Abstract
Purpose
To report the vitamin D status in adults from seven European countries and to identify behavioural correlates.
Methods
In total, 1075 eligible adult men and women from Ireland, Netherlands, Spain, Greece, UK, Poland and Germany, were included in the study.
Results
Vitamin D deficiency and insufficiency, defined as 25-hydroxy vitamin D3 (25-OHD3) concentration of <30 and 30–49.9 nmol/L, respectively, were observed in 3.3 and 30.6% of the participants. The highest prevalence of vitamin D deficiency was found in the UK and the lowest in the Netherlands (8.2 vs. 1.1%, P < 0.05). In addition, the prevalence of vitamin D insufficiency was higher in females compared with males (36.6 vs. 22.6%, P < 0.001), in winter compared with summer months (39.3 vs. 25.0%, P < 0.05) and in younger compared with older participants (36.0 vs. 24.4%, P < 0.05). Positive dose–response associations were also observed between 25-OHD3 concentrations and dietary vitamin D intake from foods and supplements, as well as with physical activity (PA) levels. Vitamin D intakes of ≥5 μg/day from foods and ≥5 μg/day from supplements, as well as engagement in ≥30 min/day of moderate- and vigorous-intensity PA were associated with higher odds (P < 0.05) for maintaining sufficient (≥50 nmol/L) 25-OHD3 concentrations.
Conclusions
The prevalence of vitamin D deficiency varied considerably among European adults. Dietary intakes of ≥10 μg/day of vitamin D from foods and/or supplements and at least 30 min/day of moderate- and vigorous-intensity PA were the minimum thresholds associated with vitamin D sufficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last 15 years, vitamin D has attracted increased attention from the scientific community, the food industry, policy makers and the public [1]. This is mainly due to new discoveries about the impact of vitamin D on several health outcomes beyond its known metabolic actions on bone and mineral metabolism [2]. Specifically, the presence of the vitamin D receptor in many body tissues supported evidence linking vitamin D deficiency to increased risk of certain auto-immune diseases, cancers, cardiovascular disease, diabetes and psychiatric disorders [3–5]. In addition to its effects on health and metabolism, vitamin D has raised interest because of the large variation in the prevalence of vitamin D deficiency across countries worldwide, with estimates ranging from 2 to 90% [6–9].
The major source of vitamin D for humans is endogenous synthesis via skin exposure to solar ultraviolet B radiation (wavelength, 290–315 nm). Skin exposure to sunlight stimulates the conversion of 7-dehydrocholesterol to pre-vitamin D3 and its subsequent conversion to vitamin D3 (one of the two major forms of vitamin D that is synthesized endogenously and is also found in animal food sources) [10]. However, several environmental factors, including seasonality, latitude and prevailing weather conditions, determine whether sufficient sunlight may induce cutaneous vitamin D3 synthesis [11]. In addition, sex, age, adiposity status and skin pigmentation, as well as clothing habits, sunscreen use and physical activity (PA) levels, as a proxy of outdoor activities and consequently sunlight exposure, have also been associated with vitamin D status [12, 13]. Regarding PA, positive associations irrespective of sun exposure have also been reported with vitamin D status [14, 15] suggesting an independent association.
When environmental conditions, personal traits or lifestyle prevent adequate exposure to sunlight, dietary intake of vitamin D from fortified foods and/or supplements is considered as a good alternative to reach and to maintain blood 25-hydroxy vitamin D (25-OHD) concentrations (the main index of vitamin D status) in the normal range. In countries where availability of vitamin D fortified foods is low, it is very difficult to meet the recommended dietary intakes of vitamin D from its limited natural food sources (e.g. oily fish) [16]. This is the case for several European countries where dietary intakes of vitamin D from fortified foods is particularly low [17].
Although the evidence on vitamin D status worldwide is increasing, there is large heterogeneity between studies, mainly due to differences in the methods used to estimate vitamin D concentration in blood [9]. The scarcity, as well as the heterogeneity of data regarding vitamin D intake and status in Europe, highlights the need for multi-centre studies that can provide relevant data for free-living populations in a consistent and standardized manner. The primary aim of the present study was to assess the prevalence of vitamin D deficiency and insufficiency in adults from seven European countries who participated in the baseline measurements of the Food4Me ‘Proof of Principle’ study [18]. The secondary aim was also to identify behavioural correlates of vitamin D status in these populations.
Methods
Study design
The Food4Me ‘Proof of Principle’ study was a 6-month, four-arm, randomized controlled trial (RCT) conducted across seven European countries to compare the effects of three levels of Personalized Nutrition (PN) with standard population advice on health-related outcomes. The current study presents data obtained at baseline from the study participants.
Recruitment
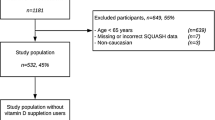
Participants were recruited in seven European countries (Ireland, the Netherlands, Spain, Greece, the UK, Poland and Germany) using identical standardised protocols in all recruitment centres, as described in detail elsewhere [19]. In brief, local and national advertising of the study via the Internet, radio, newspapers, posters, e-flyers, social media and word of mouth, were used to recruit adult men and women. Prior to participation, an information sheet was provided to potential volunteers who completed an online informed consent form before submitting personal data. This signed online consent form was automatically directed to the study coordinator to be counter-signed and archived. In total, 5562 volunteers were screened online between August 2012 and August 2013 [20]. A second online informed consent form was completed before randomisation to the intervention study only for participants who met the inclusion criteria. A total of 1607 study participants aged ≥18 years were recruited [19]. The current study presents results on 1075 participants with full data on dietary intake, PA, anthropometrics, genetics and 25-hydroxy vitamin D3 (25-OHD3) concentrations.
Ethics approval
The Research Ethics Committees at each University or Research Centre granted ethics approval for the study.
Eligibility
Regarding eligibility criteria, volunteers aged >18 years were included in the study. In all these volunteers, the following exclusion criteria were applied in identifying eligible study participants: pregnant or lactating women; no or limited access to the Internet; following a prescribed diet for any reason, including weight loss, in the last 3 months; diabetes, coeliac disease, Crohn’s disease, or any metabolic disease or condition altering nutritional requirements such as thyroid disorders (if condition was not controlled); allergies or food intolerances. Exclusion based on prescribed diet or specific diseases was to avoid that participating in the intervention study could be disadvantageous.
Measurements
To ensure that procedures were similar, standardized operating procedures were adopted in the recruiting centres and for all study procedures and researchers were trained in their use [19]. Two screening questionnaires, including a Food Frequency Questionnaire (FFQ) that was specifically developed and validated for the purposes of this study [21, 22], were used to identify participants for the Food4Me study. Participants self-reported online their ethnicity and occupations. Occupations were grouped according to the European classifications of occupations and the respective salaries of these occupations, as described in details elsewhere [23]. Based on this classification, the following groups and group names were generated: “Professional and managerial”; “Intermediate”; “Routine and manual”. Categories for “Students” and “Retired and unemployed” were also added. Participants also provided health and anthropometric data at screening and detailed information on dietary intake and food preferences [19]. Anthropometric and PA data, as well as blood and buccal cell samples were collected from all study participants and the latter were used for metabolic marker analysis and genotyping, respectively. Detailed information on the inclusion/exclusion criteria used and the information collected are provided elsewhere [19].
Anthropometry
Participants self-measured their height and body weight and uploaded their anthropometric measurements to their personal Food4Me online account [19]. Standardised instructions on how to perform these measurements were provided to participants in printed and digital format. Validation of the self-reported anthropometry is described elsewhere [24]. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2), whereas participants with a BMI ≥30 kg/m2 were categorized as obese.
Food frequency questionnaire (FFQ)
Habitual dietary intake was quantified using an online FFQ, developed for this study, which included food items consumed frequently in each of the seven recruitment countries. The average daily intakes of foods and nutrients consumed over the last month were computed in real time using a food composition database based on McCance & Widdowson’s ‘‘The composition of foods’’ [25]. For each one of the seven countries participating in the Food4Me study, the McCance & Widdowson food composition database was updated with the nutritional composition of local foods and recipes included in the FFQ. Following this procedure, dietary intakes of vitamin D from individual food items and supplements were computed and used in the current analysis. Nevertheless, it should be noted that the agreement between the FFQ and the 4-day weighted food record used to assess the validity of the FFQ in estimating dietary intakes was lower for vitamin D compared to other nutrients [21]. More information on the design, reproducibility, validity and computations of food and nutrient intakes of the online FFQ has been previously described [21, 22].
Metabolic markers
Blood samples were collected from all eligible study participants at their baseline evaluation that took place some time within the period from August 2012 to August 2013. Finger-prick blood samples were collected by the participants using a collection pack provided by Vitas Ltd, Oslo, Norway. Before spotting blood, cards for vitamin D analysis (Whatman Protein Saver 903 Card; GE Healthcare) were pre-treated with 1% of 2,6-di-tert-butyl-4-methylphenol (BHT) dissolved in methanol (MeOH); 30 μL of 1% BHT in MeOH were pipetted to each circle on the card and allowed to dry for at least 30 min at room temperature. These pre-treated cards were packed in an airtight aluminium bag (Whatman Foil Bags, item no. 10534321; Whatman Inc.) with a drying agent (Sorb-it, item no. 10548234; Süd-Chemie) and stored at room temperature until analysis. To help with blood collection, participants had access to an online video demonstration, written instructions and frequently asked questions in the local language. For the finger pricks, 2.0-mm contact-activated lancets (BD Microtainer; Becton, Dickinson and Company) were used. Each participant was asked to fill two Dry Blood Spot cards (equivalent to five drops of blood or up to 250 μL of blood per card) at each collection time point. When the 10 blood spots were filled, participants were instructed to leave the cards to dry at room temperature for at least 2 h, but not longer than 4 h, before samples were put in the airtight aluminium bag with drying sachet and returned by post to the corresponding recruiting centre. The centres shipped the samples to DSM (DSM Nutritional Products Ltd, Switzerland) for measurements of vitamin D (25-OHD2 and 25-OHD3). Although the shipments were done at ambient temperature, the closed bags were stored at the centres and at DSM at nominal −20 °C. Calibration was carried out using whole-blood samples received from blood donors of the ‘Blutspendezentrum SRK beider Basel’ (Blood Donation Centre at Basel Hospital), including haematocrit values for each sample. More information on the procedures followed for the calibration is provided in detail elsewhere [19]. Before analysis, the samples were assessed to check whether they met the quality criteria. Samples meeting quality criteria, which are described elsewhere [19], were prepared for analysis. Chromatography was performed using an Ascentis Express C18 column (Supelco), and detection was carried out by an AB Sciex 5500 Qtrap instrument with APPI positive mode and MRM scan type at unit resolution. The resulting 25-OHD3 concentration was corrected for sex-specific mean haematocrit values. The corrected 25-OHD3 values are used in the present study.
Vitamin D status in study participants was assessed using the threshold values recently proposed by the Institute Of Medicine (IOM) Dietary Reference Intake (DRI) Committee [26]. More specifically, vitamin D deficiency, insufficiency and sufficiency were defined as 25-OHD3 concentrations <30 nmol/L, 30–49.9 nmol/L and ≥50 nmol/L, respectively.
Gene analyses
Buccal cell samples were collected by participants at baseline using Isohelix SK-1 DNA buccal swabs and Isohelix Dri-capsules (LGC Genomics, Hertfordshire, UK). Samples were returned to the recruiting centres and shipped to LGC Genomics, where DNA was extracted and competitive allele-specific polymerase chain reaction (KASP) genotyping assays were used to provide biallelic scoring of single nucleotide polymorphisms (SNPs) rs1544410 and the rs2228570 in the Vitamin D Receptor (VDR) gene [19] among other SNPs. These two VDR SNPs were used as covariates in the associations of vitamin D status with dietary intake and PA levels examined in the present study.
Physical activity
Physical activity was measured objectively using the DirectLife triaxial accelerometer for movement registration (TracmorD) (Philips Consumer Lifestyle, the Netherlands) [27–29]. The PA monitor was sent by post to each participant. Online video demonstrations as well as digital and printed instructions were provided at baseline. Participants were instructed to wear the monitor throughout the 6 months intervention and to upload their PA data fortnightly via an online interface. Data were recorded with a time-sampling interval of 1 min. A day was considered valid if the participant had worn the PA monitor for at least 10 h, but not longer than 18 h. Wear time was defined as 24 h minus non-wear time. To define non-wear time, the recommendations of Choi et al. [30] were adapted to the TracmorD. The R software version 3.1.2 was used for PA data processing.
Activity energy expenditure (AEE) and time spent in PAs of different intensity were derived from accelerometers [31]. Classification into sedentary activities and light-, moderate- and vigorous- intensity PA was based on the application of thresholds for AEE [31]. Time spent in sedentary activities, as well as in light-, moderate- and vigorous-intensity PA was calculated. Lastly, to account for the fact that 1 min of vigorous-intensity PA is equivalent to 2 min of moderate-intensity PA [32], the time spent in moderate-equivalent PA was also calculated as follows: Moderate-equivalent PA (min/day) = Moderate-intensity PA (min/day) + 2 x Vigorous-intensity PA (min/ day).
Statistical analysis
Normality of the distribution of continuous variables was evaluated using the Kolmogorov–Smirnov test. Continuous variables were expressed as mean values ± standard deviations (sd), whereas categorical variables were presented as frequencies (%). Differences in mean values of continuous variables were examined using the one-way Analysis Of Variance (ANOVA) or the non-parametric Kruskal–Wallis test in the case of normally and non-normally distributed variables, respectively. Differences in frequencies were tested using the chi square (χ 2) test and the two-sample z-test for proportions for multiple post-hoc comparisons. Analyses of co-variance and multivariate logistic regression analysis were also performed to examine the dose-response effect on 25-OHD3 concentrations and the likelihood of vitamin D sufficiency derived from different doses of vitamin D intake from foods or supplements, as well as different moderate-equivalent PA levels. These analyses were adjusted for age, sex, dietary energy intake (kcal per day), VDR rs1544410, VDR rs2228570, BMI, study site and seasonality. To model the seasonal variation at the study sites, the study centre and the interaction of study centre with the functions sin (sample year × 2 × π) and cos (sample year × 2 × π) were included as confounders in the analyses. The 20th January was the consensus date across all study centres when the 25-OHD3 concentrations reached their nadir. To simplify the subsequent modelling and interpretation, a single normalised sine function was derived, which oscillated between −1.0 when the 25-OHD3 concentration was at its lowest on the 20th January and + 1.0 when the 25-OHD3 concentration was at its highest on the 21st July. Including a single season function and its interaction with each centre in the model assumes synchronised timing of seasons across all study centres and enables the model to differentiate mean concentrations and seasonal amplitudes by study centre. More details are provided elsewhere [19]. All reported P-values were based on two-sided tests. The level of statistical significance in all analyses was set at P < 0.05. SPSS version 22.0 (SPSS Inc., Texas, USA) was used for all statistical analyses.
Results
Vitamin D status by country, sex, age, season, weight status, ethnicity and occupation
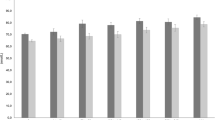
The prevalence of vitamin D deficiency and insufficiency in each of the seven participating countries is presented in Fig. 1. The highest prevalence of vitamin D deficiency, 25-OHD3 concentration of <30 nmol/L, was observed in the UK, while the lowest prevalence was observed in the Netherlands (8.2 vs. 1.1%, P < 0.05). Supplementary Table 1 presents more information on the sociodemographic characteristics of the Food4Me study participants as well as the seasonal distribution of the measurements. In addition, Table 1 presents the prevalence of vitamin D deficiency and insufficiency in the total sample, by sex, seasonality, age and weight status groups. Overall, the prevalence of vitamin D deficiency and insufficiency was 3.3 and 30.6%, respectively. Furthermore, the prevalence of vitamin D deficiency and insufficiency was significantly higher in females compared with males (5.4 vs. 0.7%, P < 0.001 for vitamin D deficiency; 36.6 vs. 22.6%, P < 0.001 for vitamin D insufficiency). Regarding seasonal differences, the prevalence of vitamin D insufficiency was higher from January to March (i.e. typical winter months) as compared to the periods from April to June (i.e. typical spring months) and July to September (i.e. typical summer months) (39.3 vs. 25.6% and 25.0%, P < 0.001). In addition, younger study participants (18–35 years) and students had higher prevalence of vitamin D insufficiency compared with older ones (≥ 51 years) and participants with “routine and manual” occupations (36.0 vs. 24.4%, P < 0.001 and 40.7 vs. 24.1%, P = 0.030 respectively). There were no statistically significant differences in the prevalence of vitamin D deficiency and insufficiency between obese and non-obese participants as well as between Caucasians, who represent ~ 97% of the total study sample, and other ethnic groups.
Dietary intake of vitamin D and PA levels by vitamin D status groups
Table 2 summarizes the differences in mean dietary intake of vitamin D from food and/or dietary supplements and in the mean time spent in PAs of different intensity among study participants with deficient (<30 nmol/L), insufficient (30–49.9 nmol/L) and sufficient (≥50 nmol/L) 25-OHD3 concentrations. Participants with sufficient 25-OHD3 concentrations had significantly higher dietary intakes of vitamin D from foods and/or dietary supplements compared with participants with vitamin D insufficiency and deficiency (P < 0.01). Regarding food-derived vitamin D, participants with sufficient concentrations of 25-OHD3 had higher dietary vitamin D intake from meat and fish (P = 0.001), as well as from fats and spreads (P = 0.047) compared with their vitamin D-insufficient and -deficient counterparts and a significant difference was reached for eggs (P = 0.030) compared to vitamin deficient participants. Furthermore, study participants with vitamin D sufficiency spent less time on sedentary activities (P = 0.026) and more time on light- (P = 0.004) and moderate-intensity PA (P = 0.003), as well as in moderate equivalent PA (P = 0.013) in comparison with vitamin D deficient and/or insufficient study participants.
Associations of different intakes of vitamin D and PA levels with vitamin D status
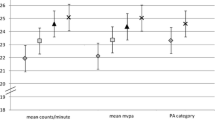
Table 3 displays the dose–response effect of different intakes of vitamin D, derived from foods or supplements, and different durations/ amounts of time spent in moderate equivalent PA with 25-OHD3 concentrations. Mean 25-OHD3 concentrations were higher with higher intakes of vitamin D from foods (P = 0.035) and supplements (P < 0.001), as well as with more time spent in moderate equivalent PA (P = 0.007). Furthermore, Table 3 presents the likelihood (adjusted OR, 95% CIs and P-values) of having sufficient 25-OHD3 concentrations for different intakes of vitamin D, derived from foods or supplements, and different amounts of time spent in moderate equivalent PA. The odds of having sufficient 25-OHD3 concentration was 1.58 (95% CI 1.01–2.52) times more likely for study participants with 5–9.9 μg/day of vitamin D intake derived from foods compared to their counterparts with dietary vitamin D intake <2.5 μg/day. The odds for having sufficient 25-OHD3 concentrations were 1.87 (95% CI 1.05–3.35), 5.49 (95% CI 1.87–16.1) and 14.2 (95% CI 1.86–36.2) times higher for study participants with 5–9.9, 10–19.9 and 20–80 μg/day, respectively, of vitamin D intake from dietary supplements compared with participants with <2.5 μg/day of vitamin D intake from supplements. Lastly, study participants engaged in 30–59.9 and ≥60 min/day of moderate equivalent PA had 1.79 (95% CI 1.24–2.58) and 1.78 (95% CI 1.23–2.57) higher odds to be vitamin D-sufficient compared to study participants spending <30 min/day on moderate-equivalent PA.
Discussion
The available literature on vitamin D status among populations in Europe is characterised by a high degree of variability among countries [6, 9]. Differences among European countries in the prevalence of vitamin D deficiency and insufficiency may be explained in part by the confounding effect of different methods/ assays used to measure 25-OHD3 concentrations in serum or plasma, as well as by different thresholds used to define deficiency and insufficiency [33–35]. Nevertheless, despite the use of the same methods/ assays to measure 25-OHD3 and application of the same thresholds for vitamin D insufficiency for all centres, the current study confirmed similar variability in the prevalence of vitamin D insufficiency and deficiency in seven European countries, ranging from 23.5 and 1.1% in the Netherlands to 34 and 8.2% in the UK, respectively (Fig. 1).
The current study also reported sex, seasonal, and other socio-demographic differences in 25-OHD3 concentrations (Table 1). In this context, the prevalence of vitamin D insufficiency and deficiency was higher in females than males, but these sex differences were smaller than reported by other studies [9]. Regarding seasonal differences, as expected the highest and lowest prevalence rates of vitamin D insufficiency were observed during typical winter (January to March) and summer (July to September) months, respectively. The prevalence of vitamin D insufficiency was lower in the older (≥51 years) compared with the younger (18–35 years) participants. The relevant evidence available in the literature concerning age-specific trends in vitamin D status across the lifespan is inconsistent since higher and lower prevalence rates of poor vitamin D status have been reported for both younger and older adults [9, 35, 36]. In the very old age (usually ≥85 years) several factors such institutionalization, especially when combined with concurrent health and mobility problems, declining efficiency of the skin to endogenously produce vitamin D [37], as well as poor dietary vitamin D intake, and general nutritional status [38], usually lead to a high prevalence of vitamin D deficiency [39]. However, in the present study older study participants were in the age range of 50 to 79 years, were healthy, and also quite physically active, which may explain their better vitamin D status as compared to younger study participants.
Humans obtain vitamin D from the diet, dietary supplements and from endogenous synthesis in the skin due to sunlight exposure, often in an ascending order [3]. The present study confirmed the relatively low contribution of foods to meeting the Estimated Average Requirement (EAR) value of 10 μg/day for vitamin D proposed by the IOM [26]. Specifically, vitamin D intakes from meat, fish, fats, spreads and eggs were significantly higher in participants with sufficient vitamin D concentrations compared with their counterparts with vitamin D insufficiency and/or deficiency (Table 2). Among the limited food sources of vitamin D, either natural or fortified ones, the consumption of foods mentioned above has been reported also by other studies to be linked to better vitamin D status [40]. In addition, even after adjusting for several potential confounders, a dose–response association was observed between dietary vitamin D intakes from foods with 25-OHD3 concentrations (Table 3). Nevertheless, the contribution of foods in the total dietary intake of vitamin D seems to be particularly low [41] and this is also supported by our observations showing that the average dietary intake of vitamin D derived from foods was less than the recommended EAR threshold of 10 μg/day.
Taking into account the low contribution of foods to total dietary vitamin D intake, the findings of the present and those of other European studies [42, 43], highlight the significant role of other sources, notably dietary supplements. The present study showed that the average vitamin D intake from dietary supplements in participants with sufficient vitamin D levels was 3.14 μg/day compared with only 0.63 and 0.53 μg/day by vitamin D insufficient and deficient participants, respectively (Table 2). Data from the National Adult Nutrition Survey in Ireland showed that the average dietary vitamin D intake coming from supplements was 9 μg/day and was much higher compared with that coming from foods, which in the Irish survey was found to be 4 μg/day [44]. This relatively low average vitamin D intake from foods (i.e. exactly 4 μg/day) was also observed in the present study of adults in different parts of Europe and together with the observed high prevalence of vitamin D insufficiency, may indicate the need for more effective dietary strategies to enhance vitamin D intake. With the exception of fatty fish, increasing the intake of natural (non-fortified) food sources of vitamin D is the least likely strategy to counteract low dietary vitamin D intake [45]. As a consequence, the use of dietary supplements has been proposed by many as the second most effective strategy (with food fortification proposed as the primary one) [46]. However, the degree to which the use of vitamin D dietary supplements can increase 25-OHD3 concentrations depends on the dose of vitamin D in the supplements [47]. In this regard, the present study showed that vitamin D intakes of ≥10 μg/day from foods and/or supplements can ensure sufficient 25-OHD3 concentrations.
Because dietary vitamin D intake from its natural food sources and from supplements cannot account for the total variability of serum or plasma 25-OHD3 concentrations, another major determinant of vitamin D status is sun exposure [48–50]. Depending on the time of the day, season, latitude and skin pigmentation, daily exposure of the skin, e.g. of arms or legs for 5 to 30 min can promote adequate endogenous synthesis of vitamin D3 [51]. Physical activity, when executed outdoors, can be a proxy measure of sunlight exposure and probably explains the significant positive association observed in the present study between time spent on moderate-equivalent PA and 25-OHD3 concentrations (Table 3). Similar positive linear associations between PA and circulating 25-OHD3 concentrations have been reported also by other recent studies [14, 49, 53]. Interestingly, some studies have reported significant positive associations between vitamin D status and PA even after adjusting for sun exposure [14]. Furthermore, other studies have found similar significant positive associations with both outdoor and indoor activities [15]. In addition to the wider health benefits from increased PA, the present study showed that at least 30 min per day spent on MVPA is related to sufficient 25-OHD3 concentrations. This observation is very important from a public health perspective, because it is in line with the daily target of moderate- and/or vigorous-intensity PA proposed by the American College of Sports Medicine and the American Heart Association for adults [54].
The findings of the current study should be interpreted in light of its strengths and limitations. Regarding strengths, the web-based design of the Food4Me study facilitated participation by volunteers regardless of distance from the research centre. In addition, the Food4Me research team developed and implemented a novel remote system for data and biological sample collection enabling study participants to provide dietary, anthropometric, PA and other health-related information via the Internet, as well as biological samples (dry blood spots and buccal cells) for nutritional, metabolic and genotypic measurements. In addition, the dried blood spot methodology used to measure 25-OHD3 concentrations was applied for the first time in a fairly large study population and demonstrated to be highly applicable, cost effective and reliable [19]. Regarding limitations, because of the cross-sectional design of the current study, we cannot attribute causality to our observations. Furthermore, most data were self-reported or derived from biological samples collected remotely with the potential for introduction of measurement errors and change of samples. However, studies examining the reliability of data collected in web-based interventions [55, 56], including the present one [24], have shown good agreement between self-reported and objectively measured indices. Moreover, in order to minimize measurement errors, all measurement protocols in the present study were standardized across all centres and were provided in the native languages of each recruitment country. Participants were assisted in recording of information and in sample collection by the provision of detailed instructions, video clips and a frequently asked questions leaflet. Lastly, participants were a convenience sample of those who volunteered for the Food4Me intervention study and are not necessarily nationally representative of the countries involved, which limits generalizability of findings from the present study. However, in several respects, participants were broadly similar to those of the adult population in Europe [19].
Conclusions
In conclusion, the present study reported a considerable variability in vitamin D status among adults examined in seven European countries. The highest prevalence of vitamin D insufficiency and/or deficiency was observed in the UK compared with the Netherlands, in females compared with males, in winter (January to March) compared with summer (July to September) months and in younger (18–35 years) compared with older (≥51 years) study participants. Regarding behavioural correlates of vitamin D status, there were positive dose–response associations between 25-OHD3 concentrations and dietary vitamin D intake from foods and supplements, as well as with physical activity PA levels, which most likely represent a proxy of sun exposure. Dietary intakes of ≥10 μg/day of vitamin D from foods and/or supplements, as well as >30 min/day of moderate-equivalent PA were the minimum thresholds for ensuring sufficient circulating 25-OHD3 concentrations.
References
Taylor CL, Thomas PR, Aloia JF, Millard PS, Rosen CJ (2015) Questions about vitamin D for primary care practice: input from an NIH conference. Am J Med. doi:10.1016/j.amjmed.2015.05.025
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP (2014) Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 348:g2035. doi:10.1136/bmj.g2035
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281. doi:10.1056/NEJMra070553
Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS (2008) Vitamin D deficiency and risk of cardiovascular disease. Circulation 117(4):503–511. doi:10.1161/CIRCULATIONAHA.107.706127
Gulseth HL, Gjelstad IM, Birkeland KI, Drevon CA (2013) Vitamin D and the metabolic syndrome. Curr Vasc Pharmacol 11(6):968–984
van Schoor NM, Lips P (2011) Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab 25(4):671–680. doi:10.1016/j.beem.2011.06.007
Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, Vestergaard P (2009) Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int 20(1):133–140. doi:10.1007/s00198-008-0626-y
Lips P (2007) Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol 103(3–5):620–625. doi:10.1016/j.jsbmb.2006.12.076
Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann K (2014) A systematic review of vitamin D status in populations worldwide. Br J Nutr 111(1):23–45. doi:10.1017/S0007114513001840
Holick MF (2006) Resurrection of vitamin D deficiency and rickets. J Clin Invest 116(8):2062–2072. doi:10.1172/JCI29449
Engelsen O, Brustad M, Aksnes L, Lund E (2005) Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol 81(6):1287–1290. doi:10.1562/2004-11-19-rn-375
Moschonis G, Tanagra S, Koutsikas K, Nikolaidou A, Androutsos O, Manios Y (2009) Association between serum 25-hydroxyvitamin D levels and body composition in postmenopausal women: the postmenopausal Health Study. Menopause 16(4):701–707. doi:10.1097/gme.0b013e318199d5d5
Lips P, van Schoor NM, de Jongh RT (2014) Diet, sun, and lifestyle as determinants of vitamin D status. Ann N Y Acad Sci 1317:92–98. doi:10.1111/nyas.12443
Touvier M, Deschasaux M, Montourcy M, Sutton A, Charnaux N, Kesse-Guyot E, Assmann KE, Fezeu L, Latino-Martel P, Druesne-Pecollo N, Guinot C, Latreille J, Malvy D, Galan P, Hercberg S, Le Clerc S, Souberbielle JC, Ezzedine K (2015) Determinants of vitamin D status in caucasian adults: influence of sun exposure, dietary intake, sociodemographic, lifestyle, anthropometric, and genetic factors. J Invest Dermatol 135(2):378–388. doi:10.1038/jid.2014.400
Wanner M, Richard A, Martin B, Linseisen J, Rohrmann S (2015) Associations between objective and self-reported physical activity and vitamin D serum levels in the US population. Cancer Causes Control 26(6):881–891. doi:10.1007/s10552-015-0563-y
Calvo MS, Whiting SJ, Barton CN (2005) Vitamin D intake: a global perspective of current status. J Nutr 135(2):310–316
Flynn A, Hirvonen T, Mensink GB, Ocke MC, Serra-Majem L, Stos K, Szponar L, Tetens I, Turrini A, Fletcher R, Wildemann T (2009) Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr Res. doi:10.3402/fnr.v53i0.2038
Celis-Morales C, Livingstone KM, Marsaux CF, Forster H, O’Donovan CB, Woolhead C, Macready AL, Fallaize R, Navas-Carretero S, San-Cristobal R, Kolossa S, Hartwig K, Tsirigoti L, Lambrinou CP, Moschonis G, Godlewska M, Surwillo A, Grimaldi K, Bouwman J, Daly EJ, Akujobi V, O’Riordan R, Hoonhout J, Claassen A, Hoeller U, Gundersen TE, Kaland SE, Matthews JN, Manios Y, Traczyk I, Drevon CA, Gibney ER, Brennan L, Walsh MC, Lovegrove JA, Alfredo Martinez J, Saris WH, Daniel H, Gibney M, Mathers JC (2015) Design and baseline characteristics of the Food4Me study: a web-based randomised controlled trial of personalised nutrition in seven European countries. Genes Nutr 10 (1):450. doi:10.1007/s12263-014-0450-2
Hoeller U, Baur M, Roos FF, Brennan L, Daniel H, Fallaize R, Forster H, Gibney ER, Gibney M, Godlewska M, Hartwig K, Kolossa S, Lambrinou CP, Livingstone KM, Lovegrove JA, Macready AL, Manios Y, Marsaux CF, Martinez JA, Celis-Morales C, Moschonis G, Navas-Carretero S, O’Donovan CB, San-Cristobal R, Saris WH, Surwillo A, Traczyk I, Tsirigoti L, Walsh MC, Woolhead C, Mathers JC, Weber P (2015) Application of dried blood spots to determine vitamin D status in a large nutritional study with unsupervised sampling: the Food4Me project. Br J Nutr. doi:10.1017/S0007114515004298
Livingstone KM, Celis-Morales C, Navas-Carretero S, San-Cristobal R, O’Donovan CB, Forster H, Woolhead C, Marsaux CF, Macready AL, Fallaize R, Kolossa S, Tsirigoti L, Lambrinou CP, Moschonis G, Godlewska M, Surwillo A, Drevon CA, Manios Y, Traczyk I, Gibney ER, Brennan L, Walsh MC, Lovegrove JA, Alfredo Martinez J, Saris WH, Daniel H, Gibney M, Mathers JC (2015) Profile of European adults interested in internet-based personalised nutrition: the Food4Me study. Eur J Nutr. doi:10.1007/s00394-015-0897-y
Fallaize R, Forster H, Macready AL, Walsh MC, Mathers JC, Brennan L, Gibney ER, Gibney MJ, Lovegrove JA (2014) Online dietary intake estimation: reproducibility and validity of the Food4Me food frequency questionnaire against a 4-day weighed food record. J Med Internet Res 16(8):e190. doi:10.2196/jmir.3355
Forster H, Fallaize R, Gallagher C, O’Donovan CB, Woolhead C, Walsh MC, Macready AL, Lovegrove JA, Mathers JC, Gibney MJ, Brennan L, Gibney ER (2014) Online dietary intake estimation: the Food4Me food frequency questionnaire. J Med Internet Res 16(6):e150. doi:10.2196/jmir.3105
Livingstone KM, Celis-Morales C, Navas-Carretero S, San-Cristobal R, Macready AL, Fallaize R, Forster H, Woolhead C, O’Donovan CB, Marsaux CF, Kolossa S, Tsirigoti L, Lambrinou CP, Moschonis G, Godlewska M, Surwillo A, Drevon CA, Manios Y, Traczyk I, Gibney ER, Brennan L, Walsh MC, Lovegrove JA, Saris WH, Daniel H, Gibney M, Martinez JA, Mathers JC, Food4Me S (2016) Effect of an Internet-based, personalized nutrition randomized trial on dietary changes associated with the Mediterranean diet: the Food4Me Study. Am J Clin Nutr 104(2):288–297. doi:10.3945/ajcn.115.129049
Celis-Morales C, Livingstone KM, Woolhead C, Forster H, O’Donovan CB, Macready AL, Fallaize R, Marsaux CF, Tsirigoti L, Efstathopoulou E, Moschonis G, Navas-Carretero S, San-Cristobal R, Kolossa S, Klein UL, Hallmann J, Godlewska M, Surwillo A, Drevon CA, Bouwman J, Grimaldi K, Parnell LD, Manios Y, Traczyk I, Gibney ER, Brennan L, Walsh MC, Lovegrove JA, Martinez JA, Daniel H, Saris WH, Gibney M, Mathers JC (2015) How reliable is internet-based self-reported identity, socio-demographic and obesity measures in European adults? Genes Nutr 10 (5):476. doi:10.1007/s12263-015-0476-0
McCance RAW (2002) Royal Society of Chemistry; McCance and Widdowson’s the composition of foods, 7th edn. London.
Institute of Medicine Food and Nutrition Board (2011) Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academy Press.
Bonomi AG, Plasqui G, Goris AH, Westerterp KR (2010) Estimation of free-living energy expenditure using a novel activity monitor designed to minimize obtrusiveness. Obesity (Silver Spring) 18(9):1845–1851. doi:10.1038/oby.2010.34
Plasqui G, Joosen AM, Kester AD, Goris AH, Westerterp KR (2005) Measuring free-living energy expenditure and physical activity with triaxial accelerometry. Obes Res 13(8):1363–1369. doi:10.1038/oby.2005.165
Plasqui G, Westerterp KR (2007) Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity (Silver Spring) 15(10):2371–2379. doi:10.1038/oby.2007.281
Choi L, Liu Z, Matthews CE, Buchowski MS (2011) Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc 43(2):357–364. doi:10.1249/MSS.0b013e3181ed61a3
Marsaux CF, Celis-Morales C, Fallaize R, Macready AL, Kolossa S, Woolhead C, O’Donovan CB, Forster H, Navas-Carretero S, San-Cristobal R, Lambrinou CP, Moschonis G, Surwillo A, Godlewska M, Goris A, Hoonhout J, Drevon CA, Manios Y, Traczyk I, Walsh MC, Gibney ER, Brennan L, Martinez JA, Lovegrove JA, Gibney MJ, Daniel H, Mathers JC, Saris WH (2015) Effects of a web-based personalized intervention on physical activity in European adults: a randomized controlled trial. J Med Internet Res 17(10):e231. doi:10.2196/jmir.4660
World Health Organization (2010) Global Recommendations on Physical Activity for Health. Recommended population levels of physical activity for health. World Health Organization, World Health
Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK (2004) Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 89(7):3152–3157. doi:10.1210/jc.2003-031979
Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt C, Ashwell M (2010) Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids 75(7):477–488. doi:10.1016/j.steroids.2010.02.012
Cashman KD, Dowling KG, Skrabakova Z, Gonzalez-Gross M, Valtuena J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Molgaard C, Jorde R, Grimnes G, Moschonis G, Mavrogianni C, Manios Y, Thamm M, Mensink GB, Rabenberg M, Busch MA, Cox L, Meadows S, Goldberg G, Prentice A, Dekker JM, Nijpels G, Pilz S, Swart KM, van Schoor NM, Lips P, Eiriksdottir G, Gudnason V, Cotch MF, Koskinen S, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Kiely M (2016) Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. doi:10.3945/ajcn.115.120873
Lanham-New SA, Buttriss JL, Miles LM, Ashwell M, Berry JL, Boucher BJ, Cashman KD, Cooper C, Darling AL, Francis RM, Fraser WD, de Groot CP, Hypponen E, Kiely M, Lamberg-Allardt C, Macdonald HM, Martineau AR, Masud T, Mavroeidi A, Nowson C, Prentice A, Stone EM, Reddy S, Vieth R, Williams CM (2011) Proceedings of the rank forum on Vitamin D. Br J Nutr 105(1):144–156. doi:10.1017/S0007114510002576
MacLaughlin J, Holick MF (1985) Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 76(4):1536–1538. doi:10.1172/JCI112134
Lips P, van Ginkel FC, Jongen MJ, Rubertus F, van der Vijgh WJ, Netelenbos JC (1987) Determinants of vitamin D status in patients with hip fracture and in elderly control subjects. Am J Clin Nutr 46(6):1005–1010
Hill TR, Granic A, Davies K, Collerton J, Martin-Ruiz C, Siervo M, Mathers JC, Adamson AJ, Francis RM, Pearce SH, Razvi S, Kirkwood TB, Jagger C (2016) Serum 25-hydroxyvitamin D concentration and its determinants in the very old: the Newcastle 85+ study. Osteoporos Int 27(3):1199–1208. doi:10.1007/s00198-015-3366-9
DeGroot LJ, Jameson JL (2001) Endocrinology. 4th edn. W.B. Saunders Co., Philadelphia
Roman Vinas B, Ribas Barba L, Ngo J, Gurinovic M, Novakovic R, Cavelaars A, de Groot LC, van’t Veer P, Matthys C, Serra Majem L (2011) Projected prevalence of inadequate nutrient intakes in Europe. Ann Nutr Metab 59(2–4):84–95. doi:10.1159/000332762
Kiely M, Black LJ (2012) Dietary strategies to maintain adequacy of circulating 25-hydroxyvitamin D concentrations. Scand J Clin Lab Invest Suppl 243:14–23. doi:10.3109/00365513.2012.681893
Skeie G, Braaten T, Hjartaker A, Lentjes M, Amiano P, Jakszyn P, Pala V, Palanca A, Niekerk EM, Verhagen H, Avloniti K, Psaltopoulou T, Niravong M, Touvier M, Nimptsch K, Haubrock J, Walker L, Spencer EA, Roswall N, Olsen A, Wallstrom P, Nilsson S, Casagrande C, Deharveng G, Hellstrom V, Boutron-Ruault MC, Tjonneland A, Joensen AM, Clavel-Chapelon F, Trichopoulou A, Martinez C, Rodriguez L, Frasca G, Sacerdote C, Peeters PH, Linseisen J, Schienkiewitz A, Welch AA, Manjer J, Ferrari P, Riboli E, Bingham S, Engeset D, Lund E, Slimani N (2009) Use of dietary supplements in the European prospective investigation into cancer and nutrition calibration study. Eur J Clin Nutr 63(Suppl 4):S226–S238. doi:10.1038/ejcn.2009.83
Black LJ, Walton J, Flynn A, Cashman KD, Kiely M (2015) Small increments in vitamin D intake by Irish adults over a decade show that strategic initiatives to fortify the food supply are needed. J Nutr 145(5):969–976. doi:10.3945/jn.114.209106
Cashman KD (2015) Vitamin D: dietary requirements and food fortification as a means of helping achieve adequate vitamin D status. J Steroid Biochem Mol Biol 148:19–26. doi:10.1016/j.jsbmb.2015.01.023
Calvo MS, Whiting SJ (2006) Public health strategies to overcome barriers to optimal vitamin D status in populations with special needs. J Nutr 136(4):1135–1139
Autier P, Gandini S, Mullie P (2012) A systematic review: influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J Clin Endocrinol Metab 97(8):2606–2613. doi:10.1210/jc.2012-1238
Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Thomsen J, Charles P, Eriksen EF (2000) Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med 247(2):260–268
Jones G, Dwyer T (1998) Bone mass in prepubertal children: gender differences and the role of physical activity and sunlight exposure. J Clin Endocrinol Metab 83(12):4274–4279. doi:10.1210/jcem.83.12.5353
Sato Y, Iwamoto J, Kanoko T, Satoh K (2005) Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimer’s disease: a randomized controlled trial. J Bone Miner Res 20(8):1327–1333. doi:10.1359/JBMR.050402
Holick MF (2011) Vitamin D deficiency in 2010: health benefits of vitamin D and sunlight: a D-bate. Nat Rev Endocrinol 7(2):73–75. doi:10.1038/nrendo.2010.234
Berger C, Greene-Finestone LS, Langsetmo L, Kreiger N, Joseph L, Kovacs CS, Richards JB, Hidiroglou N, Sarafin K, Davison KS, Adachi JD, Brown J, Hanley DA, Prior JC, Goltzman D (2012) Temporal trends and determinants of longitudinal change in 25-hydroxyvitamin D and parathyroid hormone levels. J Bone Miner Res 27(6):1381–1389. doi:10.1002/jbmr.1587
Thuesen B, Husemoen L, Fenger M, Jakobsen J, Schwarz P, Toft U, Ovesen L, Jorgensen T, Linneberg A (2012) Determinants of vitamin D status in a general population of Danish adults. Bone 50(3):605–610. doi:10.1016/j.bone.2011.12.016
Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39(8):1423–1434. doi:10.1249/mss.0b013e3180616b27
Pursey K, Burrows TL, Stanwell P, Collins CE (2014) How accurate is web-based self-reported height, weight, and body mass index in young adults? J Med Internet Res 16(1):e4. doi:10.2196/jmir.2909
Lassale C, Peneau S, Touvier M, Julia C, Galan P, Hercberg S, Kesse-Guyot E (2013) Validity of web-based self-reported weight and height: results of the Nutrinet-Sante study. J Med Internet Res 15(8):e152. doi:10.2196/jmir.2575
Acknowledgements
Study approval: The Food4Me study has been reviewed and approved by seven independent ethical research committees in each of the countries involved in the trial. Patient consent: All participants, both screened and randomised, have given consent to take part in this study. Funding statement: This work was supported by the European Commission under the Food, Agriculture, Fisheries and Biotechnology Theme of the 7th Framework Programme for Research and Technological Development [265494].
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manios, Y., Moschonis, G., Lambrinou, C.P. et al. Associations of vitamin D status with dietary intakes and physical activity levels among adults from seven European countries: the Food4Me study. Eur J Nutr 57, 1357–1368 (2018). https://doi.org/10.1007/s00394-017-1415-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1415-1