Abstract
Cancer cases have increased worldwide. Cutaneous melanoma (CM), a highly metastatic skin cancer, largely contributes to global statistical cancer death data. Research has shown that rosmarinic acid (RA) is a promising phenolic compound with antineoplastic properties. Thus, we investigated the effects of RA on apoptosis–inducing in melanoma cells, purinergic signaling modulation, and cytokine levels. We treated SK–MEL–28 cells for 24 h with different concentrations of RA and assessed the apoptosis, CD39, CD73, and A2A expression, and cytokine levels. We found RA–induced apoptosis in melanoma cells. Regarding the purinergic system, we verified that RA downregulated the expression of CD73 and A2A, specially at high concentrations of treatment. Additionally, RA increased IL–6, IL–4, IL–10, IFN–γ, and TNF–α levels. Our in vitro results confirm RA's potential to be used to induce melanoma cell apoptosis, having CD73 and A2A as targets when reversion of immune suppression is desired. Further studies in animal models and clinical trials focusing on RA's modulation of purinergic signaling in melanoma are required.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer incidence has increased globally in the twenty-first century [40]. Among them, cutaneous melanoma (CM) has become a public health problem due to its ability to metastasize and its significant contribution to the global statistics data on cancer deaths [28, 38]. This neoplasm’s pathology originates from the malignant transformation of epidermal melanocytes mainly due to excessive or unprotected sunbathing, which leads to DNA mutations by ultraviolet (UV) radiation [7, 31].
Even with new therapeutic possibilities, several side effects and possible pharmacological resistance still limit the effectiveness of cancer treatments [18]. When CM progresses to stages III and IV, these problems are more pronounced [30]. In this context, phenolic compounds, such as rosmarinic acid (RA), have been indicated promising in an adjuvant therapeutic perspective associated with pharmacological use [10]. This phenolic acid is easily and naturally found in plants from Boraginaceae and Lamiaceae families, such as rosemary (Rosmarinus officinalis L.) [34]. Some research has already shown the anticancer effect of RA in colon carcinoma [20], prostate cancer [19], and breast cancer [29]. Although works are showing the effects of RA in the field of cancer and of CM, the effects of RA in humans are still poorly understood.
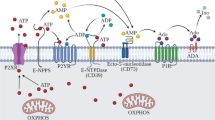
Recently, the purinergic system, a ubiquitous and sophisticated cell–cell communication extracellular signaling pathway [6], has been shown to play an essential role in cancer pathophysiology, such as in lung cancer [49], glioblastoma [1] and melanoma [27]. The molecules involved in this cell signaling pathway mainly include the nucleotides adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP) and the nucleoside adenosine (Ado), whose levels are regulated by ectonucleotidases NTPDase (CD39), 5′-nucleotidase (CD73) and adenosine deaminase (ADA). The signaling molecule’s action on P1 and P2 receptors is implicated in several cellular outcomes [5, 6].
Although other signaling pathways have shown a solid association with the anticancer action of RA, the potential for purinergic signaling modulation has yet to be explored in the experimental context. In a previous study, we proposed hypothetically that RA may be a modulator of the purinergic system [10]. Continuing to research RA properties, in this research we investigated the effects of RA on melanoma cell viability, purinergic signaling modulation, and cytokine levels. Thus, this is the first study that focused on experimentally investigating the purinergic signaling as a target of RA in melanoma context.
Materials and methods
Chemicals, reagents and equipment
All chemicals and reagents used were of analytical grade, purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA) and Merck (Darmstadt, Germany). RA (99% purity) (Fig. 1) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell culture plates and flasks used for culture procedures were obtained from Gibco™ Thermo Fisher Scientific (Grand Island, NY, USA) and Invitrogen Life Technologies (Carlsbad, CA, USA). Molecular biology reagents were purchased from Invitrogen and Applied Biosystems (Waltham, Massachusetts, USA). Flow cytometry was analyzed in an Accuri™ C6 Plus cytometer (BD Biosciences) and by the FlowJo V10 software.
Cell culture and treatment with RA
The human metastatic melanoma cell line SK–MEL–28 was purchased from the Cell Bank of Rio de Janeiro (CBRJ), Brazil. Cells were grown in flasks with Dulbecco’s modified Eagle’s medium (DMEM) with glucose (4,500 mg/L), L–glutamine, containing antibiotics/antifungal (1% penicillin/streptomycin) and supplemented with 10% fetal bovine serum. The cells were grown under adequate conditions in a humidified and controlled atmosphere of 5% carbon dioxide (CO2) at 37 °C. RA was dissolved in the appropriate culture medium to obtain different concentrations, and the cells were treated for 24 h at concentrations of 400 µM and 800 µM according to previous studies that found optimal RA concentrations to be used [9]. The negative control group cells received only culture medium.
Apoptosis assay
The detection of the apoptosis-inducing capacity of RA was assessed by an Annexin V–FITC and propidium iodide (PI) apoptosis detection kit (BD Biosciences), used according to the manufacturer’s instructions. Briefly, after exposure to RA, cells were harvested and washed twice with cold PBS. Then, cells were resuspended at 1 × 106 cells/mL in 100 μL binding buffer, mixed with 5 μL Annexin V–FITC and 5 μL PI (20 μg/mL), and then incubated for 15 min at room temperature in the dark. Finally, the reaction was mixed with 400 μL binding buffer and read on the BD Accuri™ C6 Plus at every 20,000 events.
Gene expression of CD39 and CD73
We used the real-time quantitative polymerase chain reaction (RT–qPCR) analysis to assess the gene expression of CD39 and CD73. First, we obtained the RNA from cell culture samples with TRIzol™ reagent (Invitrogen™) and quantified it spectrophotometrically (Thermo Scientific™ Varioskan™ LUX). Reverse transcription into cDNA was performed with a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems™) by the addition to each 10 μL sample of 10 μL of a mix containing 1 μL of MultiScribe™ Reverse Transcriptase. The steps of the reaction were 25 °C for 10 min, 37 °C for 120 min, 85 °C for 5 min, and a final hold step of 4 °C for 30 min, performed using a thermal cycler. The RT–qPCR reaction was performed using 17 μL of a mix containing the SYBR Green PCR Master Mix (Applied Biosystems™) and 3 μL of the cDNA sample. The parameters used were a pre-activation step of 10 min at 95 °C, followed by a cycling step of 40 cycles at 95 °C for 15 s, 60 °C for 60 s, and finally, a melting step with a melting curve of 60 °C to 95 °C with an increase of 1 °C every 5 s. The relative expression of each gene was represented as the fold expression about the control and calculated using the comparative ΔΔCT value. GAPDH was used as the housekeeping gene to normalize gene expression. The forward and reverse sequences of oligos (5′-3′) used for each gene were as follows: GAPDH (F): CTCCTCACAGTTGCCATGTA; GAPDH (R): GTTGAGCACAGGGTACTTTATTG; CD39 (F): GCCCTGGTCTTCAGTGTATTAG; CD39 (R): CTGGCATAACCTACCTACTCTTTC; CD73 (F): GTGCCTTTGATGAGTCAGGTAG; CD73 (R): TTCCTTTCTCTCGTGTCCTTTG.
Assessment of CD39, CD73 and A2A protein expression
SK–MEL–28 cells were cultured under treatment conditions for 24 h, followed by trypsinization using trypsin, and counted in a hemocytometer. Around 1 × 106 cells were centrifuged for 5 min at 400 g and washed twice with PBS with 10% FBS. The cell pellets were suspended and incubated for 30 min with purified mouse anti–human CD39 (clone A1 (RUO), catalog Nº. 567157), anti–human CD73 (clone AD2 (RUO), catalog Nº. 550257), and anti-human A2A (catalog Nº. MA5–31611) antibodies conjugated with fluorescent phycoerythrin (PE) (BD Pharmingen TM) (1:10). Then, the same number of cells was incubated without antibodies (non–stained). All samples were washed with PBS, and 10,000 events were immediately acquired by flow cytometry (BD ACCURI C6) and analyzed by FlowJo V10 software. Each target (CD39, CD73, and A2A) was analyzed separately and incubated with respective antibodies. The results were expressed as a percentage (%) of CD39 or CD73 or A2A positive cells relative to the control.
Enzymatic activity of ectonucleotidases
The hydrolysis of nucleotides such as ATP, ADP, AMP, and the nucleoside Ado was employed to evaluate the alterations in purinergic system enzyme activities. Briefly, after protein adjustments of cells, 20 μL of samples were added to the reaction mixture of each enzyme and pre-incubated at 37 °C for 10 min. The enzymatic reaction was initiated by adding the specific substrates for each enzyme: ATP and ADP for ATPase/ADPase and AMP for AMPase. After incubation at 37 °C for 70 min, the reactions were stopped by the addition of trichloroacetic acid (TCA, 10%), and the released inorganic phosphate due to ATP, ADP, and AMP hydrolysis was determined by using malachite green as the colorimetric reagent. A standard curve was prepared with KH2PO4. Controls were performed to correct for non-enzymatic hydrolysis. The absorbance was measured at 630 nm, and enzyme-specific activities were reported as nmol/Pi/min/mg of protein [24, 39].
For the Ado breakdown, assessed by ADA activity, was performed based on the measurement of ammonia produced when this enzyme is activated, following a previously published method (Giusti and Galanti, 1984). In brief, 50 μL of cell suspension reacted with 21 mM of adenosine (pH 6.5) at 37 °C for 60 min. After incubation, the reaction was stopped by adding 167.8 mM sodium nitroprusside, 106.2 mM phenol, and a sodium hypochlorite solution. Lastly, absorbance was read at 620 nm, and values were expressed as units/liter (U/L).
Assessment of cytokine levels by flow cytometry
The levels of cytokines released from melanoma cells, interleukin–4 (IL–4), interleukin–6 (IL–6), interleukin–10 (IL–10), interferon–γ (IFN–γ), and tumor necrosis factor–α (TNF–α), were verified by BD™ CBA Human Th1/Th2 Cytokine Kit II (catalog Nº. 551,809) following the manufacturer’s recommendations. The method principle of the kit is based on the formation of sandwich complexes (capture bead/cytokine/fluorescent detection reagent) of cytokines with conjugated specific antibody beads of known size and fluorescence, whose intensity can be measured in flow cytometry equipment and is proportional to the cytokine level. Briefly, 50 µL of supernatant samples were mixed with 50 µL of Human Th1/Th2–II PE Detection Reagent in a microtube, followed by 3 h of incubation at room temperature and protected from light. After time elapsed, the samples were washed with 1 mL of washing buffer and centrifuged at 200 g for 5 min. Then, the supernatant from each test tube was discarded, and the sandwich bead pellet was resuspended with 300 µL of buffer for analysis by flow cytometry.
Protein determination
We used the Bradford [4] method to assess the protein concentration in samples using bovine serum albumin as the standard. When necessary, the protein of samples was adjusted according to each protocol.
Statistical analysis
All measurements were statistically performed by analysis of variance followed by the appropriate post hoc test using GraphPad Prism 9 software. The differences between the untreated (CT) and treated cells were evaluated by unpaired Student’s t–test or through the variance analysis one–way ANOVA followed by Tukey’s post hoc test. All data are expressed as mean ± standard deviation. The differences in the probability of rejecting the null hypothesis at < 5% (P < 0.05) were considered statistically significant. Statistical significance was defined for p–values of *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Results
RA induces apoptosis in melanoma cells
The apoptosis-induced in melanoma cells after 24 h of RA treatment is shown in Fig. 2. It is notably that RA significantly induced apoptosis in melanoma cells at concentrations of 400 µM (P < 0.0001) and 800 µM (P < 0.0001) in comparison to control (Fig. 2A–B). These results showed that RA exhibits strong antineoplastic effects on melanoma cells through induction of apoptosis.
Apoptosis-induced melanoma cells after treatment with RA. RA also significantly induced apoptosis of melanoma cells at concentrations of 400 µM and 800 µM (A–B). Control (CT). All experiments were performed independently at least three times and in three replicates. Data are presented as mean ± SD. Statistical analysis: one–way ANOVA followed by a post hoc Tukey’s multiple comparisons test. Values with P < 0.05 were considered statistically significant. *(P < 0.05); **(P < 0.01); ***(P < 0.001); ****(P < 0.0001). All data indicate differences from the control group
RA modulates expression of purinergic signaling components in melanoma cells
We searched for a possible modulatory effect of RA on the gene and protein expression of CD39 and CD73 in melanoma cells (Fig. 3A–F). After 24 h, the treatment with RA downregulated the gene expression of CD39 at concentration of 800 µM (P = 0.0183) when compared to the control group (Fig. 3A). However, this effect was no observed on the protein expression of CD39 (Fig. 3B–C). Regarding the CD73, we also found a reduction in gene expression at concentration of 800 µM (P = 0.0003), but in contrast to CD39, the protein expression of CD73 also was strongly reduced (P < 0.0001) in this treatment concentration (Fig. 3D–F).
Gene and protein expression of the CD39 and CD73. We employed RT–qPCR to assess the gene expression of CD39 and CD73 in melanoma cells. To verify the protein expression, we used flow cytometry. At concentration of 800 µM, the treatment with RA reduced the gene expression of CD39 (A) and of CD73 (D). Similarly, the same RA treatment concentration was able to decreased of CD73 protein expression (E–F). There was no statistical significance for CD39 protein expression (B–C). Control (CT). All experiments were performed independently at least three times and in three replicates. Data are presented as mean ± SD. Statistical analysis: one–way ANOVA followed by a post hoc Tukey’s multiple comparisons test. Values with P < 0.05 were considered statistically significant. *(P < 0.05); **(P < 0.01); ***(P < 0.001); ****(P < 0.0001). All data indicate differences from the control group
In addition, we investigated the effect of RA on the protein expression of the A2A receptor (Fig. 4A–B). Interestingly, we found that RA treatment was able to reduce A2A expression in melanoma cells at concentrations of 400 µM (P < 0.0001) and 800 µM (P < 0.0001) when compared to control.
Protein expression of the receptor A2A. After 24 h of treatment with RA, the expression of the receptor A2A significantly decreased at both concentrations of 400 µM and 800 µM in melanoma cells (A–B). Control (CT). All experiments were performed independently at least three times and in three replicates. Data are presented as mean ± SD. Statistical analysis: one–way ANOVA followed by a post hoc Tukey’s multiple comparisons test. Values with P < 0.05 were considered statistically significant. *(P < 0.05); **(P < 0.01); ***(P < 0.001); ****(P < 0.0001). All data indicate differences from the control group
RA modulates activity of ectonucleotidases in melanoma cells
Figure 5 (A–D) shows the enzymatic activity of ectonucleotidases in the hydrolysis of nucleotides ATP, ADP and AMP, and the Ado nucleoside after 24 h of treatment with RA. Curiously, at concentration of 800 µM, the treatment significantly decreased the ADP hydrolysis (P < 0.0001) (Fig. 5B). Likewise, the treatment with RA at concentration of 800 µM greatly reduced the AMP hydrolysis (P < 0.0001) (Fig. 5C). No statistical significance was observed in ATP hydrolysis and Ado breakdown (Fig. 5A and D).
Enzymatic activity of ectonucleotidases. We assessed the ATPase/ADPase/AMPase activity on ATP, ADP, and AMP hydrolysis, respectively. In addition, we also assessed the breakdown of adenosine (Ado) by the ADA activity. Treatment of 800 µM for 24 h o RA significantly decreased the hydrolysis of ADP (B) and AMP (C) in melanoma cells. There was no statistical significance for ATP hydrolysis (A) and Ado breakdown (D) in both tested concentrations of RA. All experiments were performed independently at least three times and in three replicates. Data are presented as mean ± SD. Statistical analysis: one–way ANOVA followed by a post hoc Tukey’s multiple comparisons test. Values with P < 0.05 were considered statistically significant. *(P < 0.05); **(P < 0.01); ***(P < 0.001); ****(P < 0.0001). All data indicate differences from the control group
RA modulates the cytokine levels in melanoma cells
We also investigated the levels of cytokines after 24 h of RA treatment (Fig. 6A–E). We found that RA is a phenolic compound that modulates the levels of cytokines in melanoma cells, such as IL–4, that had increased levels at concentrations of 400 µM (P = 0.0002) and 800 µM (P = 0.0005) in comparison to the control group (Fig. 6A). The levels of IL–6 were also increased at concentrations of 400 µM (P = 0.0045) and 800 µM (P = 0.0010) (Fig. 6B). We also found increased levels for IL–10 at concentrations of 400 µM (P = 0.0011) and 800 (P = 0.0016) of RA (Fig. 6C). The levels of IFN–γ were increased at concentration of 800 µM (P = 0.0152) (Fig. 6D). In addition, we detected elevated levels of TNF–α after treatment with RA in melanoma cells in 400 µM (P = 0.0007) and 800 µM (P = 0.0004) (Fig. 6E).
Cytokine levels. RA treatment increased the levels of cytokines IL–4 (A), IL–6 (B), IL–10 (C), and TNF–α (E) in both concentrations of 400 µM and 800 µM. In the case of INF–ɣ, treatment with RA increased the levels of this cytokine at concentrations of 800 µM (D). Control (CT). All experiments were performed independently at least three times and in three replicates. Data are presented as mean ± SD. Statistical analysis: one–way ANOVA followed by a post hoc Tukey’s multiple comparisons test. Values with P < 0.05 were considered statistically significant. *(P < 0.05); **(P < 0.01); ***(P < 0.001); ****(P < 0.0001). All data indicate differences from the control group
Discussion
Several studies have shown the multiple biological properties of RA, such as anti-inflammatory [25], anti–oxidant [35], and neuroprotection [16]. In the field of oxidative stress, Venza et al. [44] showed that the molecule ROS has a significant role in melanoma, being that high levels of ROS are involved in the pathophysiology of this cancer type. Interestingly, in a recent article, we proved that RA can reduce both intracellular and extracellular ROS levels in melanoma cells besides modulates genes related to cell death [9].
An important cell signaling pathway called purinergic signaling, has been linked to pathophysiology of CM [27, 49]. Unfortunately, there is a poor prognosis in the advanced stages of CM due to insufficient effectiveness of available treatments [45]. Even if the evidence about RA's antineoplastic potential is highlighted, research on purinergic signaling as a target of RA in the CM context needs to be improved. In a previous study, we hypothesized that RA may be a modulator of the purinergic system in melanoma context [10]. In this ground–breaking study, we proved that RA is able to downregulates the expression of CD73 and A2A, and modulates the ectonucleotidases activity. Taken all together, these purinergic signaling modulation cause increasing in the levels of several cytokines that culminates in apoptosis of melanoma cells.
In previous experimental work, we reported that RA has an antineoplastic effect on melanoma cells by reducing viability [9]. Here we found that RA induces apoptosis at concentrations of 400 µM and 800 µM (Fig. 2A–B). Similar, a study involving CT26 colorectal cancer cells showed that RA reduces cell viability after 24 h of treatment [17]. Reports from the literature also showed that RA can induce apoptosis in breast [29] and prostate [19] cancer cells, but this effect had not been well explored in SK–MEL–28 lineage until then. Thus, our results reinforce the antineoplastic effect of RA and add new findings in the melanoma context.
Sequentially, we continue searching for a possible modulatory effect on the components of the purinergic system. We found that RA significantly reduced the expression of the CD73 at concentration of 800 µM (Fig. 3D–F). In an animal model, tumor size reduction was related to CD73 inhibition [13]. In humans, overexpression of CD73 has been described in many cancer cell types, such as breast cancer, colorectal cancer, ovarian cancer, gastric cancer, and gallbladder cancer [14]. The expression and activity of CD73 seem to be associated with tumor invasion and metastasis [8]. It was also shown that patients with advanced melanoma have high rates of CD73 expression [32]. Thus, our results bring to light the possibility of RA pharmacotherapeutic applications having the CD73 as a target against melanoma. In addition, although associated with a hydrolytic activity, CD73 also has a non–enzymatic action, promoting cell migration in the extracellular matrix (ECM) through the activation of focal adhesion kinase (FAK) in melanoma cells [41]. Thus, together with our previous study in which we found that RA inhibits cell migration [9], now we have support to the hypothesis that this inhibition of adhesion of melanoma cells by may occurs by the effect of RA on CD73 expression.
Alterations in the enzymatic activity of ectonucleotidases were highlighted in previous studies involving patients with lung cancer [50], uterine cervical neoplasia [26], and melanoma [27]. Considering the studies above and the results presented in this work, which indicated that RA is a phenolic compound that modulates the expression of components of the purinergic system, we advanced our study to understand its action on the purinergic enzymatic cascade in detail. For this, assays to verify the enzymatic activity of ectonucleotidases were performed. After 24 h of treatment, we found that RA interfered in the enzymatic activity of ectonucleotidases, reducing significantly the hydrolysis of ADP (Fig. 5B), but did not have the same effect on ATP hydrolysis (Fig. 5A). Although CD39 is an enzyme capable of hydrolyzing ATP into ADP and ADP into AMP [12], it is not the only enzyme with this function. Alkaline phosphatases (APs) can also specifically hydrolyze ADP into AMP [2], which may justify the reduction in ADP hydrolysis that we found, not being strictly related to a previous hydrolysis of ATP by CD39. The hydrolysis of AMP was also decreased at a concentration of 800 µM (Fig. 5C). These outcomes are explained by reduction in CD73 expression after treatment with RA (Fig. 3E–F), which promotes changes in the purinergic signaling cascade and reduces Ado formation.
Regarding Ado, the CD73/ADA enzyme axis tightly regulates the levels of this nucleoside at the end of the purinergic cascade. This is due to CD73 promoting the conversion of AMP into Ado, while ADA converts Ado into inosine by an irreversible deamination reaction [3]. Thus, the ADA is crucial in controlling Ado levels [8]. The presence of increased levels of Ado in the tumor microenvironment (TME) is related to tumor immunosuppression [11]. Reduced ADA activity and increased P1 receptor expression (activated by Ado) in patients with advanced–stage lung cancer have been attributed to pro-tumor effects by increasing IL–6 and TNF–α levels and decreasing IL–17 and INF–γ [49]. However, in this study, we did not find statistical significance for ADA activity after treatment with RA (Fig. 5D), and thus, in opposition to the studies mentioned above, our results indicated that purinergic cascade was more prone to a decrease in Ado formation in melanoma cells. Hence, our results relative to CD73 downregulation indicate that RA’s antineoplastic effect may be linked to two mechanisms: reducing Ado production and inhibiting cell adhesion.
As shown, CD73 is responsible for converting AMP into Ado and thus exerts essential control over the immune response against tumors [8]. In cancer cells, the activation of the A2A receptor by Ado also participates in the immune response, promoting a pro–tumoral condition via the PIK3/AKT signaling pathway [42], with an increase in angiogenesis, tumor growth, and reduction of the immune response, such as IL–2, IL–6, and IFN–γ production [33, 47]. Recently, it has been shown that simultaneous inhibition of CD73 and A2AR exhibits a synergistic effect against cancer [46]. Thus, the blockade of A2AR may be an exciting cancer immunotherapy [48]. In melanoma, blocking A2A is linked to restoring the antitumor immune response [36]. Kim et al. [21] suggested hypothetically that RA plays a negative allosteric role in regulating the A2A receptor. Intriguingly, found that RA significantly reduces the expression of A2A (Fig. 4A–B).
We must highlight that purinergic signaling closely connects with the immune system, promoting many physiological mechanisms. Interestingly, in this field, evidence has shown that melanocytes exhibit macrophage phagocytic-like functions and may actively participate in immune responses, particularly as antigen-processing or antigen–presenting cells [22]. Moreover, this cell type can produce cytokines, such as IL–4, IL–6, and IFN–γ, in inflammatory processes [15]. Considering that CM is derived from melanocytes and our results regarding the CD73 and A2A receptors, we searched for cytokine levels in melanoma cells after treatment with RA. In this in vitro study, we found that RA treatment increased the levels of IL–4, IL–6, IL–10, IFN–γ, TNF–α (Fig. 6A–E) at the same concentrations as RA significantly decreased A2A receptor expression.
Two cytokines involved in the immune response that play critical roles in the inflammatory process are IL–6 and IL–10. While IL–6 is an important pro-inflammatory mediator that induces acute phase responses and the production of other cytokines, IL–10 is related to the suppression of inflammation [37, 43]. Our study also showed increased cytokine levels, emphasizing IL–6 and IL–10. Although simultaneous increases in both IL–6 and IL–10 levels can be explained by the assumption that cytokine release is an inflammation regulatory balance, data from the literature supports that IL–6 may have growth-inhibitory effects against melanoma [23].
Conclusion
In an unprecedented-like manner, we proved that RA downregulated expression of CD73 and A2A in melanoma cells. Reduction in CD73 expression promoted a modulation in hydrolysis of ADP and AMP. All this purinergic signaling modulations by RA, increased the levels of cytokines, reversing the immunosuppression, and led melanoma cells to apoptosis. Thus, our in vitro results confirm RA's potential to be used to induce melanoma cell apoptosis, having CD73 and A2A as target when reversion of immune suppression is desired. We suggest further studies in animal models and clinical trials focusing on the modulation of purinergic signaling by RA in melanoma.
Data availability
All main text or supplementary materials data are available.
Abbreviations
- ADA:
-
Adenosine deaminase
- ADP:
-
Adenosine diphosphate
- AMP:
-
Adenosine monophosphate
- ATP:
-
Adenosine triphosphate
- CBRJ:
-
Cell Bank of Rio de Janeiro
- CM:
-
Cutaneous melanoma
- DAMP:
-
Damage-associated molecular pattern
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ECM:
-
Extracellular matrix
- FAK:
-
Focal adhesion kinase
- IL-4:
-
Interleukin-4
- IL-6:
-
Interleukin-6
- IL-10:
-
Interleukin-10
- IFN-γ:
-
Interferon-γ
- PE:
-
Phycoerythrin
- PI:
-
Propidium iodide
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- RA:
-
Rosmarinic acid
- TCA:
-
Trichloroacetic acid
- TME:
-
Tumor microenvironment
- TNF-α:
-
Tumor necrosis factor-α
- UV:
-
Ultraviolet
References
Azambuja JH, Gelsleichter NE, Beckenkamp LR, Iser IC, Fernandes MC, Figueiró F, Battastini AMO, Scholl JN, De Oliveira FH, Spanevello RM, Sévigny J, Wink MR, Stefani MA, Teixeira HF, Braganhol E (2019) CD73 downregulation decreases in vitro and in vivo glioblastoma growth. Mol Neurobiol 56:3260–3279. https://doi.org/10.1007/s12035-018-1240-4
Azpiazu D, Gonzalo S, Villa-Bellosta R (2019) Tissue non-specific alkaline phosphatase and vascular calcification: a potential therapeutic target. CCR 15:91–95. https://doi.org/10.2174/1573403X14666181031141226
Blackburn MR, Kellems RE (2005) Adenosine deaminase deficiency: metabolic basis of immune deficiency and pulmonary inflammation. In: Adv Immunol Elsevier, pp. 1–41. https://doi.org/10.1016/S0065-2776(04)86001-2
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Burnstock G, Knight GE (2004) Cellular distribution and functions of p2 receptor subtypes in different systems. In: Int Rev Cytol Elsevier, pp. 31–304. https://doi.org/10.1016/S0074-7696(04)40002-3
Burnstock G, Verkhratskiĭ AN (2012) Purinergic signalling and the nervous system. Springer, Heidelberg
Carr S, Smith C, Wernberg J (2020) Epidemiology and risk factors of melanoma. Surg Clin North Am 100:1–12. https://doi.org/10.1016/j.suc.2019.09.005
Chen S, Wainwright DA, Wu JD, Wan Y, Matei DE, Zhang Y, Zhang B (2019) CD73: an emerging checkpoint for cancer immunotherapy. Immunotherapy 11:983–997. https://doi.org/10.2217/imt-2018-0200
Da Silva GB, Manica D, Da Silva AP, Marafon F, Moreno M, Bagatini MD (2023) Rosmarinic acid decreases viability, inhibits migration and modulates expression of apoptosis-related CASP8/CASP3/NLRP3 genes in human metastatic melanoma cells. Chem Biol Interact 375:110427. https://doi.org/10.1016/j.cbi.2023.110427
da Silva GB, Yamauchi MA, Zanini D, Bagatini MD (2022) Novel possibility for cutaneous melanoma treatment by means of rosmarinic acid action on purinergic signaling. Purinergic Signalling 18:61–81. https://doi.org/10.1007/s11302-021-09821-7
Di Virgilio F (2012) Purines, Purinergic Receptors, and Cancer. Cancer Res 72:5441–5447. https://doi.org/10.1158/0008-5472.CAN-12-1600
Di Virgilio F, Adinolfi E (2017) Extracellular purines, purinergic receptors and tumor growth. Oncogene 36:293–303. https://doi.org/10.1038/onc.2016.206
Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock IM, Maiolino P, Arra C, Cicala C, Pinto A, Morello S (2012) Inhibition of CD73 Improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol 189:2226–2233. https://doi.org/10.4049/jimmunol.1200744
Gao Z, Dong K, Zhang H (2014) The Roles of CD73 in cancer. Biomed Res Int 2014:1–9. https://doi.org/10.1155/2014/460654
Gasque P, Jaffar-Bandjee MC (2015) The immunology and inflammatory responses of human melanocytes in infectious diseases. J Infect 71:413–421. https://doi.org/10.1016/j.jinf.2015.06.006
Ghasemzadeh Rahbardar M, Hosseinzadeh H (2020) Effects of rosmarinic acid on nervous system disorders: an updated review. Naunyn-Schmiedeberg’s Arch Pharmacol 393:1779–1795. https://doi.org/10.1007/s00210-020-01935-w
Han Y-H, Kee J-Y, Hong S-H (2018) Rosmarinic acid activates ampk to inhibit metastasis of colorectal cancer. Front Pharmacol 9:68. https://doi.org/10.3389/fphar.2018.00068
Hwang S-Y, Chae J-I, Kwak A-W, Lee M-H, Shim J-H (2020) Alternative options for skin cancer therapy via regulation of AKT and related signaling pathways. IJMS 21:6869. https://doi.org/10.3390/ijms21186869
Jang Y-G, Hwang K-A, Choi K-C (2018) Rosmarinic acid, a component of rosemary tea, induced the cell cycle arrest and apoptosis through modulation of HDAC2 expression in prostate cancer cell lines. Nutrients 10:1784. https://doi.org/10.3390/nu10111784
Karthikkumar V, Sivagami G, Viswanathan P, Nalini N (2015) Rosmarinic acid inhibits DMH-induced cell proliferation in experimental rats. J Basic Clin Physiol Pharmacol 26. https://doi.org/10.1515/jbcpp-2014-0044
Kim T-H, Bormate KJ, Custodio RJP, Cheong JH, Lee BK, Kim HJ, Jung Y-S (2022) Involvement of the adenosine A1 receptor in the hypnotic effect of rosmarinic acid. Biomed Pharmacother 146:112483. https://doi.org/10.1016/j.biopha.2021.112483
Le Poole IC, Mutis T, van den Wijngaard RM, Westerhof W, Ottenhoff T, de Vries RR, Das PK (1993) A novel, antigen-presenting function of melanocytes and its possible relationship to hypopigmentary disorders. J Immunol 151:7284–7292
Lu C, Kerbel RS (1993) Interleukin-6 undergoes transition from paracrine growth inhibitor to autocrine stimulator during human melanoma progression. J Cell Biol 120:1281–1288. https://doi.org/10.1083/jcb.120.5.1281
Lunkes GI, Lunkes D, Stefanello F, Morsch A, Morsch VM, Mazzanti CM, Schetinger MRC (2003) Enzymes that hydrolyze adenine nucleotides in diabetes and associated pathologies. Thromb Res 109:189–194. https://doi.org/10.1016/S0049-3848(03)00178-6
Luo C, Zou L, Sun H, Peng J, Gao C, Bao L, Ji R, Jin Y, Sun S (2020) A Review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front Pharmacol 11:153. https://doi.org/10.3389/fphar.2020.00153
Maldonado PA, Negrini LA, Ethur JDS, Oliveira L, Corrêa MDC, Becker LV, Zanin RF, Morsch VM, Schetinger MRC (2010) Nucleotide degrading enzymes in platelets from uterine cervical neoplasia patients treated with conization or radiotherapy. Biomed Pharmacother 64:499–504. https://doi.org/10.1016/j.biopha.2010.02.007
Manica A, Da Silva AM, Cardoso AM, Moreno M, Leal DB, Dutra Da Silva A, Schetinger MRC, Morsch VMM, Bagatini MD (2018) High levels of extracellular ATP lead to chronic inflammatory response in melanoma patients. J Cell Biochem 119:3980–3988. https://doi.org/10.1002/jcb.26551
Markovic SN, Erickson LA, Rao RD, McWilliams RR, Kottschade LA, Creagan ET, Weenig RH, Hand JL, Pittelkow MR, Pockaj BA, Bardia A, Vachon CM, Schild SE, Laman SD, Maples WJ, Pulido JS, Cameron JD (2007) Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc 82:364–380. https://doi.org/10.4065/82.3.364
Messeha SS, Zarmouh NO, Asiri A, Soliman KFA (2020) Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur J Pharmacol 885:173419. https://doi.org/10.1016/j.ejphar.2020.173419
Michielin O, Van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U (2019) Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1884–1901. https://doi.org/10.1093/annonc/mdz411
Milac, A.L., Negroiu, G., 2018. The Multiple Roles of Tyrosinase-Related Protein-2/L- Dopachrome Tautomerase in Melanoma: Biomarker, Therapeutic Target, and Molecular Driver in Tumor Progression. In: Blumenberg M (ed), Human Skin Cancers - Pathways, Mechanisms, Targets and Treatments. InTech. https://doi.org/10.5772/intechopen.70513
Monteiro I, Vigano S, Faouzi M, Treilleux I, Michielin O, Ménétrier-Caux C, Caux C, Romero P, de Leval L (2018) CD73 expression and clinical significance in human metastatic melanoma. Oncotarget 9:26659–26669. https://doi.org/10.18632/oncotarget.25426
Montinaro A, Iannone R, Pinto A, Morello S (2013) Adenosine receptors as potential targets in melanoma. Pharmacol Res 76:34–40. https://doi.org/10.1016/j.phrs.2013.07.002
Nunes S, Madureira R, Campos D, Sarmento B, Gomes AM, Pintado M, Reis F (2015) Therapeutic and nutraceutical potential of rosmarinic acid - cytoprotective properties and pharmacokinetic profile. Crit Rev Food Sci Nutr 00–00. https://doi.org/10.1080/10408398.2015.1006768
Oğuz A, Böyük A, Ekinci A, Alabalik U, Türkoğlu A, Tuncer MC, Ekingen A, Deveci E, Gültürk B, Aday U (2020) Investigation of antioxidant effects of rosmarinic acid on liver, lung and kidney in rats: a biochemical and histopathological study. Folia Morphol 79:288–295. https://doi.org/10.5603/FM.a2019.0087
Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MKK, Huang X, Caldwell S, Liu K, Smith P, Chen J-F, Jackson EK, Apasov S, Abrams S, Sitkovsky M (2006) A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA 103:13132–13137. https://doi.org/10.1073/pnas.0605251103
Ouyang W, O’Garra A (2019) IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity 50:871–891. https://doi.org/10.1016/j.immuni.2019.03.020
Paddock LE, Lu SE, Bandera EV, Rhoads GG, Fine J, Paine S, Barnhill R, Berwick M (2016) Skin self-examination and long-term melanoma survival. Melanoma Res 26:401–408. https://doi.org/10.1097/CMR.0000000000000255
Pilla C, Emanuelli T, Frassetto SS, Battastini AMO, Dias RD, Sarkis JJF (1996) ATP diphosphohydrolase activity (apyrase, EC 3.6.1.5) in human blood platelets. Platelets 7:225–230. https://doi.org/10.3109/09537109609023582
Roy P, Saikia B (2016) Cancer and cure: A critical analysis. Indian J Cancer 53:441. https://doi.org/10.4103/0019-509X.200658
Sadej R, Skladanowski AC (2012) Dual, enzymatic and non-enzymatic, function of ecto-5’-nucleotidase (eN, CD73) in migration and invasion of A375 melanoma cells. Acta Biochim Pol 59:647–652
Sun C, Wang B, Hao S (2022) Adenosine-A2A Receptor Pathway in Cancer Immunotherapy. Front Immunol 13:837230. https://doi.org/10.3389/fimmu.2022.837230
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb Perspect Biol 6:a016295–a016295. https://doi.org/10.1101/cshperspect.a016295
Venza I, Venza M, Visalli M, Lentini G, Teti D, d’Alcontres FS (2021) ROS as Regulators of cellular processes in melanoma. Oxid Med Cell Longev 2021:1–19. https://doi.org/10.1155/2021/1208690
Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, Grob J-J, Butler MO, Middleton MR, Maio M, Atkinson V, Queirolo P, Gonzalez R, Kudchadkar RR, Smylie M, Meyer N, Mortier L, Atkins MB, Long GV, Bhatia S, Lebbé C, Rutkowski P, Yokota K, Yamazaki N, Kim TM, de Pril V, Sabater J, Qureshi A, Larkin J, Ascierto PA (2017) Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377:1824–1835. https://doi.org/10.1056/NEJMoa1709030
Willingham SB, Hotson AN, Miller RA (2020) Targeting the A2AR in cancer; early lessons from the clinic. Curr Opin Pharmacol 53:126–133. https://doi.org/10.1016/j.coph.2020.08.003
Xia C, Yin S, To KKW, Fu L (2023) CD39/CD73/A2AR pathway and cancer immunotherapy. Mol Cancer 22:44. https://doi.org/10.1186/s12943-023-01733-x
Yu F, Zhu C, Xie Q, Wang Y (2020) Adenosine A 2A receptor antagonists for cancer immunotherapy: miniperspective. J Med Chem 63:12196–12212. https://doi.org/10.1021/acs.jmedchem.0c00237
Zanini D, Manfredi LH, Pelinson LP, Pimentel VC, Cardoso AM, Carmo Araújo Gonçalves V do, Santos CB dos, Gutierres JM, Morsch VM, Leal DBR, Schetinger MRC (2019) ADA activity is decreased in lymphocytes from patients with advanced stage of lung cancer. Med Oncol 36, 78. https://doi.org/10.1007/s12032-019-1301-1
Zanini D, Schmatz R, Pimentel VC, Gutierres JM, Maldonado PA, Thomé GR, Cardoso AM, Stefanello N, Oliveira L, Chiesa J, Leal DBR, Morsch VM, Schetinger MRC (2012) Lung cancer alters the hydrolysis of nucleotides and nucleosides in platelets. Biomed Pharmacother 66:40–45. https://doi.org/10.1016/j.biopha.2011.09.003
Acknowledgements
We thank the Federal University of Fronteira Sul (GBS and MDB) and the State University of Santa Catarina (GBS and APK).
Funding
This work is funded by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (MDB; Grant: 310606/2021–7), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) (MDB; Grant: 2021TR1543).
Author information
Authors and Affiliations
Contributions
Conceptualization: GBS. Methodology: GBS, DM, PD, RAN, JVC, FM, APS and APK. Investigation: GBS and DM. Visualization: MDB, APK, MM. Funding acquisition: MDB. Project administration: GBS and DM. Supervision: MDB. Writing – original draft: GBS. Writing – review and editing: GBS. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, G.B., Manica, D., Dallagnol, P. et al. Rosmarinic acid modulates purinergic signaling and induces apoptosis in melanoma cells. Purinergic Signalling (2024). https://doi.org/10.1007/s11302-024-10040-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11302-024-10040-z