Abstract
Serratia marcescens is an emerging opportunistic bacterium that can cause healthcare-associated infections. The high rate of multidrug resistance and the ability to produce a set of virulence factors, by which it can produce infectious diseases makes it urgent to find an alternative approach to the treatment of such infections. Disarming of virulence by targeting of quorum sensing (QS) as the regulating mechanism of virulence is a promising approach that has no effect on bacterial growth that is considered a key factor in emergence of resistance. This study was designed to investigate the ability of sub-inhibitory concentrations (sub-MICs) of sotolon to attenuate virulence of a clinical isolate of S. marcescens. Sotolon at 25 and 50 μg/ml inhibited 35.2 and 47.5% of biofilm formation, respectively. The inhibition of swimming motility were 41.4 and 69.3%, while that of swarming motility were 77.6 and 86.8% at 25 and 50 µg/ml, respectively. Moreover, sotolon reduced prodigiosin production by 76.6 and 87.6% at concentrations of 25 and 50 µg/ml, respectively. Protease activity was reduced by 25 µg/ml of sotolon by 54.8% and was completely blocked at 50 µg/ml. The relative expression of genes regulating virulence factors decreased by 40% for fimA, 29% for fimC, 59% for flhC, 57% for flhD, 39% for bsmB, 37% for rssB, 49% for rsmA, 54% for pigP, and 62% for shlA gene in the presence of 50 µg/ml sotolon. In conclusion, sotolon is an anti-virulence agent that could be used for the treatment of S.marcescens hospital-acquired infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serratia marcescens is a facultative anaerobic motile Gram-negative bacillus that belongs to the family Enterobacteriaceae. It is of great importance due to the fact of being an opportunistic human pathogen that can cause a variety of healthcare-associated infections (Kida et al. 2007). The infections include central line associated blood stream infections, pneumonia, endocarditis, urinary tract infections and osteomyelitis (Van Houdt et al. 2007; Redondo-Bravo et al. 2019). Moreover, it is considered a food-borne pathogen because of its ability to colonize the gastrointestinal tract (Cristina et al. 2019). Furthermore, the common multidrug resistance nature of S. marcescens complicates the treatment of its infections (González-Juarbe et al. 2015).
Many virulence factors contribute to the ability of S. marcescens to cause infections such as several extracellular enzyme including proteases, nucleases, lipases and hemolysin, as well as their swimming and swarming motilities (Sethupathy et al. 2017). S. marcescens also produces a characteristic red pigment; prodigiosin (2-methyl-3- pentyl-6-methoxyprodigiosin) that exerts antibacterial, anticancer, antiprotozoal, and immunosuppressant activities (Yip et al. 2019).
Many Gram-negative pathogens employ a cell-to-cell communication system called quorum sensing (QS). QS is a cell density dependent regulatory system of gene expression by employing signaling molecules or autoinducers termed N-acyl-homoserine lactones (AHLs) (Boyle et al. 2015; Bucio-Cano et al. 2015; Busetti et al. 2015). The LuxI protein family is responsible for the synthesis of AHLs that bind to receptors of the LuxR protein family. Such binding regulates the expression of many genes responsible for bioluminescence, production of pigments or antibiotics and virulence factors secretion (Morohoshi et al. 2008; Abisado et al. 2018). QS system enables bacteria to behave in a community-like manner to perform many physiological functions, amongst which are the migration to more suitable environments, biofilm formation, virulence factors secretion, bacteriocin and antibiotic production, bioluminescence and pigment production (Skandamis and Nychas 2012; Martins et al. 2014).
QS is an attractive target for inhibition to control the virulence and pathogenesis of bacteria (Krzyżek 2019). QS inhibition can be achieved either by interfering with autoinducers synthesis, secretion or degradation, as well as by interfering with their recognition by proteins receptor such as those of the LuxR type homologs. As a result, the control of QS can be of great importance in control of infections (Jiang et al. 2019). The advantage of QS inhibition strategy is the lack of targeting the bacterial growth and imposing selective pressure on cells that leads to development of resistance (Asfour 2018). Thus, QS inhibition can be a good strategy for the treatment for S. marcescens infections that avoids the emergence of resistant strains as in the case with antibiotics.
Many natural compounds were used as QS inhibitors. One of the first used QS inhibitors were the halogenated furanones produced by the red alga Delisea pulchra. These compounds were found to inhibit quorum sensing in a number of bacteria (Zhang and Li 2015). In Serratia, halogenated furanones could block the swarming motility in S. liquefaciens MG1 strain (Rasmussen et al. 2000).
Sotolon (3-hydroxy-4,5-dimethyl-2(5H)-furanone) is a lactone compound that has an extremely powerful aroma. It bears the typical smell of fenugreek or curry when used at high concentrations, while it has maple syrup, caramel, or burnt sugar odor at lower concentrations. Sotolon is the major aroma and flavor component of fenugreek seed and lovage (Blank and Schieberle 1993).Sotolon was first isolated in 1975 from the herb fenugreek (Rijkens and Boelens 1975). Its name came from soto- ”raw sugar” in Japanese and -olon signifies that the molecule is an enol lactone because it was found to be responsible for the flavor of raw cane sugar. Sotolon can pass through the body relatively unchanged, and when sotolon-rich foods such as fenugreek are consumed by human, a maple syrup aroma can be imparted to one’s sweat and urine (Yukiko et al. 1980).
In this work, the anti-virulence activities of sotolon as a furanone compound was investigated against a clinical isolate of S. marcescens from a patient with nosocomial pneumonia.
Materials and methods
Bacterial strain
A clinical isolate of S. marcescens recovered from a patient with nosocomial pneumonia at the intensive care unit at Zagazig University Hospital by endotracheal aspiration and identified using the MALDI-TOFF apparatus at the Clinical Pathology Department, Faculty of Medicine, Zagazig University was used in this investigation (Abbas and Hegazy 2017).
Determination of minimum inhibitory concentration (MIC) of sotolon
The broth microdilution method was used to determine the minimum inhibitory concentration (MIC) of sotolon (CLSI 2012). S. marcescens was cultivated in Mueller–Hinton (MH) broth (Oxoid, Hampshire, UK) at 37 °C for 18 h. Then, this culture was diluted with MH broth to have a turbidity equivalent to 0.5 McFarland standard and, then diluted (1:100) to have a final cell density of approximately 106 CFU/ml. Different dilutions of sotolon (400, 200, 100, 50, 25, 12.5 µg/ml) were prepared in MH broth, then aliquots of 100 µl aliquots of these dilutions were transferred into the wells of 96-well microtiter plate. To the wells containing sotolon dilutions, aliquots of 100 µl of the prepared S. marcescens suspension were added. The microtiter plate was incubated at 37 °C and also at 28 °C for 20 h and MIC of sotolon was calculated by determination of the lowest concentration of sotolon that showed no visible growth.
Sub-inhibitory concentrations (sub-MICs) of sotolon (50 and 25 μg/ml corresponding to 1/4 and 1/8 MIC values, respectively) were used to assess the effect of sotolon on virulence of S. marcescens.
Biofilm formation assay
Biofilm was allowed to form in the presence and absence of sub-MICs of sotolon (Abraham et al. 2011). S. marcescens was grown in tryptone soya broth (TSB; Oxoid, Hampshire, UK) for 18 h at 28 °C and TSB was used to dilute the culture to have a suspension with optical density at 600 nm (OD600) of 0.4 measured using Biotek spectrofluorometer (Biotek, USA). Aliquots of 10 µl of this suspension were added to 1 ml of fresh TSB with sotolon and control TSB and 100 µl of TSB preparations were transferred into the wells of 96-well microtiter plate. The plate was incubated at 28 °C for 24 h and the planktonic cells were removed, then the wells were washed with distilled water and dried. The adherent cells were methanol-fixed for 20 min and stained with 1% crystal violet. After 20 min, the wells were washed and the attached stain was dissolved using glacial acetic acid (33%). The absorbance of treated and untreated samples was measured at 590 nm using Biotek spectrofluorometer and the percentage of biofilm inhibition was calculated using the following formula:
Swimming and swarming motilities assay
To detect the possible anti-swarming and anti-swimming activities of sotolon, sotolon-containing swimming Luria–Bertani (LB) agar (Lab M Limited, Lancashire, United Kingdom) plates (0.3% agar) and sotolon-containing swarming LB agar plates (0.5% agar) and control plates were prepared. Culture of S. marcescens in LB broth was prepared by culturing the bacteria at 28 °C for 18 h, from which 5 µl aliquots of were inoculated into the center of the swimming plates, and were point inoculated in the swarming plates. The plates were incubated at 28 °C for 20 h and both the swimming and swarming zones were measured (Matsuyama et al. 1992).
Prodigiosin production assay
Quantification of prodigiosin production by S. marcescens was performed in the presence and absence of sotolon. The bacterial strain was grown in LB broth for 16 h at 37 °C, diluted to have a suspension with OD600 equal to 0.4 as measured by Biotek Spectrofluorometer and then inoculated in 2 ml fresh LB broth with and without sotolon. LB broth tubes were incubated at 28 °C for 18 h and then centrifuged at 13,000 rpm for 10 min to collect the cell pellets. Prodogiosin was extracted by acidified ethanol (4 ml of 1 M HCl in 96 ml of ethanol). and the tubes were centrifuged again to separate the supernatants. The absorbance of the supernatants was measured at 534 nm using Biotek Spectrofluorometer and the degree of prodogiosin inhibition was determined. The inhibition percentage was calculated as % of prodigiosin inhibition = [absorbance of control – absorbance in presence of sotolon] x 100/absorbance of control (Gowrishankar et al. 2014).
Proteolytic activity assay
To assess the inhibition of proteolytic activity of the tested S. marcescens by sotolon, the skim milk agar method was used. Wells were made in skim milk LB agar plates (5% skim milk). S. marcescens was grown in LB broth for 16 h at 37 °C and then diluted to have a suspension with OD600 equal to 0.4 as measured by Biotek spectrofluorometer. The prepared suspension was used to inoculate fresh LB broth with and without sotolon that was incubated at 28 °C for 18 h. The suspensions were centrifuged at 10,000 rpm for 15 min to separate the supernatants. Aliquots of 100 µl of the supernatants of treated and untreated samples were transferred to the wells and the plates were incubated for 18 h at 37 °C. The proteolytic activity was determined by measuring the clear zones around the wells. (Hassett et al. 1995).
Quantification of the expression of virulence genes by quantitative real-time qRT-PCR
For RNA extraction, S. marcescens was grown in LB broth with 50 μg/ml of sotolon and in control LB broth and incubated for 18 h at 28 °C. The suspensions were centrifuged at 10,000 rpm for 15 min to separate the pellets. The bacterial cells were lysed using RNA lysis buffer, then total RNA was isolated with RNAeasy Mini Kit (Qiagen, Germany) and further analyzed for quantity and quality with the spectrophotometer (Beckman, USA).
Total RNA from each sample (10 ng) was used for cDNA synthesis by reverse transcription using High-capacity cDNA Reverse Transcriptase kit (Applied Biosystem, USA). Then, cDNA was amplified using the Syber Green I PCR Master Kit (Fermentas, USA) in a 48-well plate by employing the Step One instrument (Applied Biosystem, USA) as follows: enzyme activation (10 min at 95 °C), followed by amplification (40 cycles of 15 s at 95 °C, 20 s at 55–65 °C and 30 s at 72 °C). The 2−∆∆Ct method was used to measure the changes in the expression of each target gene that were normalized relative to the mean critical threshold (CT) values of the housekeeping rplU gene (Salini and Pandian 2015). Aliquots of 1 μM of both primers specific for each target gene were used. The sequence of the primers sequence and the annealing temperature specific for each gene are demonstrated in Table 1.
Statistical analysis
The experiments were made in triplicates except for the qRT-PCR of the virulence genes expression which was made in duplicate. One way ANOVA test, Graph Pad Prism 5 was used for statistical analysis of the inhibitory activities of sotolon on virulence factors of S. marcescens. P values < 0.05 were considered statistically significant.
Results
Antibacterial activity of sotolon
Sotolon inhibited the growth of S. marcescens at 200 µg/ml. the potential inhibitory effects of sotolon against virulence factors of S. marcescens was investigated at two sub-MICs; 25 and 50 µg/ml that are equivalent to 1/8 and 1/4 MIC values, respectively.
Sotolon reduced biofilm formation
In previous study on S. marcescens clinical isolate, it was found that it had a strong ability to form biofilm (Abbas and Hegazy 2017). Sotolon significantly reduced biofilm formation by S. marcescens (p < 0.05)by 35.2% at 25 µg/ml and 47.5% at 50 µg/ml (Fig. 1).
Sotolon inhibited swimming and swarming motilities
Swimming and swarming motility are related to the adhesion of bacteria to host tissues and the ability to form biofilms. The zones of swimming and swarming formed in swimming and swarming LB agar plates with and without sotolon were measured. At 25 µg/ml of sotolon, swimming motility was reduced by 41.4%, while swarming motility decreased by 77.6%. Moreover, swimming and swarming motilities were reduced by 69.3 and 86.8%, respectively, in the presence of 50 µg/ml of sotolon (Figs. 2 and 3).
Sotolon inhibited prodigiosin production
The intracellular red prodigiosin pigment is produced by S. marcescens and its production is under the control of the QS machinery. Prodigiosin inhibition by sotolon was found to be remarkable. The inhibition achieved ranged between 76.6 and 87.6% at concentrations of 25 and 50 µg/ml, respectively (Fig. 4).
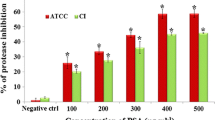
Sotolon diminished protease activity
To investigate the possible inhibition of protease activity by sotolon, the skim milk agar method was used and the diameters of the clear zones surrounding the wells made in skim milk LB agar plates were determined. Protease activity was reduced by 54.8% at 25 µg/ml of sotolon and was completely blocked at 50 µg/ml (Fig. 5).
Sotolon reduced the expression of virulence genes in S. marcescens
For confirmation of the ability of sotolon to inhibit the virulence factors in S. marcescens at the molecular level, qRT-PCR was used (Fig. 6). Interestingly, it was found that sotolon significantly reduced the levels of expression of all tested virulence genes as shown by comparing the expression levels in sotolon treated S. marcescens and in control S. marcescens by the 2−∆∆Ct method. The relative expression of genes regulating virulence factors production decreased by 40% for fimA, 29% for fimC, 59% for flhC, 57% for flhD, 39% for bsmB, 37% for rssB, 49% for rsmA, 54% for pigP, and 62% for shlA gene.
Discussion
S. marcescens is one of the clinically significant bacteria because it is frequently involved in diverse healthcare-associated infections and it can also cause food infection (Van Houdt et al. 2007; Cristina et al. 2019). S. marcescens pathogenesis is complicated by its ability to secrete several virulence factors such as proteases, lipases, nucleases and hemolysin, its swimming and swarming motilities and the emergence of multidrug resistance (Abbas and Hegazy 2020). The high rate of multidrug resistance necessitates extensive research to find new drugs to treat resistant bacterial infections. Several approaches are now investigated, among which the targeting of the intercellular communication system termed QS that regulates the production of virulence factors (Gebreyohannes et al. 2019; Mion et al. 2019).
In this study, the natural compound sotolon was investigated for its virulence attenuating activity.
Sotolon was tested as S. marcescens virulence attenuating agent at sub-MICs (25 µg/ml equivalent to 1/4 MIC and 50 µg/ml equivalent to 1/8 MIC) to avoid any possible bacterial growth inhibitory activity.
Biofilm formation represents a major cause of antibiotic resistance in S. marcescens. By adhesion to living surfaces or medical devices, S. marcescens can form biofilms resulting in life-threatening infections (Shanks et al. 2007; Satpathy et al. 2016). Sotolon was found to significantly inhibit biofilm formation. Moreover, the ability of S. marcescens to swim and to swarm that are linked to biofilm formation capability was blocked by sotolon. The swarming inhibiting activity of sotolon was more pronounced than its swimming inhibiting one.
S. marcescens strains were found to produce the red prodigiosin pigment (Lin et al. 2019). Prodigiosin production is directly regulated by QS. Sotolon could reduce prodigiosin production to a significant extent. This indicates the possible anti-QS activity of sotolon.
S. marcescens can modulate the immunity of the host by secretion of extracellular proteases (Sethupathy et al. 2016). Protease activity in the presence of 25 µg/ml of sotolon decreased by 54.8% and was completely abolished at 50 µg/ml.
Further confirmation of the anti-virulence activity of sotolon was performed at the molecular level by the qRT-PCR analysis. When the effect of sotolon on the expression of fimA, fimC, flhC, flhD, rssB and rsmA genes was performed, it was found that sotolon remarkably downregulated these genes that are involved in adhesion and swimming and swarming motilities. In S. marcescens, fimA and fimC are the major fimbrial subunits. The knock out of the fimA gene produced a mutant strain that lost the ability to form fimbriae (Labbate et al. 2007). Moreover, swimming and swarming motilities in addition to cell division and differentiation are regulated by the global gene regulators flhD and flhC that are produced under the control of the flhDC master operon. Furthermore, the flhDC promoter is regulated by the RssAB two compartment system (Srinivasan et al. 2017).
Sotolon also decreased the expression level of bsmB gene in S. marcescens clinical isolate. Mutation in bsmB resulted in the inability of the mutant to form biofilm or to secrete lipase and protease (Labbate et al. 2007).
Sotolon reduced the level of the expression of pigP gene. The master transcriptional regulator pigP is responsible for the regulation of production of the QS-controlled S. marcescens prodigiosin pigment (Gristwood et al. 2011).
Sotolon-treated cells showed reduced expression of shlA gene. The major virulence gene shlA that was found to exert cytotoxic action against fibroblasts and epithelial cells (Di Venanzio et al. 2014). Mutants of shlA gene showed a significant decrease in the virulence of S. marcescens in mice (González-Juarbe et al. 2015).
Impeding QS by natural compounds is a promising approach for treating infections caused by S. marcescens. Examples of natural agents that could successfully interfere with QS are O-methyl ellagic acid and alpha-bisabolol (Salini and Pandian 2015; Sethupathy et al. 2016). QS inhibitors could also synergize antibiotics (Borges et al. 2014).
Sotolon is a furanone compound that has celery, nutty, spicy aroma. It is the major flavor compound of dried fenugreek seeds (Blank et al. 1997). The reason for selection of sotolon is its structural similarity to halogenated furanones produced by the marine algae Delisea pulchra. These furanones are quorum sensing inhibitors that were found to interfere with the algal surface colonization by other marine organisms (Dworjanyn et al. 2006). By mimicking the AHL signal through occupying the binding site on the putative regulatory protein, furanones make it highly unstable leading to fast disruption of the quorum sensing-mediated gene regulation (Eberl et al. 1999, Zhang and Li 2015). Also, synthetic furanones were found to block many bacterial quorum sensing and help decrease the severity of pulmonary infections (Wu et al. 2004). Ascorbic acid is a natural furanone that was found to block quorum sensing in Pseudomonas aeruginosa (El-Mowafy et al. 2014).
In conclusion, sotolon is a natural agent that could attenuate the virulence of S. marcescens and the mechanism of action can be the inhibition of QS. Thus, sotolon may be a promising anti-pathogenic natural agent to fight infections caused by S. marcescens in humans. To the best of our knowledge, this is the first study of sotolon against the virulence of S. marcescens.
References
Abbas HA, Hegazy WAH (2017) Targeting the virulence factors of Serratia marcescens by ambroxol. Roum Arch Microbiol Immunol 76(2):27–32
Abbas HA, Hegazy WAH (2020) Repurposing anti-diabetic drug “Sitagliptin” as a novel virulence attenuating agent in Serratia marcescens. PLoS ONE 15(4):e0231625. https://doi.org/10.1371/journal.pone.0231625
Abisado RG, Benomar S, Klaus JR, Dandekar AA, Chandler JR (2018) Bacterial quorum sensing and microbial community interactions.mBio. 9(3): e02331-17. https://doi.org/10.1128/mbio.02331-17
Abraham SV, Palani A, Ramaswamy BR, Shunmugiah KP, Arumugam VR (2011) Antiquorum sensing and antibiofilm potential of Capparis spinosa. Arch Med Res 42(8):658–668. https://doi.org/10.1016/j.arcmed.2011.12.002
Asfour HZ (2018) Anti-quorum sensing natural compounds. J Microsc Ultrastruct 6:1–10. https://doi.org/10.1016/j.jmau.2017.02.001
Blank, I, Lin J, Devaud S, Fumeaux R, Fay LB(1997) The principal flavor components of fenugreek(Trigonella foenum-graecum L.) In ACSSymposium Series 660:12–28. https://doi.org/10.1021/bk-1997-0660.ch003
Blank I, Schieberle P (1993) Analysis of the seasoning-like flavour substances of a commercial lovage extract. Flavour Frag J 8(4):191–195. https://doi.org/10.1002/ffj.2730080405
Borges A, Serra S, Abrue AC, Saavedra MJ, Salgado A, Simões M (2014) Evaluation of the effects of selected phytochemicals on quorum sensing inhibition and in vitro cytotoxicity. Biofouling 30(2):183–195. https://doi.org/10.1080/08927014.2013.852542
Boyle KE, Monaco H, van Ditmarsch D, Deforet M (2015) Xavier JB (2015) Integration of metabolic and quorum sensing signals governing the decision to cooperate in a bacterial social trait. PLoS Comput Biol 11:e1004279
Bucio-Cano A, Reyes-Arellano A, Correa-Basurto J, Bello M, Torres-Jaramillo J, Salgado-Zamora H et al (2015) Targeting quorum sensing by designing azoline derivatives to inhibit the N-hexanoyl homoserine lactone-receptor CviR: synthesis as well as biological and theoretical evaluations. Bioorg Med Chem 23(24):7565–7577. https://doi.org/10.1016/j.bmc.2015.10.046
Busetti A, Shaw G, Megaw J, Gorman SP, Maggs CA, Gilmore BF (2015) Marine-derived quorum-sensing inhibitory activities enhance the antibacterial efficacy of tobramycin against Pseudomonas aeruginosa. Mar drugs 13:1–28. https://doi.org/10.3390/md13010001
Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approvated standard, CLSI Document M07-A9, Vol. 2012; 32, No. 3. Wayne, PA, USA
Cristina ML, Sartini M, Spagnolo AM (2019) Serratia marcescens infections in neonatal intensive care units (NICUs). Int J Environ Res Public Health 16(4). https://doi.org/10.3390/ijerph16040610
Di Venanzio G, Stepanenko TM, Véscovi EG (2014) Serratia marcescens ShlA pore-forming toxin is responsible for early induction of autophagy in host cells and is transcriptionally regulated by RcsB. Infect Immun 82:3542–3554. https://doi.org/10.1128/IAI.01682-14
Dworjanyn SA, de Nys R, P. D. Steinberg PD (2006) Chemically mediated antifouling in the red alga Delisea pulchra Mar Ecol Prog Ser 318: 153–163
El-Mowafy SA, Shaaban MI, Abd El Galil KH (2014) Sodium ascorbate as a quorum sensing inhibitor of Pseudomonas aeruginosa. J Appl Microbiol 117(5):1388–1399. https://doi.org/10.1111/jam.12631
Gebreyohannes G, Nyerere A, Bii C, Sbhatu DB (2019) Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon. 5(8):e02192. https://doi.org/10.1016/j.heliyon.2019.e02192
González-Juarbe N, Mares CA, Hinojosa CA, Medina JL, Cantwell A, Dube PH et al (2015) Requirement for Serratia marcescens cytolysin in a murine model of hemorrhagic pneumonia. Infect Immun 83(2):614–624. https://doi.org/10.1128/IAI.01822-14
Gowrishankar S, Poornima B, Pandian SK (2014) Inhibitory efficacy of cyclo(L-leucyl-L-prolyl) from mangrove rhizosphere bacterium-Bacillus amyloliquefaciens (MMS-50) toward cariogenic properties of Streptococcus mutans. Res Microbiol 165(4):278–289. https://doi.org/10.1016/j.resmic.2014.03.004
Gristwood T, McNeil MB, Clulow JS, Salmond GP, Fineran PC (2011) PigS and PigP regulate prodigiosin biosynthesis in Serratia via differential control of divergent operons, which include predicted transporters of sulfur-containing molecules. J Bacteriol 193:1076–1085. https://doi.org/10.1128/JB.00352-10
Hassett DJ, Schweizer HP, Ohman DE (1995) Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol 177(22):6330–6337. https://doi.org/10.1128/jb.177.22.6330-6337.1995
Jiang Q, Chen J, Yang C, Yin Y, Yao K (2019) Quorum sensing: a prospective therapeutic target for bacterial diseases. Biomed Res Int 4:2015978. https://doi.org/10.1155/2019/2015978
Kida Y, Inoue H, Shimizu T, Kuwano K (2007) Serratia marcescens serralysin induces inflammatory responses through protease-activated receptor. Infect Immun 75:164–174. https://doi.org/10.1128/IAI.01239-06
Krzyżek P (2019) Challenges and limitations of anti-quorum sensing therapies. Front Microbiol 10:2473. https://doi.org/10.3389/fmicb.2019.02473
Labbate M, Zhu H, Thung L, Bandara R, Larsen MR, Willcox MD et al (2007) Quorum-sensing regulation of adhesion in Serratia marcescens MG1 is surface dependent. J Bacteriol 189:2702–2711. https://doi.org/10.1128/JB.01582-06
Lin C, Jia X, Fang Y, Chen L, Zhang H, Lin R et al (2019) Enhanced production of prodigiosin by Serratia marcescens FZSF02 in the form of pigment pellets. Electron J Biotechnol. https://doi.org/10.1016/j.ejbt.2019.04.007
Martins ML, Pinto UM, Riedel K, Vanetti MCD, Mantovani HC, Araújo EF (2014) Lack of AHL-based quorum sensing in Pseudomonas fluorescens isolated from milk. Brazil J Microbiol 45(3):1039–1046. https://doi.org/10.1590/s1517-83822014000300037
Matsuyama T, Kaneda K, Nakagawa Y, Isa K, Hara-Hotta H, Yano I (1992) A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol 174(6):1769–1776. https://doi.org/10.1128/jb.174.6.1769-1776.1992
Mion S, Rémy B, Plener L, Brégeon F, Chabrière E, Daudé D (2019) Quorum quenching lactonase strengthens bacteriophage and antibiotic arsenal against Pseudomonas aeruginosa clinical isolates. Front Microbiol 10:2049. https://doi.org/10.3389/fmicb.2019.02049
Morohoshi T, Kato M, Fukamachi K, Kato N, Ikeda T (2008) N -Acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC12472. FEMS Microbiol Lett 279:124–130. https://doi.org/10.1111/j.1574-6968.2007.01016.x
Rasmussen TB, Manefield M, Andersen JB, Eberl L, Anthoni U, Christophersen C et al (2000) How Delisea pulchra furanones affect quorum sensing and swarming motility in Serratia liquefaciens MG1. Microbiol 146:3237–3244. https://doi.org/10.1099/00221287-146-12-3237
Redondo-Bravo L, Gutiérrez-González E, San Juan-Sanz I, Fernández-Jiménez I, Ruiz-Carrascoso G, Gallego-Lombardo S et al (2019) Serratia marcescens outbreak in a neonatology unit of a Spanish tertiary hospital: risk factors and control measures. Am J Infect Control 47(3):271–279
Rijkens F, Boelens H (1975) The future of aroma research. In: H. Maarse and P.J. Groenen (eds) Proceedings of the international symposium on aroma research. Wageningen, Netherlands: Pudoc, pp 203-220
Salini R, Pandian SK (2015) Interference of quorum sensing in urinary pathogen Serratia marcescens by Anethum graveolens. Pathogen Dis 73(6):ftv038. https://doi.org/10.1093/femspd/ftv038
Satpathy S, Sen SK, Pattanaik S, Raut S (2016) Review on bacterial biofilm: an universal cause of contamination. Biocatal Agric Biotechnol 7:56–66. https://doi.org/10.1016/j.bcab.2016.05.002
Sethupathy S, Shanmuganathan B, Devi KP, Pandian SK (2016) Alpha-bisabolol from brown macroalga Padina gymnospora mitigates biofilm formation and quorum sensing controlled virulence factor production in Serratia marcescens. J Appl Phycol 28:1987–1996. https://doi.org/10.1007/s10811-015-0717-z
Sethupathy S, Ananthi S, Selvaraj A, Shanmuganathan B, Vigneshwari L, Balamurugan K et al. (2017) Mahalingam S, Pandian SK. Vanillic acid from Actinidia deliciosa impedes virulence in Serratia marcescens by affecting S-layer, flagellin and fatty acid biosynthesis proteins. Sci Rep 7(1):16328. https://doi.org/10.1038/s41598-017-16507-x
Shanks RM, Stella NA, Kalivoda EJ, Doe MR, O’Dee DM, Lathrop KL et al (2007) A Serratia marcescens OxyR homolog mediates surface attachment and biofilm formation. J Bacteriol 189(20):7262–7272. https://doi.org/10.1128/JB.00859-07
Skandamis PN, Nychas GJ (2012) Quorum sensing in the context of food microbiology. Appl Environ Microbiol 78(16):5473–5482. https://doi.org/10.1128/AEM.00468-12
Srinivasan R, Mohankumar R, Kannappan A, Karthick Raja V, Archunan G, Karutha Pandian S et al (2017) Exploring the anti-quorum sensing and antibiofilm efficacy of phytol against Serratia marcescens associated acute pyelonephritis infection in Wistar rats. Front Cell Infect Microbiol 7:498. https://doi.org/10.3389/fcimb.2017.00498
Van Houdt R, Givskov M, Michiels CW (2007) Quorum sensing in Serratia. FEMS Microbiol Rev 31(4):407–424. https://doi.org/10.1111/j.1574-6976.2007.00071.x
Wu H, Song Z, Hentzer M, Andersen JB, Molin S, Givskov M et al (2004) Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J Antimicrob Chemother 53(6):1054–1061. https://doi.org/10.1093/jac/dkh223
Yip CH, Yarkoni O, Ajioka J, Wan KL, Nathan S (2019) Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl Microbiol Biotechnol 103(4):1667–1680. https://doi.org/10.1007/s00253-018-09611-z
Yukiko T, Akio K, Tei Y, Shigeru M (1980) Studies on the “sugary flavor” of raw cane sugar. III. Key compound of the sugary flavor. Proc Japan Acad series B 56 (7): 457-462.
Zhang W, Li C (2015) Exploiting Quorum sensing interfering strategies in Gram-Negative bacteria for the enhancement of environmental applications. Front Microbiol 6:1535. https://doi.org/10.3389/fmicb.2015.01535
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbas, H.A., Goda, R.M. Sotolon is a natural virulence mitigating agent in Serratia marcescens. Arch Microbiol 203, 533–541 (2021). https://doi.org/10.1007/s00203-020-02039-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02039-y