Abstract
Lakes are multifunctional waterbodies in terrestrial hydrosphere ecosystems and sediment plays a key role in the liquid–solid junction. The rapid economic development in the post-industrial era had manifold heavy metal concentration compared to background values. The non-lithogenic forms of heavy metals possess toxicity to living beings through bioaccumulation and bio-magnification processes. Chilika Lake (India) is a diversified hotspot region and habitat for many endangered species. Over the years, the lake was affected by anthropogenic activities. So, the present study was undertaken to study the dynamic relationship between heavy metals (Cr, Ni, Cd, and Pb) and sediment matrix and their mobilization behavior to the water phase influenced by sediment properties (pH and TOC). A sequential extraction procedure (SEP) was used to evaluate each chemical form's metal content. The ecological risk of metals was assessed by contamination factor (CF), geo-accumulation index (Igeo), Nemerow index (Iin), pollution load index (PLI), potential ecological risk index (PER), ratio of primary phase to secondary phase (RSP), and mobility factor (MF). Source identification (PCA) was done to chalk out the remedial plan. The spatial distribution revealed greater heterogeneity in metal concentration in the lake. Sediment properties control the mobility of metals thus protecting their quality. The mean concentration (mg kg−1) of metals follows the order of Cr (54.35) > Ni (34.95) > Pb (19.53) > Cd (0.93). The dominance of metal content in the labile fraction was found in Cd (97%) and Pb (70%), which indicates a toxic effect on biota. The average value of MF follows the order of Cd (48%) > Pb (13%) > Cr (11%) > Ni (9%) indicating a dynamic equilibrium between sediment and aqueous phase. Source analysis indicates that Cr and Ni came mostly from natural sources, while Cd and Pb originate from man-made activities. The study revealed that the majority of threat is coming from Cd and Pb. Thus, regular monitoring of sediments, treatment of influx water, use of high-quality fuel in the motorboats and the development of a drainage system are essential to eliminate heavy metal toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lakes are rich in natural resources and play a significant role in the hydrosphere and ecosystem of the planet. They are accountable for supplying water, irrigating farms, commuting, shipping, and preserving the ecosystem's balance (Yue-Fang et al., 2022). Hazardous materials have been released into aquatic waterbodies through a variety of channels in recent decades due to the faster economic development of industry and agriculture as well as the intensification of human developmental activities. This has caused the aquatic ecosystem to rapidly deteriorate and frequently polluted by these materials and among them, heavy metals (HMs) are important (Wang et al., 2021). Large-scale global attention has been drawn to HMs pollution in waterbodies like lakes in recent years due to an increase in anthropogenic activities and a boom in population (Li et al., 2022a; Zou et al., 2022).
Because HMs are highly biotoxic, long-lasting, and inert, they pose a potential risk to the environment's ecological balance (Li et al., 2023) and around 23 heavy metals have been found hazardous and toxic when present at high concentrations in the environment (Jaishankar et al., 2014). Heavy metals can harm the ecological balance and public health irreversibly through the bioaccumulation and biomagnification of the food chain (Kabir et al., 2021). It is well recognised that heavy metal exposure in the population can result in major health issues like cancer, damage to the nervous and organ systems, autoimmune diseases, and in rare cases, even death. The four metals examined in this paper- Cr, Ni, Pb, and Cd are the most frequently found heavy metals in the environment and are thought to be harmful to humans and ecosystems in high concentrations. For instance, research has demonstrated that exposure to high concentrations of Cd in human results in kidney illness, infertility, mental, intestinal, and cancer issues (Xu et al., 2017). Nickel toxicity causes cancer, heart disease, and respiratory failure. Pb can trigger several diseases such as neuronal dysfunction (especially in children), deficits in renal function, hypertension and heart disease, and disorders of the reproductive system. Exposure to Cr causes kidney and liver damage, pulmonary disorder and hypoglycemia (Calmuc et al., 2021).
Heavy metals make their entry into the environment from anthropogenic and natural sources. The natural sources include volcanic eruption, mineral weathering and erosion processes, while anthropogenic sources include atmospheric fallout, use of fertilizer and pesticide in agriculture, disposal of municipality sewage sludge, mine effluents and industrial discharge (Baran et al., 2016; Kluska and Jabłońska, 2023). Heavy metals entering into aquatic ecosystems are distributed in 3 phases viz. water phase (dissolved phase), sediment phase, and suspended particulate matter (SPM) phase. More metal content in the aqueous phase indicates significant toxicity to flora and fauna (Luo et al., 2019). Because of SPM's large specific surface area, reactivity, and ability to absorb dissolved heavy metals, the concentration of HMs in SPM and sediment is typically higher when compared to water phases (Li et al., 2023). The present investigation is based on the evaluation of heavy metals concentration in sediments.
Sediment is present in the solid–liquid junction of aquatic ecosystems and behaves as the biggest reservoir of HMs. About 99% of heavy metals entering to aquatic ecosystem get deposited in sediments making it a sink of HMs. Sediments contain an HM concentration of 4–5 times more than the water phase (Xu et al., 2018). So, sediment quality reflects HM's pollution status in aquatic ecosystems. Both sediment and aqueous phases are in dynamic equilibrium; upon changing the environmental condition (pH, salinity, texture, total organic matter, redox potential, and types of chelating agents) of the ecosystem, HMs present in sediments release into dissolved phase causing secondary pollution. In this way, sediments also act as a source of pollution (Li et al., 2020; Nazneen et al., 2019).
Heavy metals get deposited in sediments in various geochemical forms viz get adsorbed on oxy-hydroxides of Fe/Mn and also in surfaces of clay minerals, occluded in amorphous minerals and complexed/ precipitated in lattice of primary and secondary minerals as well as in organic matter. These chemical forms govern their solubility and intensity of release to the dissolved phase upon changes in environmental conditions in the aquatic ecosystem (Morillo et al., 2007; Tack & Verloo, 1995). These geochemical forms can be classified as lithogenic (metal concealed in lattice and not easily mobilized) and non-lithogenic (metal easily remobilized to water column) (Panda et al., 1995). Heavy metal concentrations in sediments are not an independent variable; rather, they interact with other environmental factors. Evaluation of the impacts of heavy metals on the ecosystem and clarification of the pollution features of the local environment have both benefited from research on the interactions between heavy metals and numerous environmental parameters (Wijesiri et al., 2019). Heavy metal content in sediment can be determined in two ways, such as total content and metal content in each chemical form. To determine the metal content in each chemical form sequential extraction procedure (SEP) was used. Determining metal content in these individual chemical forms helps to assess the potential ecological risk associated with these forms and to evaluate the pollution status of the ecosystem.
Chilika Lake is located in Odisha (India) and is the largest lagoon in the country. It is a biodiversity-enriched aquatic ecosystem connected to the Bay of Bengal by a narrow channel. It is a nesting ground for many migratory birds and other endangered species like the Irrawaddy dolphin (Orcaella brevirostris) (Kannan et al., 2005), which attracts millions of visitors. Its productiveness is facing threats from heavy metals originating from multiple natural and anthropogenic sources. Since, 1980 the lagoon has attracted numerous researchers to study the sediments of Chilika Lake (Barik et al., 2018), but only a few worked on heavy metals (Barik et al., 2018; Mohanty et al., 2017; Nazneen et al., 2019; Panda et al., 1995, 2006, 2010; Sarkar et al., 1981; Zachmann et al., 2009). Their efforts were mostly focused on total metal content rather than on speciation. For this reason, the present study was undertaken to determine the metal content in the different geochemical forms and assess the potential ecological risk associated with these geochemical forms. Therefore, the study area is investigated with following objectives (i) to determine the metal content in each geochemical form, (ii) to determine spatial distribution of total metal content in the lake, (iii) to assess the heavy metal pollution by calculating different synergistic and individual indices, and (iv) to determine the relationship among heavy metals by multivariate statistical analysis to identify their possible sources. The findings of the study will help to take reasonable management strategies to control the heavy metal pollution in the lake.
2 Materials and Methods
2.1 Study Area
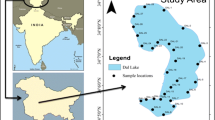
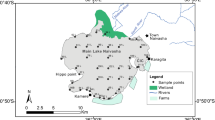
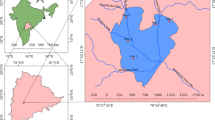
Chilika Lake, the largest brackish water lake in the Asian sub-continent, is situated along India's east coast and has been classified as a Ramsar site in 1981 (19° 28′—19° 54′ N latitude and 80° 05′—85° 82′ E longitude). The lake is divided in four ecological areas, the northern sector (NS), southern sector (SS), central sector (CS), and outer channel (OC). It's a shallow body of water that is roughly 65 km in length and 20 km in breadth. During the summer and monsoon seasons, the water spread area is predicted to be 704 km2 and 1020 km2, respectively (Gupta et al., 2008). The lagoon has a unique hydrological effect viz. (i) silt-loaded fresh water discharged by Mahanadi distributary systems, (ii) drainage from western and southern catchment regions, and (iii) exchange of water between the Bay of Bengal and the lagoon (Nazneen et al., 2019). The lagoon also receives a total annual rainfall of 1238 mm in 72 rainy days.
This unique assemblage of marine, fresh, and brackish water makes it one of India's biodiversity hotspots region and home to numerous IUCN-listed species that are near threatened, vulnerable, endangered, and critically endangered (Kannan et al., 2005; Sahu et al., 2014). The lagoon receives 1.5 million MT of silt per year carried by the Mahanadi River distributary system, whereas 0.3 million MT per year of sediment enters from the western basin. Domestic wastewater from 141 villages around the Chilika watershed, as well as 550 MLD of untreated domestic wastewater from five sewage discharge zones in the capital city of Bhubaneswar, were discharged into the lagoon (Ghosh et al., 2006). The lake's drainage area is around 3560 km2, and 52 rivers and rivulets empty into it. Most of the time during the monsoon season, rivers like Mandakini, Kansari, Salia, and tiny streams from the hills on the western side (part of the Eastern Ghats), supply freshwater to the lake. The Mahanadi River system empties into the lake on its northern side, and during the wet season, the deltaic region is drowned (Barik et al., 2016). In the lake, there are over 7000 fishing boats in operation (Mohanty et al., 2017).
2.2 Sampling and Preparation for Analysis
Nineteen samples were collected from the four ecological divisions (Fig. 1). According to the established protocol (USEPA, 2001), sediment samples were taken in December 2018 (winter season) using a 250 cc KC Denmark A/S grab sampler. The samples were collected and preserved in an acid-rinsed polypropylene bottle in a refrigerator set to 4 °C. The sediment samples were air dried, mashed with a mortar and pestle, sieved through a 2 mm sieve, and then placed in a polythene bag with labels. All glass-wares and plastic-wares used in this investigation were cleaned of any contamination by being submerged in 14% (v/v) HNO3 for 24 h, then rinsed with double-distilled water. For solution preparation, all the chemicals were used AR grade supplied by MERCK and Milli-Q (Millipore, USA).
2.3 Physicochemical Parameters Analysis
Physicochemical parameters like pH and total organic carbon were determined by following standard methods. For pH determination, a 1: 2.5 soil water suspension ratio was used (Jackson, 1973). Total organic carbon (TOC) measured in percentage was determined by using the wet oxidation method of Walkley and Black (1934).
2.4 Metal Fractionation
The most popular technique for determining the speciation of HMs was Tessier's five-step continuous extraction (Tessier et al., 1979) and this speciation procedure includes five geochemical forms viz exchangeable form (F1/ EXC), carbonate bound forms (F2/ CAR). Fe/Mn oxide bound (F3/ FMO), organic matter bound (F4/ OM), and residual bound (F5/ RES) (Table 1). In our present study, we made slight modifications in the shaking time of CAR fraction i.e. from 5 to 2 h as Tessier et al. (1979) mentioned that shaking time depends on sediment particle size and carbonate content and 90% of carbonate dissolution occured in 2 h. To determine the metal content in RES form, HF – HClO4 digestion was performed by taking 0.5 g sediment from the residue of OM fraction in a PTFE beaker placed in an infrared digestion unit (BOROSIL). The metal concentration of each form of SEP was determined using an air/ acetylene burner of atomic absorption spectrophotometer (240FSAA, Agilent technologies). Total metal content was determined by the sum of metal measured in five geochemical forms. The bioavailability of metal follows the order: highly bioavailable form (EXC + CAR) > potentially bioavailable form (FMO + OM) > bio-unavailable form (RES).
2.5 Indices of Heavy Metal Pollution Assessment
2.5.1 Contamination Factor (CF) and Degree of Contamination (Cd)
The contamination factor assesses the pollution level of the individual element while the degree of contamination is a cumulative index of n number of heavy metals (Hakanson, 1980). These two indices are calculated based on Eqs. (1) and (2):
Cn is the concentration of metal “n”; Bn is the background value of metal “n”. Metal concentration (mg kg−1) at the southernmost point of the lagoon was taken as background value (Cr = 81.63, Ni = 36.13, Cd = 0.1, and Pb = 7.35) as this point is adjacent to the land and least polluted. The classification of CF and Cd (Luo et al., 2007) were presented in Table S1.
2.5.2 Pollution Load Index (PLI)
Pollution load index is an integrated approach given by Tomlinson et al. (1980) to assess the quality of sediment. It is defined as the nth root of multiplications of contamination factor of heavy metals viz. Cr, Ni, Cd and Pb. It is calculated as,
The PLI value of less than one indicates no pollution, while more than one indicates progressive deterioration of the quality of sediment.
2.5.3 Index of Geoaccumulation (Igeo)
The geoaccumulation index not only depicts the HMs distribution in its natural state, but it also shows how anthropogenic influences and natural diagenesis have an impact on the background value. In actuality, this index indicates the degree of HMs enrichment in sediments. The calculation formula is as follows (Muller, 1969):
where Cn is the metal concentration in sediment samples and Bn is the background concentration of that element (Cr = 81.63, Ni = 36.13, Cd = 0.1, and Pb = 7.35). To minimize the effect of variation in background values, mainly the lithogenic variation in sediments, factor 1.5 is introduced (Barik et al., 2018). This index was classified into seven enrichment classes to give an idea of the extent of metal pollution in Table S2.
2.6 Nemerow Index (I in)
Nemerow index is a cumulative pollution index, developed by Nemerow and Sumitomo (1970). This index integrates average value and maximum value of geoaccumulation index and gave an environmental quality index based on multiple weighted factors. This index is calculated based on Eq. (5):
where Igeomax is the maximum Igeo value among n number heavy metals of station i and Igeoavg is the average value of Igeo value among n number heavy metals of station i. The index is classified in to five classes presented in Table S3.
2.6.1 Potential Ecological Risk Index (PER)
This index was developed by Hakanson (1980) and it estimates the ecological risk associated with heavy metals. The PER is summing total of potential ecological risk factor (Er) of individual heavy metals. This index covers the risk factor (Er), contamination factor (CF) and toxic response factor (Tr) (Duodu et al., 2016). The PER is calculated as follows:
where Er is the potential ecological risk factor of individual metal, CF is the contamination factor and Tr is the toxic response factor (Cr = 2, Ni = Pb = 5, and Cd = 30) (Hakanson, 1980). The classification of Er and PER (Luo et al., 2007) is shown in Table S4.
2.6.2 Adverse Effect Index (AEI)
The adverse effect index is defined as the ratio between the total measured concentration of metal “m” to the threshold effect level of metal “m”. Its value of more than 1 indicates an adverse effect on the biota while less than 1 indicates no significant adverse effect on the biota (Koukina & Lobus, 2020)
Cm is the total metal concentration of metal “m” and TELm is the TEL of metal “m”. The TEL value was given by MacDonald et al. (2000).
2.6.3 Mobility Factor (MF)
This index is calculated as metal content in EXC and CAR fraction to total metal content in percentage scale. It represents the highly bioavailable form of heavy metals that quickly mobilize to the water phase. It is calculated as follows (Kabala & Singh, 2001):
where MCexc and MCcar are metal concentrations in EXC and CAR fractions and TC is the total metal concentration. This index is classified into 5 classes (Perin et al., 1985) presented in Table S5.
2.6.4 Ratio of Secondary Phase to Primary Phase (RSP)
This index represents both the highly bioavailable forms and potentially bioavailable forms of heavy metals i.e. the secondary phase. It is calculated as follows (Xu et al., 2022):
where, Msec is metal content in the secondary phase (EXC, CAR, FMO, and OM) and Mpri is metal content in the primary phase (RES). It is classified into four groups presented in Table S6.
2.7 Data Analysis
Multivariate statistical analysis like PCA (principal component analysis), correlation, regression, cluster diagram, and production of graphs and maps were performed in SPSS 20.0, Origin 9.0, Arc Gis 10.5, and Microsoft Excel 2016.
3 Results and Discussion
3.1 Concentration of Heavy Metals and Physicochemical Properties of Sediments
The concentration and descriptive statistics of four heavy metals (Cr, Ni, Cd and Pb) are presented in Fig. 2 and Table 2 respectively. The average concentration (ranges) of heavy metals (Cr, Ni, Cd, and Pb) in the lagoon sediments are 54.35 (24.7 – 95.3), 34.95 (12.37 – 52.48), 0.93 (0.03 – 2.55) and 19.53 (5.6 – 36.95) mg kg−1 respectively. The average concentration of four heavy metals follows the order Cr > Ni > Pb > Cd. The coefficient of variation (CV) lower than 50% indicate less anthropogenic influence, while more than 50% indicate higher influence of human activities. Among the four heavy metals, Cd has the higher CV i.e. 88.51% which indicate that presence of this metal in the sediments is mainly due to anthropogenic activities. While other three metals have the CV viz. Cr (33.24%), Ni (25.37%) and Pb (45.27%) and the heavy metals follows the pattern Cd > Pb > Cr > Ni (Meng et al., 2023; Nezhad et al., 2015; Wu et al., 2022) (Table 2). The enrichment degree of metals was calculated by ratio between average concentration of metals to their atomic weight for this paper and the result revealed the pattern of enrichment was Cr (1.045) > Ni (0.595) > Pb (0.094) > Cd (0.008) (Wu et al., 2022).
Evaluation of the total metal (Cr, NI, Cd and Pb) concentration of sediments of the lagoon at sampling sites and assessment of the sediment quality by comparing total metal concentration with various sediment quality guidelines (BV: background value, UCC: Upper continental crust value, PEL: Probable effect level and TEL: Threshold effect level)
The total concentration of four investigated heavy metals at nineteen sampling sites shows greater variability with background values (Table 3). In the case of Cr, most of the sampling sites (except S2, S3) are far below than background values, and a similar trend for Ni is also seen (except S3, S4, S5, S6, S7, S17). But, a reverse pattern was seen in the case of Cd and Pb, where maximum sampling sites are well above the background values. It was found that the average total concentration of Cd (9.3 times) and Pb (2.65 times) was higher than the background values. When the average total concentration was compared with the upper continental crust value (UCC) the result revealed that Cr (1.55 times), Ni (1.74 times), and Cd (10 times) were higher than the UCC value (Taylor & McLennan, 1995). Sediment quality guidelines given by MacDonald et al. (2000) viz. probable effect level (PEL) and threshold effect level (TEL) were used to assess eco-toxicity level. Compared with the PEL value, in the case of Cr and Ni only a few sampling stations (Cr- S2, S3 and Ni – S3, S4, S5, S6, S7, S17) are above the PEL value which indicates in these sampling sites are contaminated with metals, but in case of Cd and Pb, all nineteen sampling sites are well below of PEL value. The threshold effect level (TEL) indicates the occurrence of adverse effects on biota above this concentration and no adverse effect below this concentration. It was found that the mean total concentration of heavy metals viz. Cr (1.45 times), Ni (1.94 times), and Cd (1.56 times) greater than the TEL value. Most of the sampling sites in the case of Cr and Ni, a few sites in the case of Cd, and only one site in the case of Pb are above the higher values (Fig. 2 and Table 3). The spatial distribution of the total concentration of heavy metals is presented in Fig. 3 and it shows greater heterogeneity in the distribution of metals in lagoon sediment.
The present study was compared with earlier studies done in the lake by Barik et al. (2018) and Panda et al. (2010) and it reveals that the concentration of metals was lower than earlier studies. The possible reasons are the opening of new channels connecting to the sea; which transfer the polluted sediments to sea, the use of high-quality fuel and paints in motor boats (Barik et al., 2018; Nazneen et al., 2019) prohibition on illegal aquaculture and establishment of new sewage treatment plants in the capital city of Odisha through which lake falling river passed. The result of the present study is compared with other studies done on lakes and rivers in India and the world and it reveals greater variability and heterogeneity in metal concentration owing to their source of pollution (Table 3).
The descriptive statistics of the physicochemical parameters are presented in Table 2. The range of pH in the sediments is 6.2–9.35 with a mean value of 7.85 ± 0.63 (Table 2). Figure 4, reveals that the pH of the sampling site's sediment varies from slightly to moderately alkaline, only site (S6) shows higher alkalinity due to its connection to the sea, and slightly acidic pH was found in the zone receiving the fresh water from the riverine system. Zhang et al., (2018a, b) found that under acidic pH, there is metal mobilization to the water phase. According to Borma et al. (2003) decrease in sediment pH, encourages metal mobility to aquatic phases due to competition between hydrogen ions and ligands (Cl−, SO42−, CO32−, S2−, OH−, and PO43−) of metals. In the present investigation, the pH of the lagoon sediment is moderately alkaline as earlier discussed by Barik et al. (2018), and the environment-inducing for the formation of carbonates and hydroxides of metals thus reducing the metal mobilization to the dissolved phase. So, the pH of the Chilika Lake sediments plays a crucial role by controlling metal mobilization and thus protecting the lake ecosystem. The total organic carbon ranges (average value) from 0.27 to 1.91% (1.01 ± 0.49) (Table 2). Most of the sampling sites in the lagoon have medium to high organic carbon content (Fig. 4) and it is mainly due to the silt load of the riverine system and drainage from the western catchment (Barik et al., 2018; Muduli et al., 2013; Nirmala et al., 2016). There is a negative correlation between TOC and Cd (f = -0.46) which indicates the adsorption of Cd by the organic matter due to the presence of carboxyl, phenolic, and hydroxyl groups (Smolders & Mertens, 2013). Organic fraction is a good reservoir of metal, the higher the organic carbon content higher be metal sorption, and the lesser be mobilization to the aqueous phase (Baran et al., 2019).
3.2 Speciation Analysis of Heavy Metals in the Sediments
The total concentration of heavy metals includes bioavailable, potentially bioavailable, and residual forms of metals. The remobilization patterns of these forms are different. Upon the sudden change in physicochemical parameters, the bioavailable forms of metal quickly remobilize from the sediment phase to the aqueous phase and exhibit eco-toxicity to biota, while residual forms of metals are not readily available as they are bound to matrix. Earlier studied sediment quality guidelines are based on total concentration which includes the matrix-bound portion of metals. So, it is necessary to quantify metal contents in each geochemical form to assess their ecological toxicity.
The geochemical distribution of four heavy metals (Cr, Ni, Cd, and Pb) is presented in Fig. 5 (in percentage scale). The extraction of metals was done using a modified Tessier sequential extraction procedure (Tessier et al., 1979). The geochemical distribution was classified into three categories and the categories are; first (EXC + CAR), second (FMO + OM), and third (RES). These three categories' mean distribution follows the pattern as such; Cr: RES (61.8%) > FMO + OM (27.31%) > EXC + CAR (10.9%), Ni: RES (55.23%) > FMO + OM (36%) > EXC + CAR (8.76%), Cd: FMO + OM (49.38%) > EXC + CAR (48%) > RES (2.64%) and Pb: FMO + OM (57%) > EXC + CAR (13.28%) > RES (29.71%). The distribution of mean concentration of metals in each fractions follows the order as; Cr: RES (61.8%) > FMO (22.02%) > EXC (6.16%) > OM (5.28%) > CAR (4.72%), Ni: RES (55.23%) > FMO (26.17%) > OM (9.82%) > CAR (7.10%) > EXC (1.65%), Cd: FMO (40.23%) > EXC (25.62%) > CAR (22.34%) > OM (9.15%) > RES (2.64%) and Pb: FMO (43.80%) > RES (29.71%) > OM (13.19%) > CAR (6.81%) > EXC (6.46%) (Fig. 5).
Geochemical distribution of heavy metals (a) Cr, (b) Ni, (c) Cd & (d) Pb in the components of the sediment matrix (F1: metal present in exchangeable form, F2: metal bound to carbonate, F3: metal bound to Fe/Mn oxide, F4: Metal content in organic matter fraction and F5: Metal present in residual form) at the sampling sites of the lagoon
The result of speciation analysis indicates more than 50% of metal content is in residual form in the case of Cr and Ni. This form of metal is fixed in a solid matrix of sediments and mostly it originates from natural sources and little from anthropogenic influence. Metal content in residual form has less impact on biota, thus not polluting the lagoon environment (Bosco et al., 2019; Nijeje et al., 2023). These insoluble forms of metals can be converted into soluble fractions by chemical reactions; that can only affect organisms (Ekwutosi et al., 2020; Islam et al., 2018; Zhang et al., 2022). But in the case of Cd and Pb, more than 50% metal is present in bioavailable (EXC + CAR) and potentially bioavailable (FMO + OM) forms. Cadmium is dominated in bioavailable and potentially bioavailable forms, making it the most polluting metal in the lagoon ecosystem, but on the other hand, Pb dominating in potentially bioavailable form makes it the second most polluting metal. More than 50% of metal in non-lithogenic form (EXC + CAR + FMO + OM) indicates the greater role of anthropogenic influence on the enrichment of metals in the sediment. Metal present in soluble form can easily pollute the aquatic ecosystem and subsequently show toxicity effects on terrestrial and aquatic organisms (Duan et al., 2019; Ren et al., 2023).
3.3 Source Identification through Multivariate Analysis
The distribution and variation in the metal concentration of sediments are significantly influenced by the potential sources. Multivariate statistical analysis like principal component analysis and correlation coefficient analysis are vital tools to identify the source of pollution. These multivariate analyses reduce data and analyze spatio-temporal changes and groupings of data (Yongo et al., 2023; Zhao et al., 2015).
3.4 Principal Component Analysis (PCA)
Based on Pearson correlation analysis (Figure S1), for further investigation of sources of metals in the lagoon sediments, PCA was run using the varimax rotation method and it yielded three components having Eigen value of more than 1 and also a cumulative variance of 84.443% (Table 4). The first principal component (PC1) yielded a variance of 31.942% with high positive loadings of Cd and Pb (consistent with correlation analysis Figure S1). These two metals contain more than 50% concentration in non-lithogenic form, so their sources are anthropogenic in origin. The possible sources of Cd and Pb in the lagoon sediments are the printing and dyeing industry (Cui et al., 2014; Satapathy & Panda, 2015), agricultural runoff carrying pesticide and fertilizer (phosphatic and nitrogenous fertilizers) (Ioannides et al., 2015; Nazneen & Patel, 2016), effluent from nearby fish market (Banerjee et al., 2017) and waste from chemical and textile industries carried by riverine system (Singh et al., 2017). The other sources of Pb in the lagoon are the use of leaded beads in the fishing net (Mohanty et al., 2017), the large number of motor boats plying in the lagoon use low-quality leaded gasoline fuel (Mohanty et al., 2017; Sahu et al., 2014) and atmospheric fallout (Nazneen & Patel, 2016; Singh et al., 2015).
The principal component 2 (PC2) exhibits strong positive loading of Cr and Ni with a variance of 27.309% (corroborates to correlation analysis, Figure S1). Both of these metals contain more than 50% of the concentration in lithogenic form so they have a common natural origin i.e. from weathering of rocks and minerals (Liang et al., 2017b; Wu et al., 2021). So the enrichment of Cr and Ni is mainly due to natural factors. Additionally, both Cr and Ni are also present in the residue of fertilizer and pesticide and they are carried through drainage from nearby agricultural fields (Ioannides et al., 2015; Nazneen & Patel, 2016). The anthropogenic influx of Ni and Cr in the lagoon is mainly due to the silt load of the Mahanadi riverine system and effluent from the western catchment (Banerjee et al., 2017; Barik et al., 2018; Zachmann et al., 2009) which contain municipal sewage, chemical industry effluent, antifouling agents and contaminated landfill. Atmospheric deposition of coal burn ash can significantly accumulate these two metals in lagoon sediments (Ke et al., 2017). The third principal component (PC3) results in the loading of pH and organic carbon with a variance of 25.192%. High pH protects the lagoon from the formation of stable complexes such as hydroxides and carbonates (Barik et al., 2018; Forstner & Wittmann, 1983). The organic matter with finer fractions has a strong affinity for metal sorption (Muduli et al., 2013).
3.5 Assessment of Metal Pollution in the Lagoon Sediments
3.5.1 Contamination Factor (CF) and Degree of Contamination (Cd)
The contamination factor (CF) is calculated as the ratio between total metal content to background values. When the value of CF is more than one, it indicates metal enrichment in the sediment. Among the four studied metals, Cr and Ni have low contamination factors (CF < 1). In the case of Cd, most of the sampling sites fall under the high contamination level category. Similarly, Pb falls under moderate to considerable contamination levels (Fig. 6 (a)). The mean contamination factor of four metals follows the pattern of Cd (9.27) > Pb (2.65) > Ni (0.96) > Cr (0.66). The degree of contamination is calculated by adding the CF value of four heavy metals. The range (mean) of degree of contamination is 3.43 to 31.05 (13.56). This index indicates except few sampling sites (S1, S2, S3, S4), the rest sites show a significant degree of contamination ranging from moderate to high category and this is attributed to the high level of contamination of Cd and Pb (Fig. 6 (b)). As these two metals come from the anthropogenic origin sources in the lagoon, they show significant enrichment in the sediment (Bai et al., 2013; Barik et al., 2018).
3.5.2 Pollution Load Index (PLI)
This index is also calculated based on CF value. The mean value of PLI in the Chilika Lagoon is 1.76. The PLI values of sampling sites are presented in Fig. 7. As per the classification, except S1, S2, S3, and S4, the rest of the sampling sites indicate sediment of the lagoon is polluted. The PLI value was found to be highest in the sites near sea linking channels (S5, S6, S7) and it signifies metal accumulation in this location mainly due to drainage from nearby agricultural field and subsequently low tidal flushing, as these connecting channels are earlier closed due to siltation. Another reason is the boat trafficking in this region. Silt load brought by the riverine system also builds up metal enrichment in the lagoon (Nazneen & Patel, 2016; Panigrahi et al., 2009; Sahu et al., 2014).
3.5.3 Index of Geoaccumulation (Igeo)
This index was introduced to measure heavy metal accumulation in sediments in an aquatic body study. The result of this index is shown in Fig. 8. The result revealed that for Cr and Ni, the values are less than zero, indicating sediments are unpolluted, while the range of Igeo value sampling sites for Cd and Pb indicate unpolluted to very strongly polluted sediment (78%) and unpolluted to moderately polluted sediments (73%) respectively. Hence, the higher enrichment of Cd signifies the role of anthropogenic sources like silt load, industrial, municipal, and fishery market effluent, runoff from agricultural fields and boats used for tourism and fishing (Banerjee et al., 2017; Mohanty et al., 2017; Zachmann et al., 2009).
3.5.4 Nemerow Index (Iin)
The value of Iin was calculated and presented in Fig. 9. The result revealed that except for three sites S2, S3, and S17, the rest of the sampling sites (84%) are slightly polluted to moderately polluted. The most polluted sampling sites are S5, S6, and S7.
3.5.5 Potential Ecological Risk Index (PER)
The potential ecological risk index (PER) and potential ecological risk factor (Er) are shown in Fig. 10. Among the four studied heavy metals Cr, Ni, and Pb, have the low ecological risk factor (Er < 40). On the contrary, Cd exhibits a very high Er value indicating toxicity to the lagoon ecosystem. The PER value of S5 (791), S6 (743.29), and S7 (733) was recorded as high among the sampling sites. Among sampling sites, 78% of sites show significant ecological risk in the lagoon environment. It indicates the progressive deterioration of the quality of sediments in the lagoon. The culprit element in the lagoon is the Cd, which is showing the highest ecotoxicity due to more metal concentration in the non-lithogenic fraction (Zhang et al., 2018a, b).
3.5.6 Adverse Effect Index (AEI)
The adverse effect index was calculated and shown in Fig. 11. Among four heavy metals, (except Pb) the remaining three metals viz. Cr, Ni, and Cd show adverse effects on the biota and the order of percentage of sampling sites showing the adverse effect follows: Cr (89%) = Ni (89%) > Cd (52%).
3.5.7 Mobility Factor (MF)
The mobility factor helps to assess the remobilization of metals present in bioavailable form (EXC + CAR). Metals from anthropogenic influence are weakly bonded in this fraction and upon chemical reaction, these metals move to the aqueous phase causing toxicity to organisms. The mobility factor was calculated and its relationship with sediment properties is shown in Fig. 12. The average value of MF follows the order Cd (48%) > Pb (13.27%) > Cr (10.89%) > Ni (8.76%). Heavy metals like Cr, Ni, and Pb are showing low to moderate risk, while Cd ranges from moderate to very high risk. Conclusively Cd is the most culprit metal having higher mobility. Around 36% of sampling sites fall under the very high-risk category class and these sampling sites are located in the zone of boat traffic and riverine discharge zone. So, the anthropogenic influence is the main reason behind the high metal mobility. From Fig. 12, it was found that with an increase in pH value and TOC content, there is a decrease in metal mobility and the risk associated with it, due to the formation of metal hydroxide and adsorption of metal on the surface of organic particles (Barik et al., 2018; Liang et al., 2017a).
3.5.8 Ratio of Secondary Phase to Primary Phase (RSP)
The value of RSP was calculated and presented in Fig. 13. As per the classification Table (S6) both Cr and Ni, at most of the sampling sites are unpolluted. Similarly, Pb at most of the sampling sites are within light to moderate pollution. But, in the case of Cd, most of the sampling sites are above severe pollution levels. Sampling sites like S13 (129) and S14 (127) are the highest value for cadmium. A large number of motor boats are plying in this area for tourism purposes which use low-quality paint and fuel, run-off from nearby agricultural fields, and effluent from fishery market and processing units are possible sources of Cd (Banerjee et al., 2017; Mohanty et al., 2017).
4 Conclusion
The study was carried out to investigate the current status of heavy metal concentration and their distribution pattern in sediments of the Chilika Lake. The heavy metals show a stronger relationship with each other and the physicochemical properties of sediment govern their solubility and remobilization to aqueous phase. Chromium dominated the total mean concentration in the lagoon sediment followed by Ni, Pb, and Cd. Chromium and nickel are found to have more metal concentration in the residual phase owing to their origin from natural sources. Cadmium and lead have higher concentrations in the non-lithogenic forms indicating their abundance due to anthropogenic inputs. Sediment properties (pH and TOC) play a key role in maintaining sediment quality by controlling the remobilization of metals to the dissolved phases. Ecological risk indices like CF, Cd, PLI, Iin, and Igeo indicate significant pollution in the sediments. The potential ecological risk index (PER) shows that eco-toxicity is mainly due to Cd as it has more metal content in the bioavailable fraction. The Adverse effect index (AEI) indicates the presence of adverse effects of metal on the lagoon ecosystem. The mobility factor (MF) indicates there is a high mobility of Cd to the dissolved phase as compared to the other three metals. The anthropogenic sources of pollution include motor boats used for tourism and fishing, illegal aquaculture, non-judicious application of fertilizer and pesticide in nearby farm fields, and discharge of silt-loaded riverine freshwater which in turn creates a potential threat to the rich diversity of the lagoon ecosystem. The bioavailability of the heavy metals is the greatest threat, so periodical monitoring of sediment along with other measures like opening channels for the drainage of polluted sediments to the sea, use of high-quality paint and fuel in the motorboats, treatment of silt-laden freshwater and effluent from nearby areas, and prohibiting illegal aquaculture is essential to cut down the metal content in the lagoon sediments.
Data Availability
The data and materials will be made available on from the corresponding author(s) upon reasonable request.
References
Aung, P. P., Mao, Y., Hu, T., Qi, S., Tian, Q., Chen, Z., & Xing, X. (2019). Metal concentrations and pollution assessment in bottom sediments from Inle Lake, Myanmar. Journal of Geochemical Exploration, 207, 106357. https://doi.org/10.1016/j.gexplo.2019.106357
Bai, J., Zhao, Q., Lu, Q., Wang, J., & Ye, X. (2013). Profile Distribution of Soil Heavy Metals in the Paludification Region of Baiyangdian Lake; A Case Study of Shaochedian Lake. Wetland Science, 11(2), 271–275.
Banerjee, S., Pramanik, A., Sengupta, S., Chattopadhyay, D., & Bhattacharyya, M. (2017). Distribution and source identification of heavy metal concentration in Chilika Lake, Odisha India: an assessment over salinity gradient. Current Science, 87–94. https://doi.org/10.18520/cs/v112/i01/87-94
Baran, A., Tarnawski, M., & Koniarz, T. (2016). Spatial distribution of trace elements and ecotoxicity of bottom sediments in Rybnik reservoir, Silesian-Poland. Environmental Science and Pollution Research, 23, 17255–17268. https://doi.org/10.1007/s11356-016-6678-1
Baran, A., Mierzwa-Hersztek, M., Gondek, K., Tarnawski, M., Szara, M., Gorczyca, O., & Koniarz, T. (2019). The influence of the quantity and quality of sediment organic matter on the potential mobility and toxicity of trace elements in bottom sediment. Environmental Geochemistry and Health, 41, 2893–2910. https://doi.org/10.1007/s10653-019-00359-7
Barik, S. K., Bramha, S. N., Mohanty, A. K., Bastia, T. K., Behera, D., & Rath, P. (2016). Sequential extraction of different forms of phosphorus in the surface sediments of Chilika Lake. Arabian Journal of Geosciences, 9, 1–12. https://doi.org/10.1007/s12517-015-2217-5
Barik, S. K., Muduli, P. R., Mohanty, B., Rath, P., & Samanta, S. (2018). Spatial distribution and potential biological risk of some metals in relation to granulometric content in core sediments from Chilika Lake, India. Environmental Science and Pollution Research, 25, 572–587. https://doi.org/10.1007/s11356-017-0421-4
Borma, L. D. S., Ehrlich, M., & Barbosa, M. C. (2003). Acidification and release of heavy metals in dredged sediments. Canadian Geotechnical Journal, 40(6), 1154–1163. https://doi.org/10.1139/t03-062
Bosco, S. J., Janvier, H., Claude, S. J., & Brigitte, M. (2019). A chemical speciation study of selected heavy metals in aquatic bottom sediment samples from Mpenge stream, Musanze District, Rwanda. African Journal of Environmental Science and Technology, 13(11), 450–455. https://doi.org/10.5897/ajest2018.2527
Calmuc, V. A., Calmuc, M., Arseni, M., Topa, C. M., Timofti, M., Burada, A., Georgescu, L. P., et al. (2021). Assessment of heavy metal pollution levels in sediments and of ecological risk by quality indices, applying a case study: The Lower Danube River, Romania. Water, 13(13), 1801. https://doi.org/10.3390/w13131801
Chakrapani, G. J., & Subramanian, V. (1990). Preliminary studies on the geochemistry of the Mahanadi river basin, India. Chemical Geology, 81(3), 241–253. https://doi.org/10.1016/0009-2541(90)90118-Q
Cui, J., Zang, S., Zhai, D., & Wu, B. (2014). Potential ecological risk of heavy metals and metalloid in the sediments of Wuyuer River basin, Heilongjiang Province, China. Ecotoxicology, 23, 589–600. https://doi.org/10.1007/s10646-014-1182-1
Duan, L., Song, J., Liang, X., Yin, M., Yuan, H., Li, X., & Yin, X. (2019). Dynamics and diagenesis of trace metals in sediments of the Changjiang Estuary. Science of the Total Environment, 675, 247–259. https://doi.org/10.1016/j.scitotenv.2019.04.190
Duodu, G. O., Goonetilleke, A., & Ayoko, G. A. (2016). Comparison of pollution indices for the assessment of heavy metal in Brisbane River sediment. Environmental Pollution, 219, 1077–1091. https://doi.org/10.1016/j.envpol.2016.09.008
Ekwutosi, K. O., Chudi, P. A. O., & Charity, E. N. (2020). Chemical speciation and potential mobility of heavy metals in the soils of Onitsha South Local Government Area Anambra Nigeria. American Journal of Applied Chemistry, 8(3), 74–81. https://doi.org/10.11648/j.ajac.20200803.12
El-Magd, S. A. A., Taha, T. H., Pienaar, H. H., Breil, P., Amer, R. A., & Namour, P. H. (2021). Assessing heavy metal pollution hazard in sediments of Lake Mariout, Egypt. Journal of African Earth Sciences, 176, 104116. https://doi.org/10.1016/j.jafrearsci.2021.104116
Forstner, U., & Wittmann, G. T. (1983). Metal pollution in aquatic environment (p. 486). Springer-Verlag, Berlin, Heidelberg, New York.
Ghosh, A. K., Pattnaik, A. K., & Ballatore, T. J. (2006). Chilika Lagoon: Restoring ecological balance and livelihoods through re-salinization. Lakes and Reservoirs: Research and Management, 11(4), 239–255. https://doi.org/10.1111/j.1440-1770.2006.00306.x
Gupta, G. V. M., Sarma, V. V. S. S., Robin, R. S., Raman, A. V., Jai Kumar, M., Rakesh, M., & Subramanian, B. R. (2008). Influence of net ecosystem metabolism in transferring riverine organic carbon to atmospheric CO2 in a tropical coastal lagoon (Chilka Lake, India). Biogeochemistry, 87, 265–285. https://doi.org/10.1007/s10533-008-9183-x
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14(8), 975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Ioannides, K., Stamoulis, K., Papachristodoulou, C., Tziamou, E., Markantonaki, C., & Tsodoulos, I. (2015). Distribution of heavy metals in sediment cores of Lake Pamvotis (Greece): A pollution and potential risk assessment. Environmental Monitoring and Assessment, 187, 1–16. https://doi.org/10.1007/s10661-014-4209-4
Islam, M. S., Proshad, R., & Ahmed, S. (2018). Ecological risk of heavy metals in sediment of an urban river in Bangladesh. Human and Ecological Risk Assessment: An International Journal, 24(3), 699–720. https://doi.org/10.1080/10807039.2017.1397499
Jackson, M. L. (1973). Soil chemical analysis. Prentice Hall of India Private Limited, New Delhi, India.
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60. https://doi.org/10.2478/intox-2014-0009
Kabala, C., & Singh, B. R. (2001). Fractionation and mobility of copper, lead, and zinc in soil profiles in the vicinity of a copper smelter. Journal of Environmental Quality, 30(2), 485–492. https://doi.org/10.2134/jeq2001.302485x
Kabir, M. M., Akter, S., Ahmed, F. T., Mohinuzzaman, M., Didar-ul-Alam, M., Mostofa, K. M., Niloy, N. M., et al. (2021). Salinity-induced fluorescent dissolved organic matter influence co-contamination, quality and risk to human health of tube well water, southeast coastal Bangladesh. Chemosphere, 275, 130053. https://doi.org/10.1016/j.chemosphere.2021.130053
Kamala-Kannan, S., Batvari, B. P. D., Lee, K. J., Kannan, N., Krishnamoorthy, R., Shanthi, K., & Jayaprakash, M. (2008). Assessment of heavy metals (Cd, Cr and Pb) in water, sediment and seaweed (Ulva lactuca) in the Pulicat Lake, South East India. Chemosphere, 71(7), 1233–1240. https://doi.org/10.1016/j.chemosphere.2007.12.004
Kannan, K., Ramu, K., Kajiwara, N., Sinha, R. K., & Tanabe, S. (2005). Organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in Irrawaddy dolphins from India. Archives of Environmental Contamination and Toxicology, 49, 415–420. https://doi.org/10.1007/s00244-005-7078-6
Ke, X., Gui, S., Huang, H., Zhang, H., Wang, C., & Guo, W. (2017). Ecological risk assessment and source identification for heavy metals in surface sediment from the Liaohe River protected area, China. Chemosphere, 175, 473–481. https://doi.org/10.1016/j.chemosphere.2017.02.029
Kluska, M., & Jabłońska, J. (2023). Variability and heavy metal pollution levels in water and bottom sediments of the liwiec and muchawka rivers (Poland). Water, 15(15), 2833. https://doi.org/10.3390/w15152833
Koukina, S. E., & Lobus, N. V. (2020). Relationship between enrichment, toxicity, and chemical bioavailability of heavy metals in sediments of the Cai River estuary. Environmental Monitoring and Assessment, 192(5), 305. https://doi.org/10.1007/s10661-020-08282-6
Kumar, S., Islam, A. R. M. T., Hasanuzzaman, M., Salam, R., Khan, R., & Islam, M. S. (2021). Preliminary assessment of heavy metals in surface water and sediment in Nakuvadra-Rakiraki River, Fiji using indexical and chemometric approaches. Journal of Environmental Management, 298, 113517. https://doi.org/10.1016/j.jenvman.2021.113517
Li, Y. Y., He, W., Liu, W. X., Yang, B., He, Q. S., Yang, C., & Xu, F. L. (2020). Impacts of anthropogenic activities on spatial variations of phthalate esters in water and suspended particulate matter from China’s lakes. Science of the Total Environment, 724, 138281. https://doi.org/10.1016/j.scitotenv.2020.138281
Li, D., Yu, R., Chen, J., Leng, X., Zhao, D., Jia, H., & An, S. (2022). Ecological risk of heavy metals in lake sediments of China: A national-scale integrated analysis. Journal of Cleaner Production, 334, 130206. https://doi.org/10.1016/j.jclepro.2021.130206
Li, X. Y., Li, P., Su, Y. W., Shi, M. M., Hu, T. P., Mao, Y., Qi, S. H., et al. (2022). Pollution and potential ecological risk assessment of heavy metals in surface sediments of Tangxun Lake. Huan Jing ke Xue= Huanjing Kexue, 43(2), 859–866. https://doi.org/10.13227/j.hjkx.202105129
Li, D., Yang, T., Zhou, R., Zhu, Z., & An, S. (2023). Assessment and sources of heavy metals in the suspended particulate matter, sediments and water of a karst lake in Guizhou Province, China. Marine Pollution Bulletin, 189, 114636. https://doi.org/10.1016/j.marpolbul.2023.114636
Liang, G., Zhang, B., Lin, M., Wu, S., Hou, H., Zhang, J., Zhou, J., et al. (2017). Evaluation of heavy metal mobilization in creek sediment: Influence of RAC values and ambient environmental factors. Science of the Total Environment, 607, 1339–1347. https://doi.org/10.1016/j.scitotenv.2017.06.238
Liang, J., Feng, C., Zeng, G., Gao, X., Zhong, M., Li, X., Fang, Y., et al. (2017). Spatial distribution and source identification of heavy metals in surface soils in a typical coal mine city, Lianyuan, China. Environmental Pollution, 225, 681–690. https://doi.org/10.1016/j.envpol.2017.03.057
Luo, W., Lu, Y., Giesy, J. P., Wang, T., Shi, Y., Wang, G., & Xing, Y. (2007). Effects of land use on concentrations of metals in surface soils and ecological risk around Guanting Reservoir, China. Environmental Geochemistry and Health, 29, 459–471. https://doi.org/10.1007/s10653-007-9115-z
Luo, Y., Wu, Y., Shu, J., & Wu, Z. (2019). Effect of particulate organic matter fractions on the distribution of heavy metals with aided phytostabilization at a zinc smelting waste slag site. Environmental Pollution, 253, 330–341. https://doi.org/10.1016/j.envpol.2019.07.015
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31. https://doi.org/10.1007/s002440010075
Masiol, M., Facca, C., Visin, F., Sfriso, A., & Pavoni, B. (2014). Inter annual heavy element and nutrient concentration trends in the top sediments of Venice Lagoon (Italy). Marine Pollution Bulletin, 89(1–2), 49–58. https://doi.org/10.1016/j.marpolbul.2014.10.036
Meng, D., Shao, Y., Luo, M., Liu, Z., Xu, D., & Ma, L. (2023). Risk Assessment and source analysis of soil heavy metal (oid) s pollution in Beijing, China. Water, Air, and Soil Pollution, 234(9), 574. https://doi.org/10.1007/s11270-023-06573-5
Mohanty, B., Muduli, P. R., Cooper, G., Barik, S. K., Mahapatro, D., Behera, A. T., & Pattnaik, A. K. (2017). Sources and variability of petroleum hydrocarbon residues in sediments of Chilika Lagoon, East Coast of India. Bulletin of Environmental Contamination and Toxicology, 99, 100–107. https://doi.org/10.1007/s00128-017-2057-y
Morillo, J., Usero, J., & Gracia, I. (2007). Potential mobility of metals in polluted coastal sediments in two bays of southern Spain. Journal of Coastal Research, 23(2), 352–361. https://doi.org/10.2112/04-0246.1
Muduli, P. R., Kanuri, V. V., Robin, R. S., Kumar, B. C., Patra, S., Raman, A. V., Subramanian, B. R., et al. (2013). Distribution of dissolved inorganic carbon and net ecosystem production in a tropical brackish water lagoon, India. Continental Shelf Research, 64, 75–87. https://doi.org/10.1016/j.csr.2013.05.014
Muller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal, 2, 108–118.
Nazneen, S., & Patel, P. (2016). Distribution and fractionation of heavy metals in surface sediments of Chilika lagoon, east coast of India. Journal of Environmental Science, Toxicology and Food Technology, 10, 63–71. https://doi.org/10.9790/2402-1006036371
Nazneen, S., Singh, S., & Raju, N. J. (2019). Heavy metal fractionation in core sediments and potential biological risk assessment from Chilika lagoon, Odisha state, India. Quaternary International, 507, 370–388. https://doi.org/10.1016/j.quaint.2018.05.011
Nemerow, N. L., & Sumitomo, H. (1970). Part A Pollution Index for Benefit Analysis (pp. 1–38). Benefits of Water Quality Enhancement. University of Syracuse.
Nezhad, M. T. K., Tabatabaii, S. M., & Gholami, A. (2015). Geochemical assessment of steel smelter-impacted urban soils, Ahvaz, Iran. Journal of Geochemical Exploration, 152, 91–109. https://doi.org/10.1016/j.gexplo.2015.02.005
Nijeje, E., Senyonjo, A., Sahan, S. J., Byamugisha, D., & Ntambi, E. (2023). Speciation of selected heavy metals in bottom sediments of River Rwizi, Mbarara City, Uganda. Water, Air, and Soil Pollution, 234(3), 193. https://doi.org/10.1007/s11270-023-06184-0
Nirmala, K., Ramesh, R., Ambujam, N. K., Arumugam, K., & Srinivasalu, S. (2016). Geochemistry of surface sediments of a tropical brackish water lake in South Asia. Environmental Earth Sciences, 75, 247. https://doi.org/10.1007/s12665-015-4964-8
Omwene, P. I., Öncel, M. S., Çelen, M., & Kobya, M. (2018). Heavy metal pollution and spatial distribution in surface sediments of Mustafakemalpaşa stream located in the world’s largest borate basin (Turkey). Chemosphere, 208, 782–792. https://doi.org/10.1016/j.chemosphere.2018.06.031
Panda, D., Subramanian, V., & Panigrahy, R. C. (1995). Geochemical fractionation of heavy metals in Chilka Lake (east coast of India) — a tropical coastal lagoon. Environmental Geology, 26, 199–210. https://doi.org/10.1007/BF00770470
Panda, U. C., Rath, P., Sahu, K. C., Majumdar, S., & Sundaray, S. K. (2006). Study of geochemical association of some trace metals in the sediments of Chilika Lake: A multivariate statistical approach. Environmental Monitoring and Assessment, 123, 125–150. https://doi.org/10.1007/s10661-006-9187-8
Panda, U. C., Rath, P., Bramha, S., & Sahu, K. C. (2010). Application of factor analysis in geochemical speciation of heavy metals in the sediments of a lake system—Chilika (India): A case study. Journal of Coastal Research, 26(5), 860–868. https://doi.org/10.2112/08-1077.1
Panigrahi, S., Wikner, J., Panigrahy, R. C., Satapathy, K. K., & Acharya, B. C. (2009). Variability of nutrients and phytoplankton biomass in a shallow brackish water ecosystem (Chilika Lagoon, India). Limnology, 10, 73–85. https://doi.org/10.1007/s10201-009-0262-z
Perin, G., Craboledda, L., Lucchese, M., Cirillo, R., Dotta, L., Zanette, M. L., & Orio, A. A. (1985). Heavy metal speciation in the sediments of northern Adriatic Sea. A new approach for environmental toxicity determination. Heavy Metals in the Environment, 2(1), 454–456.
Ren, J., Wen, Z., & Lu, G. (2023). Environmental assessment of heavy metals in the typical vegetable field and irrigation sediment in Xijiang River Basin, China: spatial distribution and ecological risk. Water, Air, and Soil Pollution, 234(9), 558. https://doi.org/10.1007/s11270-023-06538-8
Sahu, B. K., Pati, P., & Panigrahy, R. C. (2014). Environmental conditions of Chilika Lake during pre and post hydrological intervention: An overview. Journal of Coastal Conservation, 18, 285–297. https://doi.org/10.1007/s11852-014-0318-z
Sarkar, A., Bhanumathi, L., & Balasubrahmanyan, M. N. (1981). Petrology, geochemistry and geochronology of the Chilka Lake igneous complex, Orissa state, India. Lithos, 14(2), 93–111. https://doi.org/10.1016/0024-4937(81)90048-7
Satapathy, D. R., & Panda, C. R. (2015). Spatio-temporal distribution of major and trace metals in estuarine sediments of Dhamra, Bay of Bengal, India—its environmental significance. Environmental Monitoring and Assessment, 187, 1–31. https://doi.org/10.1007/s10661-014-4133-7
Selvam, A. P., Priya, S. L., Banerjee, K., Hariharan, G., Purvaja, R., & Ramesh, R. (2012). Heavy metal assessment using geochemical and statistical tools in the surface sediments of Vembanad Lake, Southwest Coast of India. Environmental Monitoring and Assessment, 184, 5899–5915. https://doi.org/10.1007/s10661-011-2389-8
Singh, S., Raju, N. J., & Nazneen, S. (2015). Environmental risk of heavy metal pollution and contamination sources using multivariate analysis in the soils of Varanasi environs, India. Environmental Monitoring and Assessment, 187, 1–12. https://doi.org/10.1007/s10661-015-4577-4
Singh, H., Pandey, R., Singh, S. K., & Shukla, D. N. (2017). Assessment of heavy metal contamination in the sediment of the River Ghaghara, a major tributary of the River Ganga in Northern India. Applied Water Science, 7, 4133–4149. https://doi.org/10.1007/s13201-017-0572-y
Smolders, E. & Mertens, J. (2013). Cadmium. In: Heavy Metals in Soils: Trace Metals and Metalloids in Soils and their Bioavailability (Alloway B.J., ed.). 3rd ed. Springer, Dordrecht. pp. 283–311.
Tack, F. M. G., & Verloo, M. G. (1995). Chemical speciation and fractionation in soil and sediment heavy metal analysis: A review. International Journal of Environmental Analytical Chemistry, 59(2–4), 225–238. https://doi.org/10.1080/03067319508041330
Taylor, S. R., & McLennan, S. M. (1995). The geochemical evolution of the continental crust. Reviews of Geophysics, 33(2), 241–265. https://doi.org/10.1029/95RG00262
Tessier, A. P. G. C., Campbell, P. G., & Bisson, M. J. A. C. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851. https://doi.org/10.1021/ac50043a017
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen, 33, 566–575. https://doi.org/10.1007/BF02414780
USEPA. (2001). Methods for collection, storage and manipulation of sediments for chemical and toxicological analyses. Technical Manual, EPA-823-B-01–002, Office of Water, Washington, DC.
Walkley, A., & Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science, 37(1), 29–38.
Wang, F., Dong, W., Zhao, Z., Wang, H., Li, W., Chen, G., Zhou, T., et al. (2021). Heavy metal pollution in urban river sediment of different urban functional areas and its influence on microbial community structure. Science of the Total Environment, 778, 146383. https://doi.org/10.1016/j.scitotenv.2021.146383
Wijesiri, B., Liu, A., Deilami, K., He, B., Hong, N., Yang, B., Goonetilleke, A., et al. (2019). Nutrients and metals interactions between water and sediment phases: An urban river case study. Environmental Pollution, 251, 354–362. https://doi.org/10.1016/j.envpol.2019.05.018
Wu, Q., Hu, W., Wang, H., Liu, P., Wang, X., & Huang, B. (2021). Spatial distribution, ecological risk and sources of heavy metals in soils from a typical economic development area, Southeastern China. Science of the Total Environment, 780, 146557. https://doi.org/10.1016/j.scitotenv.2021.146557
Wu, D., Liu, H., Wu, J., & Gao, X. (2022). Spatial distribution, ecological risk assessment and source analysis of heavy metals pollution in urban lake sediments of Huaihe River Basin. International Journal of Environmental Research and Public Health, 19(22), 14653. https://doi.org/10.3390/ijerph192214653
Xu, Y., Wu, Y., Han, J., & Li, P. (2017). The current status of heavy metal in lake sediments from China: Pollution and ecological risk assessment. Ecology and Evolution, 7(14), 5454–5466. https://doi.org/10.1002/ece3.3124
Xu, J., Chen, Y., Zheng, L., Liu, B., Liu, J., & Wang, X. (2018). Assessment of heavy metal pollution in the sediment of the main tributaries of Dongting Lake, China. Water, 10(8), 1060. https://doi.org/10.3390/w10081060
Xu, M. Y., Zhang, C., Shan, B. Q., & Liu, C. (2022). Speciation and risk of heavy metals in surface sediments of different types of water bodies in Baiyangdian Lake. Huan Jing ke Xue= Huanjing Kexue, 43(9), 4532–4542. https://doi.org/10.13227/j.hjkx.2015.06.018
Yang, H. J., Bong, K. M., Kang, T. W., Hwang, S. H., & Na, E. H. (2021). Assessing heavy metals in surface sediments of the Seomjin River Basin, South Korea, by statistical and geochemical analysis. Chemosphere, 284, 131400. https://doi.org/10.1016/j.chemosphere.2021.131400
Yongo, E., Agembe, S. W., Manyala, J. O., & Mutethya, E. (2023). Assessment of the current trophic state and water quality of Lake Naivasha, Kenya using multivariate techniques. Lakes and Reservoirs: Research and Management, 28(1), e12422. https://doi.org/10.1111/lre.12422
Yue-Fang, C. H. E. N., Qing-Yun, S. H. I., Jin-Yan, Q. U., Meng-Xue, H. E., & Qian, L. I. U. (2022). A pollution risk assessment and source analysis of heavy metals in sediments: A case study of Lake Gehu, China. Chinese Journal of Analytical Chemistry, 50(5), 100077. https://doi.org/10.1016/j.cjac.2022.100077
Zachmann, D. W., Mohanti, M., Treutler, H. C., & Scharf, B. (2009). Assessment of element distribution and heavy metal contamination in Chilika Lake sediments (India). Lakes and Reservoirs: Research and Management, 14(2), 105–125. https://doi.org/10.1111/j.1440-1770.2009.00399.x
Zhang, C., Shan, B., Zhao, Y., Song, Z., & Tang, W. (2018). Spatial distribution, fractionation, toxicity and risk assessment of surface sediments from the Baiyangdian Lake in northern China. Ecological Indicators, 90, 633–642. https://doi.org/10.1016/j.ecolind.2018.03.078
Zhang, Y., Zhang, H., Zhang, Z., Liu, C., Sun, C., Zhang, W., & Marhaba, T. (2018). pH effect on heavy metal release from a polluted sediment. Journal of Chemistry, 2018, 1–7. https://doi.org/10.1155/2018/7597640
Zhang, S., Chen, B., Du, J., Wang, T., Shi, H., & Wang, F. (2022). Distribution, assessment, and source of heavy metals in sediments of the Qinjiang River, China. International Journal of Environmental Research and Public Health, 19(15), 9140. https://doi.org/10.3390/ijerph19159140
Zhao, D., Wan, S., Yu, Z., & Huang, J. (2015). Distribution, enrichment and sources of heavy metals in surface sediments of Hainan Island rivers, China. Environmental Earth Sciences, 74, 5097–5110. https://doi.org/10.1007/s12665-015-4522-4
Zou, X., Li, Y., Wang, L., Ahmed, M. K., Chen, K., Wu, J., Lin, F., et al. (2022). Distribution and assessment of heavy metals in suspended particles in the Sundarban mangrove river, Bangladesh. Marine Pollution Bulletin, 181, 113856. https://doi.org/10.1016/j.marpolbul.2022.113856
Funding
The authors declare that no grants, funds, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panda, D., Ghosh, A.K., Ballesta, R.J. et al. Heavy Metals in the Sediments of the Chilika Lake, India: Contamination, Source and Toxicity Analysis. Water Air Soil Pollut 235, 415 (2024). https://doi.org/10.1007/s11270-024-07226-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07226-x