Abstract
Serratia marcescens is an opportunistic human pathogen causing nosocomial infections and displays expanded resistance towards the conventional antibiotics. In S. marcescens, quorum sensing (QS) mechanism coordinates the population-dependent behaviors and regulates the virulence factors production. Photodynamic inactivation (PDI) is a promising alternative for the treatment of infections caused by drug resistant bacteria. Although PDI should be applied at lethal doses, it is possible that during PDI treatment, pathogens encounter sub-lethal doses of PDI (sPDI). sPDI cannot kill microorganisms, but it can considerably influence the microbial virulence. So, in this study, the effect of methylene blue (MB)-mediated PDI on QS-mediated virulence factor production and biofilm formation of S. marcescens at lethal and sub-lethal doses was evaluated. The biofilm formation and virulence factor production of S. marcescens ATCC 13,880 and S. marcescens Sm2 were assessed before and after PDI treatment. Besides, the effect of lethal and sub-lethal PDI on expression of bsmA and bsmB (Biofilm maturation), fimA and fimC (Major fimbrial protein), flhD (Regulator of flagellar mediated swarming and swimming motility) and swrR (AHL-dependent regulator) genes were evaluated by quantitative real time polymerase chain reaction. Lethal and sub-lethal PDI resulted in a significant decrease in biofilm formation, swimming/swarming motility, and pigment and hemolysin production ability of S. marcescens strains. bsmA, bsmB, flhD and swrR genes were down-regulated after PDI treatments. In conclusion, QS-mediated virulence factor production and biofilm formation ability of the two studied S. marcescens strains decreased after both lethal and sub-lethal PDI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serratia marcescens is a gram-negative bacteria, responsible for a growing number of nosocomial infections such as urinary tract, bloodstream and central nervous system infections, septicemia and surgical wound infections especially in intensive care unit patients (Srinivasan et al. 2016). This organism forms biofilm on catheters or implants and causes persistent infections. S. marcescens presents different virulence factors such as protease, nuclease, lipase, hemolysin, and swarming and swimming motility. It also produces a red pigment that is a member of the prodigiosins (Van Houdt et al. 2007).
Bacterial species depend on quorum sensing (QS) to regulate important cellular mechanisms that are essential for survival and adaptation to their changing environments (Hawver et al. 2016). In QS system, bacteria coordinate the expression of certain genes in response to the presence of signal molecules called auto-inducers (Defoirdt 2018). Most gram-negative bacteria employ autoinducer-1N-acyl-homoserine lactones (AHLs) as signaling molecules. When the cell density increases, the extracellular concentrations of signaling molecules reach a certain threshold that allows the signaling molecule to be sensed and coordinates bacteria response to environmental stress (Van Houdt et al. 2007). QS controls virulence gene expression in S. marcescens and formation of biofilm during infection (Ramanathan et al. 2018). Some recent studies aimed to inhibit virulence factors of S. marcescens via interfering QS (Devi et al. 2018; Padmavathi et al. 2014; Srinivasan et al. 2016).
The multi-drug-resistant nature of S. marcescens confines therapeutic options, and prolongs the treatment. Thus, there is a serious demand for new therapeutic approaches that are efficient against planktonic as well as biofilm cells. The effect of photodynamic inactivation (PDI) on this opportunistic pathogen has been reported (Parente et al. 2016). PDI is a non-thermal photochemical reaction that requires the simultaneous presence of visible light, oxygen and a dye known as photosensitizer (PS). The outcome of the reaction between PS and visible light, in the presence of molecular oxygen, is reactive oxygen species (ROS) such as singlet oxygen, hydroxyl radicals, superoxide anions, and hydrogen peroxide (Alves et al. 2014).
As a wide range of microbial targets are simultaneously affected by PDI, preventing the development of resistance and inactivation of a broad-spectrum of microorganisms, independently of their antimicrobial resistance profiles, will be achieved by this method (Bartolomeu et al. 2016). PDI has multiple cellular targets, since the PS-generated ROS can non-specifically attack various molecules. Therefore, PDI appears to be encouraging and potentially applicable in very diverse contexts where PS and light can be delivered (Hu et al. 2018). Nowadays, in addition to topical infections such as wound, the internal parts of the body like urinary tract infections are targeted by PDI in animal models (Huang et al. 2018) and some bacterial strains such as S. marcescens have become candidates for inactivation by this method.
PDI should be generally applied at lethal doses to kill bacteria; however, it is likely that during the treatment of human hosts, any microorganism viable at the site of infection would be exposed to sub-lethal doses of PDI (sPDI) (Kashef and Hamblin 2017). In this study, we aimed to determine whether lethal and sub-lethal PDI using methylene blue (MB) would affect quorum sensing system and consequently, virulence factors production and biofilm formation ability of survived cells in S. marcescens. Thus, after PDI treatments, the expression of genes necessary for biofilm formation (bsmA and bsmB), attachment (fimA and fimC), motility (flhD) and QS regulatory (swrR) were evaluated.
Materials and methods
Bacterial strain and growth condition
Bacterial strains used in this study were S. marcescens ATCC 13,880 and a clinical isolate from a blood sample (Serratia sp. named Sm2). Clinical isolate was identified as S. marcescens Sm2 through 16S rRNA gene sequences with the GenBank accession number of MK371794. The two strains were maintained in Luria–Bertani (LB) broth at 37 °C and log phase cultures with an optical density (OD) of ~ 0.8 to 1 were used as an inoculum for all assays.
PS and light source
MB (Sigma-Aldrich) was used as the photosensitizing agent. MB stock solution (600 µM) was prepared in phosphate-buffered saline (PBS, pH 7.2). After sterilization with 0.22 nm filter, the stock solution was stored at 4 °C in the dark no more than 2 weeks prior to use. Stock solution was further diluted in PBS to obtain the desired concentrations. The light source used in this study was a light-emitting diode (LED) (Heguang, China) with an emission at 660 nm. The total output power provided by the device was 8 mW.
Determination of lethal and sub-lethal doses of PDI
To measure the combined effect of MB and light, 300 µl bacterial suspensions (1–2 × 108 CFU/ml) were placed in a 96-well microplate, incubated with MB at final concentrations of 25–100 µM in the dark and at room temperature for 30 min. Treated cells were exposed to light emitted diode lamp with power of 5–15 J/cm2 (660 nm). After the illumination, 100 µl of each cell suspension was spread on nutrient agar plates in tenfold serial dilutions. Colonies were counted after incubation for 24 h at 37 °C. All experiments were repeated three times in triplicate. Controls included bacterial suspensions incubated with 0.9% saline in the dark (untreated), bacterial suspensions incubated with MB (25–100 µM) in the dark (dark toxicity), and bacterial suspensions subjected to illumination (5–15 J/cm2) in the absence of MB (light alone).
Biofilm formation quantification assay
The survivors of photosensitization process were diluted 1:50 in tryptic soya broth (TSB) supplemented with 0.2% glucose in 96-well microplate and incubated for 24 h at 37 °C. After incubation, the planktonic cells were removed by rinsing the wells twice with PBS. The surface-adhered cells were stained with 200 µl of 0.4% crystal violet (CV) solution for 15 min. The wells were washed with sterile distilled water to remove excess stain and de-stained with 200 µl of 30% glacial acetic acid for 20 min. Absorbance of the solubilized dye was determined at OD490 nm (Sharma et al. 2008).
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) images were taken to evaluate the effect of PDI treatments on S. marcescens biofilms. Samples were prepared as described by Gowrishankar et al. (Gowrishankar et al. 2014) with minor modifications. Biofilms formed on the glass slides by PDI-treated and untreated cells were fixed with PBS solution (1 M) containing 2.5% glutaraldehyde for 2 h. Then, the glass slides were washed in distilled water and dehydrated using increasing concentrations of ethanol (35%, 50%, 70%, 90% and 100%) for 10 min. The biofilm samples were gold sputtered after critical point drying and examined under a SEM analysis.
Prodigiosin quantification assay
Prodigiosin, a tripyrrole red pigment, was extracted from PDI-treated and untreated cells according to the method of Sethupathy et al. (Sethupathy et al. 2016). Tubes containing LB broth medium were inoculated with 100 μl [5% (v/v)] of bacterial cells [survived cells from PDI treatments (lethal and sub-lethal doses)] and incubated at 30 °C for 24 h. 1.5 ml of PDI-treated and untreated cells was separately placed into sterile micro-centrifuge tubes and centrifuged at 10,000 rpm for 10 min. 1 ml of acidified ethanol (4% [v/v] of 1 M HCl in 96 ml absolute ethanol) was added to the pellet, and vortexed vigorously followed by centrifugation at 10,000 rpm for 10 min. The absorbance of the extracted prodigiosin in acidified ethanol was measured at 534 nm.
Lipolytic activity
LB broth medium were inoculated with 100 μl [5% (v/v)] of bacterial cells (survived cells from PDI treatments) and incubated for 24 h at 37 °C. Lipolytic activity of treated and untreated cells was quantified using p-nitro phenyl palmitate (pNPP) as the substrate. A buffered substrate was prepared as follows: 9 volumes of PBS (50 mM) were added to 1 volume of pNPP (0.5 M) in absolute ethanol and then, the substrate was emulsified by Triton X-100 (0.6% V/V). The reaction mixture contained 500 µl of substrate and 500 µl of the bacterial supernatant was incubated for 30 min at 37 °C. After incubation, the reaction was stopped by adding 1 mL of acetone–ethanol (1:1 v/v) and the mixture was centrifuged at 10,000 rpm for 10 min. Then, the absorbance of the clear solution was measured at 410 nm (Imanparast et al. 2018).
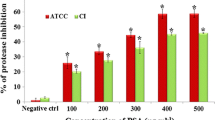
Proteolytic activity
The effect of PDI/sPDI on proteolytic activity of strains was conducted as described by Bakkiyaraj et al. with minor modification (Bakkiyaraj et al. 2012). Tubes of LB broth medium were inoculated with 100 μl [5% (v/v)] of bacterial cells (survived cells from PDI treatments) and incubated at 37 °C for 24 h. 300 µl of each treated and untreated groups was incubated in 3 ml of LB medium. After 24 h incubation at 37 °C, cultures were centrifuged (11,000 for 20 min at 4 °C) and then cell-free supernatants were collected to quantify the protease production using azocasein as substrate. To 500 μl of each supernatant, 500 μl of buffered substrate [0.3% azocasein (Sigma, USA) in 1 M potassium phosphate buffer (pH 7.0)] was added and incubated at 37 °C for 1 h. After incubation, 500 μl of trichloro acetic acid (10%) was added to each tube to terminate the reaction by incubation at − 20 °C for 20 min. The mixture was centrifuged at 12,000 rpm for 15 min. The supernatant (200 μl) was separated and 100 μl of 1 M NaOH was added and read at OD440 nm.
Swimming and swarming assays
For swimming assay, the PDI/sPDI treated and untreated cultures were stab inoculated at the center of the swimming agar medium consisting of 1% peptone, 0.5% NaCl and 0.3% agar. For swarming assays, 5 μl of culture was inoculated at the center of the swarming agar medium consisting of the same components as swimming agar medium. The plates were then incubated at 30 °C in upright position. The reduction in swimming and swarming migration was recorded by measuring the swim and swarm zones of the bacterial cells after 24 h (Salini and Pandian 2015).
Hemolysin assay
Hemolytic activity of treated and untreated cultures was measured. 100 μl [5% (v/v)] of bacterial cells [survived cells from PDI treatments] were inoculated in LB broth medium and incubated at 37 °C for 24 h. Each bacterial group (treated and untreated) in LB medium, cultures were centrifuged at 11,000 rpm for 20 min at 4 °C. To 100 μl of supernatant, 900 μl of sheep blood suspension (2% sheep blood erythrocytes in PBS; pH 7.4) was added and incubated at 37 °C for 1 h. The mixture was centrifuged at 3,000 rpm for 10 min and the absorbance of supernatant was measured at 530 nm (Devi et al. 2018).
Quantification of extracellular polysaccharides (EPS)
Survived cells of PDI/sPDI treatment were inoculated in 96-well flat-bottomed sterile polystyrene microplate to form biofilm as described previously. After incubation at 37 °C for 24 h, wells were washed with 200 μl of PBS (pH 7.4) to remove the planktonic cells. To this, 40 μl of 0.9% NaCl, 40 μl of 5% phenol and 200 μl of concentrated sulfuric acid (w/v) were added. The mixture was incubated in the dark for 1 h and the absorbance was measured at 490 nm (Dubois et al. 1956).
Total RNA isolation and quantitative real time PCR (qPCR) analysis
To assess the effect of PDI on the expression of QS regulated genes, the PDI/sPDI treated and untreated cells were harvested by centrifugation and the total RNA was extracted using RNX-PLUS reagent (SINACLONE, Iran). Extracted RNA was dissolved in 20 μl of 0.1% diethyl pyrocarbonate (DEPC) treated water. Total extracted RNA was treated by 1 U/μl of DNase I (RNase free, Thermo Scientific) for 15 min at 37 °C. The denaturation of the DNase was carried out by adding 1 μl of 50 mM EDTA. RNA samples were reverse transcribed into cDNA using cDNA reverse transcription kit (BIOFACT, South Korea) following the manufacturer's instructions. The qPCR reactions were performed with Power SYBR Green PCR Master Mix (BIOFACT, South Korea) in the real-time PCR system (Bio Molecular Systems, Australia). The expression pattern of QS regulated genes (fimA, fimC, flhD, SwrR, bsmA and bsmB) was normalized using rplU gene (50S Ribosomal gene, Housekeeping gene) as an internal control. All primers were designed by the software Primer 3, https://primer3.ut.ee/. Hairpin structure and primer dimerization were analyzed by OligoAnalyzer Tool. The length of the primers was from 18-mer to 21-mer and the expected PCR products range from 102 to 221 bp. The details of the gene-specific primers and the accession numbers of the genes used for the design are given in Table 1. The changes in gene expression were calculated as fold-change using the mathematical formula: 2−∆∆Ct, where the ∆∆Ct is the difference between the ∆Ct value of the treated group and the untreated group (control). The ∆Ct is the difference between the Ct value of the target gene and the normalization gene (rplU) (Soni et al. 2008).
Statistical analysis
All values were represented as means ± standard errors. Comparisons between means of groups were analyzed using one-way ANOVA and post hoc Tukey tests. p < 0.05 was considered statistically significant. Real time PCR data was analyzed for statistical significance using a pairwise fixed reallocation randomization test by the Relative Expression Software Tool v2.0.13 (REST 2009).
Results
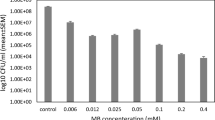
Lethal and sub-lethal doses of PDI
The use of MB (25–100 µM) and light (5–15 J/cm2) alone caused < 1 log reduction in the number of bacteria (data not shown). Figure 1 shows the effect of PDI mediated by different concentrations of MB (25–100 µM) and 15 J/cm2 light irradiation on viability of bacteria (log10 CFU/ml). The results showed that with increasing MB concentration from 25 to 100 µM, the viability of bacteria reduced by PDI treatment. Since the reduction of at least 3 log steps can be stated as a bactericide effect of a specific treatment (Boyce and Pittet 2002), MB at concentration of 25 and 50 µM and 15 J/cm2 light irradiation were used as sub-lethal and lethal PDI parameters in the following tests, respectively.
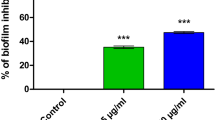
Biofilm formation quantification
As shown in Fig. 2, a significant decrease in biofilm biomass formation was observed in both strains after lethal and sub-lethal treatment (p < 0.05). Lethal dose showed 25.7% and 35.3% of biofilm inhibition in S. marcescens ATCC 13,880 and S. marcescens Sm2, respectively. Sub-lethal dose caused significant reduction (29%) only in S. marcescens Sm2 strain (p = 0.041).
Scanning electron micrographs
As shown in Fig. 3a and d, SEM images of untreated groups clearly showed a highly structured biofilm architecture, whereas both S. marcescens ATCC 13,880 and S. marcescens Sm2 showed extensive disintegration of biofilms after lethal PDI treatment (Fig. 3c, f, respectively). sPDI treatment only attenuated the biofilm formation of S. marcescens Sm2 (Fig. 3e) and did not cause equal reduction in S. marcescens ATCC 13,880 (Fig. 3b).
Scanning electron micrographs of S. marcescens biofilms. a S. marcescens ATCC 13,880 untreated group, b S. marcescens ATCC 13,880 biofilm after sub-lethal treatment, c S. marcescens ATCC 13,880 biofilm after lethal treatment, d S. marcescens Sm2 untreated group, e S. marcescens Sm2 biofilm after sub-lethal treatment, f S. marcescens Sm2 biofilm after lethal treatment
Prodigiosin production
Figure 4 shows that after sPDI/PDI treatment, reduction in prodigiosin production was observed in both S. marcescens ATCC 13,880 and S. marcescens Sm2. A maximum of 35.3% and 36.7% inhibition in prodigiosin production was occurred by lethal PDI in S. marcescens ATCC 13,880 and S. marcescens Sm2, respectively. Sub-lethal dose resulted in less reduction of pigment production of both S. marcescens ATCC 13,880 (25.9%) and S. marcescens Sm2 (34.7%) compared to lethal PDI.
Quantitative analysis of prodigiosin inhibition in S. marcescens ATCC 13,880 and S. marcescens Sm2 by lethal and sub-lethal PDI. Data are represented as percentage of prodigiosin inhibition. Mean values of triplicate independent experiments and SD are shown. *Significant at p < 0.05, **significant at p < 0.005 and ***significant at p < 0.0005
Lipolytic and proteolytic activity
The lipolytic and proteolytic activity production of both strains remained unchanged after lethal and sub-lethal PDI (data not shown).
Swimming and swarming motility
As shown in Fig. 5, control group of S. marcescens ATCC 13,880 exhibited swimming and swarming on a soft agar plate by occupying almost the whole plate, while the lethal and sub-lethal PDI treated groups showed decreased swimming and swarming motility. Swimming and swarming ability of S. marcescens Sm2 strain was also inhibited by both lethal and sub-lethal PDI.
Effect of lethal and sub-lethal PDI on S. marcescens ATCC 13,880 and S. marcescens Sm2 swimming and swarming motility; (a swimming of S. marcescens ATCC13880) (b swimming of S. marcescens Sm2) (c swarming S. marcescens ATCC 13,880) (d swarming of S. marcescens Sm2), from left to right: untreated cells; sub-lethal treated cells; lethal-treated cells
Hemolytic activity
Secreted hemolysin of S. marcescens ATCC 13,880 was significantly inhibited by lethal (57.6%) and sub-lethal PDI (53.6%) treatment (p < 0.05). Ability of hemolysin production of S. marcescens Sm2 was low, which was inhibited by lethal (33.5%) and sub-lethal (38.2%) treatment (Fig. 6).
Extracellular polysaccharide quantification
After lethal PDI treatment, EPS production significantly reduced in S. marcescens ATCC 13,880 (44.1%) and S. marcescens Sm2 (29.5%). The sub-lethal PDI also decreased the EPS formation in S. marcescens ATCC 13,880 and S. marcescens Sm2 about 48.7% and 42.6%, respectively (Fig. 7). The results of lethal and sub-lethal PDI on EPS production in both strains are not significantly different compared to each other (p > 0.05).
Quantitative real time PCR analysis
The qPCR analysis was carried out to assess the fold changes in transcriptional level of QS-mediated genes in treated samples compared to their controls. Figure 8 shows fold changes in gene expression of the studied microorganisms after PDI/sPDI compared to their untreated control. PDI/sPDI treatment led to the down-regulation of the expression of QS-controlled biofilm formation genes (bsmA and bsmB), fimbrial genes (fimA and fimC), flagellar gene (flhD) and QS gene (swrR) in S. marcescens ATCC 13,880 and S. marcescens Sm2.
Discussion
Virulence features of S. marcescens for causing infections are extracellular enzymes such as protease, gelatinase, lipase and hemolysin. Besides, the ability to swarm and swim has been shown to be pathogenic factors of these bacteria (Mahlen 2011). Quorum sensing mechanism plays a vital role in regulating the extensive ranges of virulence factors and biofilm formation in S. marcescens (Srinivasan et al. 2017). As PDI is a promising approach to current antibiotics, it is therefore important to understand how it may affect QS-regulated virulence factors.
The results showed that lethal dose of PDI could reduce the biofilm formation ability of the two studied S. marcescens strains. Reduction in biofilm formation was observed only for S. marcescens Sm2 after sub-lethal treatment. As the bacterial cells in biofilms have enhanced resistance to different antimicrobial agents and are better adapted to tolerate environmental stresses (Stanley and Lazazzera 2004), it is important that survived cells of PDI treatment do not augment their biofilm formation ability. The results of this study are consistent with the findings of recent study, which reported significant decrease in biofilm formation ability of Pseudomonas aeruginosa after MB-sPDI (Hendiani et al. 2019b).
The two signal molecules, N-butanoyl homoserine lactone and N-hexanoyl homoserine lactone of the QS system regulate the pigment formation in S. marcescens. Therefore, any interference with these QS systems leads to a reduction in prodigiosin production (Packiavathy et al. 2014). In this study, the pigment production in two S. marcescens strains decreased significantly, after both lethal and sub-lethal PDI compared to their untreated controls. Since there is a direct relationship between the pigment production and QS system, it can be assumed that the QS system was repressed by oxidative stress induced by PDI in survived cells. Furthermore, other QS-controlled traits like hemolysin production, swimming and swarming motility decreased after both lethal and sub-lethal PDI.
The lipolytic and proteolytic activity of both S. marcescens ATCC 13,880 and S. marcescens Sm2 did not change after the lethal and sub-lethal PDI treatment. Since in our study, detection of virulence factors activity was performed in surviving cells after a period of recovery, the effect of PDI treatment on the lipase and protease production may have been temporary. However, the lethal and sub-lethal PDI effect on other virulence features were persistent and continued among next generations of bacteria.
In order to determine the effect of lethal and sub-lethal PDI on treated cells at the molecular level, a real-time PCR analysis was done. The QS-controlled genes, bsmA and bsmB, regulate biofilm formation in S. marcescens. The bsmA and bsmB mutated strains were identified as poor biofilm formers and they also failed to produce EPS (Labbate et al. 2004). Upon both lethal and sub-lethal PDI, the expression of bsmA and bsmB were down regulated in S. marcescens Sm2. These results were compatible with the phenotypic assays. PDI/sPDI treatments led to considerable down-regulation in bsmA and bsmB genes (p < 0.05) in S. marcescens ATCC 13,880, while according to the CV test result, sub-lethal dose did not show substantial reduction in biofilm production of this strain (p = 0.37).
The flhD gene regulates the flagellar mediated swarming and swimming motilities in S. marcescens (Liu et al. 2000). After lethal and sub-lethal PDI treatments, the flhD transcript level decreased significantly in both strains, which supports the decline of swarming and swimming motility on agar plates.
Labbate et al. have reported the involvement of fimA and fimC genes, which encode the main fimbrial proteins, in the attachment of S. marcescens cells to surfaces and to form biofilms (Labbate et al. 2007). Both fimA and fimC genes showed insignificant reduction (p > 0.05) after PDI/sPDI treatment in S. marcescens ATCC 13,880, however, they showed considerable decline in S. marcescens Sm2.
The SwrI/SwrR QS system regulates swarming motility, biofilm formation, production of serrawettin, protease, and S-layer protein in S. marcescens (Mahlen 2011). The swrI gene encodes an AHL synthase that directs the synthesis of AHL autoinducers. Downstream of swrI, an open reading frame codes for a polypeptide (SwrR) with major similarity to members of the LuxR family of AHL-dependent regulators (Eberl et al. 1999). In this study, the swrR gene was substantially down regulated in both strains after treatments. Tan et al. study showed that 5-aminolevulinic acid (ALA)-PDI with lethal dose inhibited pyocyanin and elastase secretion in P. aeruginosa. Besides, the mRNA expression of QS-related genes (lasI, lasR, rhlI, and rhlR) and virulence factor-related genes (lasB and phzH) in P. aeruginosa reduced significantly after ALA-PDI (Tan et al. 2018). Hendiani et al. reported that oxidative stress induced by sPDI led to the down-regulation of the expression of QS genes (lasI, lasR, rhlI and rhlR) and rhamnolipid gene (rhlA) in P. aeruginosa (Hendiani et al. 2019a).
The entire results obtained by real-time PCR analysis are consistent with the findings of other studies, which used various compounds (silver nanoparticles, marine sponge extracts, Bacillus spp. supernatant, cyclodextrins) as anti-QS agents of S. marcescens (Annapoorani et al. 2012; Khadar et al. 2012; Morohoshi et al. 2013; Ravindran et al. 2018). Quorum sensing inhibition has been identified as an effective strategy, which combats bacterial virulence instead of targeting bacterial survival, and reduces the risk of evolution of drug resistant bacteria. According to the present study, exposure of S. marcescens cells to sPDI can function as an anti-QS agent, which results in an attenuation in QS system leading to the reduction of virulence and biofilm formation concurrently. However, there is no guarantee that other bacteria respond to sPDI-induced oxidative stress in the same manner. The lethal PDI can decrease the number of bacterial cells in the site of infection and also affect the remained bacteria in a way that they reduce their virulence factor production. Therefore, it is important to investigate PDI/sPDI effect on different bacterial species with other PSs and light sources in several doses.
Conclusion
In this study, we showed that both lethal and sub-lethal PDI resulted in decreasing virulence factors production and biofilm formation ability of the two studied S. marcescens strains. As the molecular mechanisms of signaling pathways activated by PDI are still unclear, more studies can help to find the keys to clarify bacterial responses to this promising antimicrobial strategy.
References
Alves E, Faustino MA, Neves MG, Cunha A, Tome J, Almeida A (2014) An insight on bacterial cellular targets of photodynamic inactivation. Future Med Chem 6:141–164
Annapoorani A, Jabbar AKKA, Musthafa SKS, Pandian SK, Ravi AV (2012) Inhibition of quorum sensing mediated virulence factors production in urinary pathogen Serratia marcescens PS1 by marine sponges. Indian J Microbiol 52:160–166
Bakkiyaraj D, Sivasankar C, Pandian SK (2012) Inhibition of quorum sensing regulated biofilm formation in Serratia marcescens causing nosocomial infections. Bioorg Med Chem Lett 22:3089–3094
Bartolomeu M, Rocha S, Cunha Â, Neves M, Faustino MA, Almeida A (2016) Effect of photodynamic therapy on the virulence factors of Staphylococcus aureus. Front Microbiol 7:267
Boyce JM, Pittet D (2002) Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force Infection Control & Hospital. Epidemiology 23:S3–S40
Defoirdt T (2018) Quorum-sensing systems as targets for antivirulence therapy. Trends Microbiol 26:313–328
Devi KR, Srinivasan S, Ravi AV (2018) Inhibition of quorum sensing-mediated virulence in Serratia marcescens by Bacillus subtilis R-18. Microb Pathog 120:166–175
Dubois M, Gilles KA, Hamilton JK, Pt R, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Eberl L, Molin S, Givskov M (1999) Surface motility of Serratia liquefaciens MG1. J Bacteriol 181:1703–1712
Gowrishankar S, Poornima B, Pandian SK (2014) Inhibitory efficacy of cyclo (l-leucyl-l-prolyl) from mangrove rhizosphere bacterium-Bacillus amyloliquefaciens (MMS-50) toward cariogenic properties of Streptococcus mutans. Res Microbiol 165:278–289
Hawver LA, Jung SA, Ng W-L (2016) Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol Rev 40:738–752
Hendiani S, Pornour M, Kashef N (2019a) Quorum-sensing-regulated virulence factors in Pseudomonas aeruginosa are affected by sub-lethal photodynamic inactivation. Photodiagn Photodyn Ther 26:8–12
Hendiani S, Pornour M, Kashef N (2019b) Sub-lethal antimicrobial photodynamic inactivation: an in vitro study on quorum sensing-controlled gene expression of Pseudomonas aeruginosa biofilm formation. Lasers Med Sci 34:1159–1165
Hu X, Huang Y-Y, Wang Y, Wang X, Hamblin MR (2018) Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front Microbiol 9:1299
Huang Y-Y, Wintner A, Seed PC, Brauns T, Gelfand JA, Hamblin MR (2018) Antimicrobial photodynamic therapy mediated by methylene blue and potassium iodide to treat urinary tract infection in a female rat model. Sci Rep 8:7257
Imanparast S, Hamedi J, Faramarzi MA (2018) Enzymatic esterification of acylglycerols rich in omega-3 from flaxseed oil by an immobilized solvent-tolerant lipase from Actinomadura sediminis UTMC 2870 isolated from oil-contaminated soil. Food Chem 245:934–942
Kashef N, Hamblin MR (2017) Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist Updates 31:31–42
Khadar SM, Shunmugiah KP, Arumugam VR (2012) Inhibition of quorum-sensing-dependent phenotypic expression in Serratia marcescens by marine sediment Bacillus spp. SS4. Ann Microbiol 62:443–447
Labbate M, Queck SY, Koh KS, Rice SA, Givskov M, Kjelleberg S (2004) Quorum sensing-controlled biofilm development in Serratia liquefaciens MG1. J Bacteriol 186:692–698
Labbate M et al (2007) Quorum-sensing regulation of adhesion in Serratia marcescens MG1 is surface dependent. J Bacteriol 189:2702–2711
Liu J-H et al (2000) Role offlhDC in the expression of the nuclease genenucA, cell division and flagellar synthesis inserratia marcescens. J Biomed Sci 7:475–483
Mahlen SD (2011) Serratia infections: from military experiments to current practice. Clin Microbiol Rev 24:755–791
Morohoshi T, Tokita K, Ito S, Saito Y, Maeda S, Kato N, Ikeda T (2013) Inhibition of quorum sensing in gram-negative bacteria by alkylamine-modified cyclodextrins. J Biosci Bioeng 116:175–179
Packiavathy IASV, Priya S, Pandian SK, Ravi AV (2014) Inhibition of biofilm development of uropathogens by curcumin: an anti-quorum sensing agent from Curcuma longa. Food Chem 148:453–460
Padmavathi AR, Abinaya B, Pandian SK (2014) Phenol, 2,4-bis (1,1-dimethylethyl) of marine bacterial origin inhibits quorum sensing mediated biofilm formation in the uropathogen Serratia marcescens. Biofouling 30:1111–1122
Parente TMAL, de Lima RE, dos Santos VCV, Barbosa FCB, Zanin ICJ (2016) Serratia marcescens resistance profile and its susceptibility to photodynamic antimicrobial chemotherapy. Photodiagn Photodyn Ther 14:185–190
Ramanathan S, Ravindran D, Arunachalam K, Arumugam VR (2018) Inhibition of quorum sensing-dependent biofilm and virulence genes expression in environmental pathogen Serratia marcescens by petroselinic acid. Antonie Van Leeuwenhoek 111:501–515
Ravindran D, Ramanathan S, Arunachalam K, Jeyaraj GP, Shunmugiah KP, Arumugam VR (2018) Phytosynthesized silver nanoparticles as antiquorum sensing and antibiofilm agent against the nosocomial pathogen Serratia marcescens: an in vitro study. J Appl Microbiol 124:1425–1440
Salini R, Pandian SK (2015) Interference of quorum sensing in urinary pathogen Serratia marcescens by Anethum graveolens. Pathog Dis 73:38
Sethupathy S, Shanmuganathan B, Kasi PD, Pandian SK (2016) Alpha-bisabolol from brown macroalga Padina gymnospora mitigates biofilm formation and quorum sensing controlled virulence factor production in Serratia marcescens. J Appl Phycol 28:1987–1996
Sharma M, Visai L, Bragheri F, Cristiani I, Gupta PK, Speziale P (2008) Toluidine blue-mediated photodynamic effects on staphylococcal biofilms. Antimicrob Agents Chemother 52:299–305
Soni K, Lu L, Jesudhasan P, Hume M, Pillai S (2008) Influence of autoinducer-2 (AI-2) and beef sample extracts on E. coli O157: H7 survival and gene expression of virulence genes yadK and hhA. J Food Sci 73:M135–M139
Srinivasan R, Devi KR, Kannappan A, Pandian SK, Ravi AV (2016) Piper betle and its bioactive metabolite phytol mitigates quorum sensing mediated virulence factors and biofilm of nosocomial pathogen Serratia marcescens in vitro. J Ethnopharmacol 193:592–603
Srinivasan R et al (2017) Exploring the anti-quorum sensing and antibiofilm efficacy of phytol against Serratia marcescens associated acute pyelonephritis infection in Wistar rats. Front Cell Infect Microbiol 7:498
Stanley NR, Lazazzera BA (2004) Environmental signals and regulatory pathways that influence biofilm formation. Mol Microbiol 52:917–924
Tan Y et al (2018) Effects of ALA-PDT on biofilm structure, virulence factor secretion, and QS in Pseudomonas aeruginosa. Photodiagn Photodyn Ther 24:88–94
Van Houdt R, Givskov M, Michiels CW (2007) Quorum sensing in Serratia. FEMS Microbiol Rev 31:407–424
Acknowledgements
This study was supported by the College of Science, University of Tehran, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors hereby declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fekrirad, Z., Kashef, N. & Arefian, E. Photodynamic inactivation diminishes quorum sensing-mediated virulence factor production and biofilm formation of Serratia marcescens. World J Microbiol Biotechnol 35, 191 (2019). https://doi.org/10.1007/s11274-019-2768-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2768-9