAP was associated with a significantly reduced level of proteinuria after 2.3 years of CPAP use.
The clinical relevance of our findings is of importance given the high prevalence of obstructive sleep apnea in CKD and ESRD populations. The early identification of sleep apnea among CKD patients and initiation of CPAP therapy constitutes a promising intervention to prevent the progression of kidney disease. Potential barriers to CPAP use that should be addressed include its perceived discomfort and the financial cost of treatment.
The findings of this study contribute to the growing evidence that sleep apnea is a novel independent risk factor for the development and progression of CKD. Sleep apnea may contribute to renal disease due to its association with vascular dysfunction and inflammation, oxidative stress, intra-renal hypoxia, dysregulation of the renin-angiotensin-aldosterone system (RAAS), and increased sympathetic nervous system activity [1–3]. It is thought that RAAS activation leads to CKD through the promotion of glomerular hyperfiltration and proteinuria, while apnea-induced chronic hypoxemia may contribute to renal disease via the mechanism of tubulointerstitial injury [3]. Sleep apnea may also play an indirect role in renal dysfunction by promoting risk factors for CKD such as hypertension, obesity, and diabetes mellitus [1]. Interestingly, it has been suggested that CKD may in turn contribute to the development of sleep apnea due to the metabolic abnormalities and hypervolemic upper airway obstruction associated with kidney dysfunction [1, 2]. For example, Elias et al. [19] found that increased nocturnal fluid shifts from the leg into the neck were associated with more severe sleep apnea in 26 patients with ESRD. There thus appears to be an interdependent relationship underlying the pathogenesis of sleep apnea and CKD which warrants further investigation.
The administration of CPAP therapy constitutes a potentially important means of reversing the noxious effects of obstructive sleep apnea on renal and vascular function. By decreasing the frequency of nocturnal apnea events, CPAP presumably improves renal hemodynamics and tissue oxygenation while normalizing RAAS and sympathetic system activity. A recent study by Nicholl et al. [20] demonstrated that CPAP use in patients with sleep apnea decreased filtration fraction and augmented the response of renal plasma flow to angiotensin II, consistent with a CPAP-induced down-regulation of RAAS activity. Although CPAP may also contribute to improved renal function by reducing systemic arterial pressure, we found no significant effect of CPAP therapy on blood pressure reduction in our small study population. We did not evaluate the effect of CPAP on other potential mediators of kidney dysfunction such as hypoxemia, RAAS activity, sympathetic tone, inflammatory markers, body mass index, and plasma glucose. Future studies might examine these variables in order to clarify the pathophysiological mechanisms underlying the relationship between sleep apnea, CPAP, and kidney dysfunction.
Several limitations of this study are to be noted. First, our results may not be applicable to the entire CKD population, as the majority of study subjects were male, obese, and had Stage 3 CKD and severe OSA. Future studies should also examine the effect of CPAP on CKD progression as a function of other prognostic variables, such as the underlying cause of kidney disease. Second, the small study population likely limited our statistical power to identify a significant relationship between CPAP use and progression of blood pressure and all-cause hospitalization. Third, although our findings are consistent with those of other studies, we cannot exclude the presence of a selection bias given the retrospective and non-randomised nature of this study. Fourth, the measurement of proteinuria by urine dipstick in this study is not sensitive to small changes in kidney protein excretion. Finally, we did not extend follow-up time to evaluate the effect of CPAP therapy on prognosis of CKD with respect to mortality and progression to renal replacement therapy.
In summary, long-term compliance with CPAP therapy is associated with a slower rate of decline of eGFR and reduced progression of proteinuria in patients with Stage 3–5 CKD and obstructive sleep apnea. Additional large-sized prospective cohort trials are warranted to confirm these findings and evaluate the effect of CPAP therapy on blood pressure and other mediators of renal dysfunction in patients with CKD.
References
Turek NF, Ricardo AC, Lash JP (2012) Sleep disturbances as non-traditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis 60(5):823–833
Ozkok A, Kanbay A, Odabas AR et al (2014) Obstructive sleep apnea syndrome and chronic kidney disease: a new cardiorenal risk factor. Clin Exp Hypertens 36(4):211–216
Hanly PJ, Ahmed SB (2014) Sleep apnea and the kidney: is sleep apnea a risk factor for chronic kidney disease? Chest 146(4):1114–1122
Kanbay A, Buyukoglan H, Ozdogan N et al (2012) Obstructive sleep apnea syndrome is related to the progression of chronic kidney disease. Int Urol Nephrol 44(2):535–539
Sim JJ, Rasgon SA, Derose SF (2010) Review article: managing sleep apnoea in kidney diseases. Nephrology 15(2):146–152
Giles TL, Lasserson TJ, Smith BJ (2006) Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 25(1):CD001106
Koga S, Ikeda S, Yasunaga T et al (2013) Effects of nasal continuous positive airway pressure on the glomerular filtration rate in patients with obstructive sleep apnea syndrome. Intern Med 52(3):345–349
Kinebuchi S, Kazama JJ, Satoh M et al (2004) Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apnoea syndrome. Clin Sci 107(3):317–322
Tahrani AA, Ali A, Raymond NT et al (2013) Obstructive sleep apnea and diabetic nephropathy: a cohort study. Diabetes Care 36(11):3718–3725
Owada T, Yoshihisa A, Yamauchi H et al (2013) Adaptive servoventilation improves cardiorenal function and prognosis in heart failure patients with chronic kidney disease and sleep-disordered breathing. J Card Fail 19(4):225–232
Koyama T, Watanabe H, Terada S et al (2011) Adaptive servo-ventilation improves renal function in patients with heart failure. Respir Med 105(12):1946–1953
Yaşar ZA, Ucar ZZ, Demir AU et al (2014) Does CPAP therapy alter urinary albumin level in adult patients with severe obstructive sleep apnea syndrome? Sleep Breath 18(3):525–532
Kidney Disease Improving Global Outcomes (KDIGO) (2012) Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3(1):19–62
The Report of an American Academy of Sleep Medicine Task Force (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22(5):667–689
Weaver TE, Grunstein RR (2008) Adherence tocontinuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 5(2):173–178
Collen J, Lettieri C, Kelly W et al (2009) Clinical and polysomnographic predictors of short-term continuous positive airway pressure compliance. Chest 135(3):704–709
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Beecroft JM, Pierratos A, Hanly PJ (2009) Clinical presentation of obstructive sleep apnea in patients with end-stage renal disease. J Clin Sleep Med 5(2):115–121
Elias RM, Bradley TD, Kasai T et al (2012) Rostral overnight fluid shift in end-stage renal disease: relationship with obstructive sleep apnea. Nephrol Dial Transpl 27:1569–1573
Nicholl DD, Hanly PJ, Poulin MJ et al (2014) Evaluation of continuous positive airway pressure therapy on renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med 190(5):572–580
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest, and no disclosure of grants or funding to make. The research protocol was approved by the institutional Research Ethics Board and designed and conducted in accordance with the Declaration of Helsinki. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Puckrin, R., Iqbal, S., Zidulka, A. et al. Renoprotective effects of continuous positive airway pressure in chronic kidney disease patients with sleep apnea. Int Urol Nephrol 47, 1839–1845 (2015). https://doi.org/10.1007/s11255-015-1113-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1113-y