Abstract
This article presents the result of the geochemical analysis of sediment from Pulicat lake, India. The analysis consists of textural composition, CaCO3, organic carbon, total phosphorus, metals such as Cr, Ni, Cu, Pb, Zn, Cd, Fe and Mn of the sediments collected. Multivariate analysis is performed on the dataset and the interpretations are used as pointers to the origin of the composition of sediment components. Analysis shows a strong intermetal relationship, with lesser correlation with sand or mud which indicates that the metals have a common origin and they were already agglomerated before reaching the lake. Contamination Factor and Geoaccumulation Index demonstrated that the sediments were moderately contaminated with Cd. The Pollution Load Index for the Cd, Cr, Cu and Pb were greater than 1, whereby a Pollution Load Index greater than 1 indicates that the system under study is undergoing progressive deterioration representing Cd>Cu>Pb>Cr>Ni>Mn>Zn. Hierarchy of EF for metals in sediments is Cd > Cu > Pb > Cr > Ni > Mn > Zn with average of 3.6 > 2.1 > 1.8 > 1.4 > 1.0 > 0.8 > 0.7, respectively. Cd concentration varies from a minimum of 0.01–1.5 mg kg−1 with a mean of 0.77 mg kg−1. Cu concentration ranges from 25 mg kg−1 and maximum of 81 mg kg−1 with a mean of 50 mg kg−1. Most of the metals are concentrated in the stations near the eastern part, which lie parallel to the sea and these regions act as hot spots for metal accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tropical brackish water lakes are very dynamic due to the effect of mixing of the tidal waters with fresh water inflow from river. Interactions of these external forces result in a complex sedimentary environment in such lakes. Sediments act as a matrix to carry toxic metals and other elements. The topmost layer of sediment is the most active zone, where microbes decompose organic matter and release simple bioactive nutrients to the upper water column. The top layer of sediment experiences regular influx of liquid, solid and gaseous states of various molecules which determines the quality of water at the bottom level. In this process, the sediment often adsorbs/absorbs various components like CaCO3, organic carbon, metals, etc. (Knoppers et al. 1990; Rajendran et al. 1992). Many of the components are essential for sustaining life, but their excess may lead to the contamination of ecosystem and can cause threat to the health and life of flora and fauna. Quality of lake sediment is a good indicator of pollution in water column, as it accumulates various chemical species introduced by fluvial and marine environments and subsequently release to the water column. Geochemical studies of the estuarine sediment often provide important information about the probable sources of various sediment components. Also, compared to water, the sediment is a more stable system and hence its study helps in assessing the health of the lake and to frame restoration strategies. Present study focuses on the geochemical analysis of sediment samples and health assessment of a tropical brackish water lake.
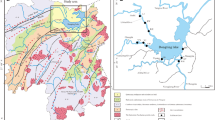
The study area was chosen to be Pulicat lake (13°20′–13°40′N latitude and 80°14′–80°15′E longitude), India’s second largest brackish water (Fig. 1) lake after the Chilika Lake in Odisha. It is located in the southeast coast, around 40 km north of Chennai city. About 84 % of the lake is in Andhra Pradesh and 16 % is in Tamil Nadu. Large spindle-shaped barrier island named Sriharikota separates the lake from the Bay of Bengal. The lake spreads over an area of about 620 km2. Out of the total area of the lake, about 360 km2 of the water body in the southern part is active, northern part is dry and the rest of the lake is now mudflat. Rain-fed rivers Kalangi and Arani are the fresh water sources to the lake. Pulicat lake connects Buckingham canal with its northern part in Andhra Pradesh and its southern part which is in Tamil Nadu (Fig. 1a). Buckingham canal is an artificial coast parallel, British-made navigational channel, which extends from Kakinada of East Godavari district of Andhra Pradesh to Marakkaanam of Tamil Nadu located in north of Pondicherry. The lake is connected to the sea through three tidal inlets, one each at Tupilipalem, Rayadoruvu and Pulicat villages, respectively, from north to south. But at present the inlet at south in Pulicat village is only active. The lake has an average depth of 1.5 m. The southern part of the lake receives substantial amount of domestic sewage, industrial effluent and aquaculture effluents from the neighbouring Chennai city through the Buckingham canal throughout the year. Fishing villages of Pulicat with its touristic attractions of remnants of Dutch dynasty of sixteenth century also exert more anthropogenic pressure to this part of the lake.
Several researchers have studied the Pulicat lake on various aspects like anthropogenic impact on the lake, conservation of biodiversity, pollution of lake water and sediments, etc. Kamala-Kannan and Krishna Moorthy (2006) have done isolation of mercury-resistant bacteria and influence of abiotic factors on bioavailability of mercury. Study stresses that if toxic heavy metals are continuously being released into the Pulicat lake, it will adversely affect the growth rate of mercury-reducing bacteria, thereby affecting the mercury reduction rate and finally lead to complete elimination of such useful microbes. Batwari et al. (2007), in their study of heavy metal Cr, Cd, Zn, Pb and Fe concentrations in four different organs in two fish species Carangoidel malabaricus and Belone stronglurus, found high levels of Fe and Pb in the liver and gills. In a different study of the avian fauna Nanda Kumar et al. (2010) have found there is a decrease in avifaunal and aquafaunal diversity and density indicating ecodegradation of habitats of lake ecosystem. Detailed study of foraminiferal test morphology, diversity and distribution of the lake done by Reddy et al. (2012), explained that Ammonia sp. were found with damaged shells and missing of few channels to the entire whorls due to pollution and siltation of lake. Basha et al. (2012) conducted a biodiversity study of lake’s flora and fauna and demonstrated that the decline in mangrove patches as one of the reason for declining richness and diversity of the lake ecosystem. They found that large amount of toxic metals such as arsenic, cadmium, mercury, lead, manganese, fluorine, beryllium, etc. reach the lake through its tributaries. The above studies indicate that the lake is getting contaminated and silted day by day due to human interference and the system’s status needs to be understood in a more extensive manner so that its productivity can be increased further and it can be conserved in a sustainable manner.
Though several studies have been carried out on various aspects, the importance of the lake as a Ramsar site, as declared by IUCN-WWF and MoEF, India’s designation as an ecosensitive zone along East coast of IndiaFootnote 1 possessing unique ecological qualities compared to other lakes and the present degrading condition inspired to work on the sediment quality of the lake again in detail. The present study has been framed to find out the proportion of total phosphorus, CaCO3, organic carbon, heavy metals such as Fe, Mn and trace metals Cr, Cu, Ni, Zn, Pb and Cd in the sediments of Pulicat lake. Granulometric study of sediments can be helpful to explain the possible association of this chemical species indicating their probable source of origin in the lake. Southern half of the Pulicat lake has been focussed for the study, since the active tidal inlet of the sea is located in this region. This region is the most dynamic zone of the lake with higher water depths. Buckingham canal extended from Marakkanam in Tamil Nadu joins the southern part bringing large volume of chemical effluents from the industrial estates in and around Chennai city. Moreover, fishing and boating activities are found to be higher in this region compared to other parts of the lake. The southern shore of the lake is comparatively more populated and also has several agricultural and aquacultural farms from which domestic discharge and effluent are drained directly to the lake.
Materials and methods
From the lake, 10 surface sediment samples were collected with Peterson grab sampler during summer 2012 (Fig. 1). Stations were fixed with handheld GPS. The sediment samples were collected with Teflon scoops. The surface sediment samples were stored in sealed ziplock envelopes and preserved at −4 °C. At the time of analysis, samples were freed from coarse shell fragments, visible organisms, sea grass leaves and roots prior to drying. For textural analysis, samples were initially washed with 30 % H2O2 to remove organic matter and 1 N HCl to remove carbonate (Van Andel and Postma 1954). This was followed by separation of sand fraction by passing through 230 ASTM sieve and then silt–clay separation and quantification done by pipette method (Carver 1971; Krumbein and Pettijohn 1938).
The organic carbon (OC), calcium carbonate (CaCO3), total phosphorus (TP) in the sediment were estimated as per Jackson (1958), Muller (1967) and Aspila et al.(1976) methods, respectively.
Finely powdered sediment (63 µm) was used for metal analysis (Loring and Rantala 1992) in flame AAS-Perkin Elmer AA 800. Quality and accuracy were ensured by calibrating with International Reference Standards BCSS-I (National Research Council, Canada). The statistical correlation analysis was done using SPSS software version 20.
Geoaccumulation index (GI), enrichment factor (EF) and pollution load index (PLI)
In the present study, Fe is considered as the acceptable normalization element (Blomqvist et al. 1992). Fe is conservative during digenesis (Berner 1980) and its geochemistry is similar to that of many trace metals both in oxic and anoxic environments. Natural concentrations of Fe in sediments are more uniform compared to Al and not under anthropogenic influence. According to Forster and Wittman (1983), in case of Fe, particularly the redox sensitive iron hydroxide and oxide under oxidation condition constitute significant sink of heavy metals in aquatic system. Even a low percentage of Fe(OH)3, in aquatic system, has a controlling influence on heavy metal distribution (Rath et al. 2005). Hence Fe is taken as a normalization element while determining the enrichment ratio ER.
The EF is calculated as per the following equation.
where M x is sediment sample concentration of the heavy metal, Fex is the Fe concentration in the sediment. M b and Feb are their concentration in a suitable back ground or baseline reference material (Salomons and Forstner 1984). GI (I geo) concept was calculated to find out the extent of metal accumulation in sediments. GI is given by (Muller 1979)
where C n is the concentration of element ‘n’. B n is the geochemical back ground value (world surface rock average given by Martin and Meyback (1979). The 1.5 is a factor for possible variation in background data due to lithogenic effect.
PLI was calculated as per Tomlinson et al.(1980). It is given by
where n is the number of metals and contamination factor (CF) is given by CF = C Metal/C background
(C metal is the corresponding metal concentration of the sample and C background metal concentration in the back ground)
Results and discussion
Water temperature recorded an average of 31.7 °C, with a mean pH of 8.48. Average depth of the study region measures as 1 m. Sediment textural composition of the study region dominated with muddy sand. Sand content varied from 15 to 95 % and the percentage of mud varied from 5 to 85 %. Station 4 has highest sand content which is located near the east and the lowest sand percentage is in station 5 (Fig. 1). Lack of strong correlation of mud with heavy metals shows insignificant role played by grain size (Silva et al. 2014).
OC content varies from 0.36 to 2.9 % (1.23 ± 0.9). Station 6 close to the Irrukam Island shows the highest OC. Higher OC in this station may be contributed by the agricultural and domestic activities of the island. The island is spread in 11,659 acres with 750 families engaged in fishing and paddy cultivation. OC reveals high positive correlation with mud (r ~0.9) (Table 1) and negative correlation with sand (r ~−0.9). Strong positive correlation of OC with mud not only exhibits the OC’s strong affinity for finer fractions but also the high rate of sedimentation and burial of organic matter before decomposition. Another source of OC is allochthonous organic matter brought by river Kalangi, which is demonstrated by the strong correlation of mud with OC. Comparatively, lesser OC in stations 4 and 8 may be due to higher rate of sedimentation and burial of organic matter before decomposition.
CaCO3 varies from a minimum of 2.68 to a maximum of 9.61 % (6.81 ± 2.2). CaCO3 reveals strong positive correlation with sand (r ~ −0.9), indicating its association with coarse fraction. Precipitation of CaCO3 by marine organisms could be the major source of carbonate rather than rock weathering. CaCO3, indicates lesser correlation with metals, which indicates that there is lesser probability of metals to be precipitated as calcite. Similarly, lack of strong correlation between CaCO3 with TP indicated lesser chances of complexing and co-precipitation as hydroxyl apatite. The TP concentration showed a wider range with a minimum of 156.3 to a maximum of 999.1 mg kg−1 (497.1 ± 274). TP shows strong positive correlation with Cr, Ni, Cu, Pb, Fe and Mn (r > 0.7).
Fe concentration varies from 2.32 to 6.42 % (4.1 ± 1.5). Highest Fe was found in station 3 followed by station 4 (Fig. 2) and all other stations showed concentration of Fe same as in the crust. The tributaries of the lake may carry weathered Fe, as the catchment areas of the lake has ultramafic rocks (Nellore and Eastern Ghat greenstone belt) which has highest Fe concentration, i.e., 9.6 % compared to basaltic (2.2 %), granitic (1.4–3 %), synetic (3.7 %) and crustal average of 7 %. Fe also shows strong positive correlation in the order of Mn > Cu > Ni > Pb > Zn.
Mn concentration ranges from 0.02 to 0.11 % (0.06 ± 0.03). The average Mn concentration of the study region is found to be lesser than globe average, i.e., 0.1 % (Turekian and Wedepohl 1961), but showed comparatively higher in some stations. On the banks of Kalangi river, there are garbage dumb fields and from which metals like Mn can reach directly and indirectly by leaching and then ultimately reaches Pulicat lake. Mn reveals a strong positive correlation with Cr, Ni, Cu and Pb (r > 0.6) (Table 1). Cr concentration ranges between 33 and 201 mg kg−1 ppm (115 ± 64.5). Station 4, which is on the eastern part of the lake shows higher concentration (Fig. 2). Cr is a low mobile element, and this may be a reason for its high concentration in sediments. Cr, Ni, Cu and Pb may come from many different sources as it is used in metal alloys and pigments for paint, cement, paper, rubber and also from agrochemicals, antifouling agent, sewage and industrial effluent as suggested by Jayaraju (2007). Cr shows strong correlation with Fe (r = 0.95). Ni concentration varies widely between 12 and 123 mg kg−1 (63.57 ± 38.1). Excess Ni concentration is crucial as excess quantity poses toxicological threat (Kim et al. 1998). Cu concentration ranges from 25 to 81 mg kg−1 (49.9 ± 89.2). Stations on the east of the lake (1–4) show comparatively higher Cu concentrations than the other stations with highest concentration in station 4 (Fig. 2). Antifouling paints coated in the fishing boats may act as source for Cu (Schuler et al. 2008; Dhanakumar et al. 2013) in the surface sediments of the Pulicat lake. Concentration of Pb ranges from 12–45 mg kg−1 (31.34 ± 12.1) in the surface sediments of Pulicat lake. Major sources of Pb in the lake includes industrial effluents, sewage, sludge, leachates and run offs from garbage yard located on river banks, pigments, petrol additives, steel products and combustion of fossil fuels. Stations lying parallel to the eastern part of the lake (1–4 along with 7) shows comparatively higher concentration. In summer months, due to closure of tidal inlets, anoxic condition prevails in the bottom of the lake, which results in the formation of free metallic complexes by forming insoluble complexes. This complex enriches the sediment by removing the Pb from the water (Mehotra et al.1991; Chakravarthy and Patgiri 2009). Sundaray et al. (2014) in his findings reports that metal concentrations in the estuarine sediment will be higher than the rivers due to absorption/accumulation of metals on sediment on saline mixing with decreasing concentration towards marine side. In alkaline, pH conditions Pb is insoluble, and there is lesser chance to be dissolved and absorbed by phytoplanktons, but may be absorbed by rooted plants. Though not bioaccumulating, Pb can be concentrated in all body organs (including brain) of animals, which may lead to poisoning (plumbism) or even death. Pb is a toxic metal and Environmental Protection Agency (EPA) has determined that lead is a probable human carcinogen.
Zn concentration varied from 20 to 92 mg kg−1 (55.16 ± 28.5). Paddy cultivation in the Pulicat village and Irukkam island which use agrochemicals containing Zn (Boxall et al. 2000) resulting in increased Zn level in the lake sediments. Zn is present in the pesticides used specifically for sugar cane cultivation, which by leaching or runoff may reach the lake through Kalangi river. Cd concentration varies from a minimum of 0.01–1.5 mg kg−1 (0.77 ± 0.5). Maximum Cd concentration is found in station 9 and 10 followed by 6 and 4, while station 1 has the minimum Cd concentration. Main land near stations 9 and 10 practices paddy cultivation with excessive usage of the agrochemicals such as phosphatic fertilizers; burning of fossil fuel might have contributed to the higher Cd concentration in this region. Similarly, Zhao et al. (2014) also found in southeastern Liaodong Bay in China, there was a drastic increase in the concentration of Pb and Cd from 1990, due to increased fossil fuel burning and agricultural applications. Cd is a non-essential element, which is toxic even at very low concentrations. The lake’s prevailing alkaline conditions may enhance the absorbance of Cd by the sea grass, which is detrimental as it can enhance bioaccumulation in higher trophic levels including human beings. Also, Cd has chemical similarity to Zn and Ca, which are essential elements for plants and animals. Environmental Protection Agency categorised Cd to be a probable human carcinogen and has classified it as a Group B1 carcinogen.Footnote 2 It can be easily bio-accumulated in organisms and Cd at exceptionally higher concentration increases chances of chronic health issues.
Hierarchical clustering
On applying hierarchical clustering for the data sets, five distinct groups were formed (Fig. 3). Cluster 1 consists of mud and organic carbon. Silt and clay being the finer fractions of sediment, with very high surface area, easily accommodate the organic matter. Association of OM with mud indicates the dominance of fluvial—autochthonous influence rather than marine input. Cluster 2 consists only of Cd showing an independent source. Cd comes to this lake through various ways such as from phosphatic fertilizers, sewage sludge and several industrial uses such as Ni–Cd batteries, plating, pigments and plastics as stabilizers in plastics (Prema et al. 2012). Cluster 3 was formed by sand and calcium carbonate. Cluster 4 was formed by Fe, Mn, Cr and TP depicts that the major source of TP is from industries and not from organic decomposition. In steel manufacturing process, Cr and Mn are often introduced to Fe in various proportions in steel industries. Phosphorus is also a major element in steel manufacturing industries, as it is one of the most important elements in strengthening solid solution of ferrite. This indicates a strong common source of Fe, Mn, Cr and TP which is discharged from steel manufacturing/related industries as all of these elements are associated with steel manufacturing processes. The positive trend in Fe and TP association denotes that, in the sediment Fe(III) along with phosphorus forms insoluble ferric phosphate. Association of Pb, Zn and Cu indicates that these elements are from sewage sludge brought from minor channels to the marshy environment (Alloway 1990). Metals such as Cu, Ni, Zn and Pb reveal positive correlation among each other (Rubio et al. 2000; Ramessur and Boodhoo 2013).
Hierarchial cluster analysis was done for different sampling stations from which three groups are formed i.e., 1–10; 5–6; 2–4,7 (Fig. 4). Cluster 1 formed by stations 1 and 8–10, indicating direct and indirect influence of anthropogenic activities of Pulicat village, explained by its proximity to these stations. Though stations 5 and 6 are at the center of the lake still this cluster shows high organic matter which is received from the settlement of Irukkam Island. Further the bottom sediments of 5 and 6 stations are muddy which has the natural ability to concentrate organic matter. The stations 2–4 and 7 lie almost in the central channel of southern part of the lake which shared the trend of higher metal and TP concentrations.
EF, GI, PLI and CF
Hierarchy of EF for metals in sediments is Cd > Cu > Pb > Cr > Ni > Mn > Zn with average of 3.6 > 2.1 > 1.8 > 1.4 > 1.0 > 0.8 > 0.7, respectively (Fig. 5). On applying Sutherland’s (2000) classification in the present study for metal enrichment in sediments, Cd indexed significant enrichment in 30 % of the stations (5 ≤ EF < 20), showing high enrichment, 40 % of the stations show moderate enrichment (2 ≤ EF < 5). The Cu shows moderate enrichment in 50 % of the stations. Similarly, Pb shows moderate enrichment for 40 % of the stations. Cr and Ni show moderate enrichment in 10 % of the sediment of the stations.
All other stations show deficiently to minimal enrichment (EF <2) for the metals analysed. Hierarchy of GI for metals in sediments is Cd > Cu > Pb > Cr > Ni > Fe > Mn > Zn with average 1.16 > 0.8 > 0.71 > 0.58 > 0.42 > 0.4 > 0.32 > 0.26, respectively (Fig. 6). As per the degree of GI class by Muller (1969), the Cd stands in the class of moderately to strongly contaminated (2–3), in station 10 and moderately contaminated in five other stations (4–6 and 8–9). Cr, Cu and Pb also show moderately contaminated in two stations (1–2). The remaining elements from all other stations fall within the category of uncontaminated to moderately contaminated (0–1). Hierarchy of CF for metals in sediments is Cd > Cu > Pb > Cr > Ni > Mn > Zn with average 2.6 > 1.8 > 1.6 > 1.3 > 0.9 > 0.7 > 0.6, respectively. Sediment in five stations (4–6 and 9–10) exhibits higher CF for Cd. Hierarchy of PLI for metals in sediments is Cd > Cu > Pb > Cr > Fe > Ni > Mn > Zn with average 1.93 > 1.67 > 1.43 > 1.07 > 0.83 > 0.77 > 0.62 > 0.5, respectively (Fig. 7). PLI for the Cd, Cr, Cu and Pb are above 1, a PLI above 1 indicates progressive deterioration stage.
Factor loading
Factor loading was applied for the data sets (Fig. 8). Factor loading 1 accounts for 53 % and depicts strong association of Fe, Mn, TP, Cr, Cu and Ni which depicts anthropogenic activities in the coastal region and that Fe/Mn acts as metal scavengers (Förstner and Salomons 1991; Millward and Moore 1982; Wang and Van Cappellen 1996; Jonathan et al. 1990). Factor loading 2 accounts for 31 % with sand, mud, CaCO3 and OC. Factor 3, (Pb, Zn and Cd) has a variance of only 8.28 % of the total variance, which could be derived from excessive amount of agrochemicals such as fertilizers and pesticides which enter into the lake through fluvial system. This is in agreement with the results of Li et al. (2014), Franz et al. (2014), etc., in their studies found that cultivated land has higher levels of heavy metals compared with uncultivated land. Guan et al. (2014) also mentions the distribution and abundance of Pb, Cu, Zn, Ni, Cr is strongly related to the human activities in an area.
Fe shows strong correlation with Mn, Cr, Ni and Pb in cluster analysis and in factor loading. Fe reacts with other heavy and trace metals here and complex molecules are formed. This suggests its origin from chemical effluents discharged by paint industries, steel industries, smelters, petrochemical, fertilizers and sewages (Basha et al. 2012; El Nemr et al. 2006; Laxmi Priya et al. 2011) from Manali, Guindy, Ambattur industrial estates and domestic sewage discharge from several parts in and around the Chennai city through Arani and Buckingham canal. Buckingham canal plays a major role in bringing heavy metal pollution to the southern side of the lake. The same canal has been reported for carrying out pollution to nearshore sediments in Kalpakkam (Deepthi et al. 2014) and Muthupet (Natesan et al. 2014b) mangrove regions. In cluster analysis, Cd forms single group, whereas in factor loading, it groups with Pb and Zn, which indicates there are two distinct sources for Cd, which is again proved by higher accumulation of Cd in two distinct and not nearby stations, explains the possibility of existence of two major sources of Cd. One source is from agriculture lands which use Cd containing pesticides and the other fossil fuel burning in nearby power plant. Fossil fuel contains Cd concentration of 0.5–1.5 mg kg−1 and phosphate fertilizers more than 10 ppm (Cook and Morrow 1995).
Effect of monsoon on sediment accumulation
During monsoon, supernumerary mouths along with the existing tidal inlet of Pulicat village open up leading to the flushing out of sediments into the sea (Sanjeevraj 2006). Annual freshwater flow through rivers and stream during monsoon raises the water level by 1.5 m (Batvari et al. 2007). Here, the tendency for flocculation and coagulation decreases with decreasing salinity. So during monsoon, the heavy metal particularly Fe chelates may have a tendency to get dissolved and flushed out to the sea. Satellite imagery studies done in this region reveals the strong influence of freshwater inflow as a strong regulatory flow over the tidal flow (Natesan et al. 2014a). During monsoon the lake acts as a positive estuary with high freshwater input (Ramesh et al. 2002), this depicts the possibility of dissolution of Fe chelates. Though there is an accumulation of sediment with pollutant load present in the lake, the metal pollution effect of summer season will be nullified due to deflocculation and also by sediment wash out to the sea. Monsoon sediment study can be conducted to find out the fate of the pollutants in the sediment as a future work.
Conclusions
The geochemical analysis carried out in the Pulicat lake indicates that there exists a strong intermetal relationship. Cd concentration varies from a minimum of 0.01–1.5 mg kg−1 with a mean of 0.77 mg kg−1. Cu concentration ranges from a minimum of 25 mg kg−1 and maximum of 81 mg kg−1 with a mean of 50 mg kg−1. Pb concentration varies from a minimum of 12 mg kg−1 and a maximum of 45 mg kg−1 with a mean of 31 mg kg−1. Cr concentration suggests a minimum of 33 mg kg−1 to a maximum of 201 mg kg−1 with an average of 115 mg kg−1. The CF and GI denote that the sediments are moderately contaminated with Cd. The hierarchy of EF for metals in sediments is in the order of Cd > Cu > Pb > Cr > Ni > Mn > Zn showing average of 3.6 > 2.1 > 1.8 > 1.4 > 1.0 > 0.8 > 0.7, respectively. The PLI for the Cd, Cr, Cu and Pb, during summer indicates that the lake ecosystem is progressively deteriorating. However, the freshwater inflow to the lake raises the water level by 1.5 m during southwest monsoon and northeast monsoon in the lake. This changes the system from negative estuary to a positive estuary diluting the metal concentrations and ultimately flushing out the pollutants to the offshore. In the opinion of the authors, one more opening through the barrier would enhance the purity of the lake water.
From the current research, it is revealed that anthropogenic activities result in changing of geomorphology of the lake like closing of tidal inlet due to siltation and geochemical changes such as increase in metal concentration etc. To maintain the equilibrium of the lake environment, the anthropogenic activities in and around the lake should be minimized and well regulated by framing proper management plans and effectively implementing them with the cooperation of local residents and industrialists. Minimization of carcinogenic Cd and Pb has to be done though they are getting diluted during monsoon as they are not favourable to the lake environment. Pulicat lake, the second largest lake in India, possesses multiple ecosystems with several sub ecosystems like island, sand dune, mud flat, sea grass, mangrove, estuary, etc., harboring and nurturing millions of fauna and flora, needs urgent attention for a better future of the global environment.
References
Alloway BJ (1990) Heavy metals in soils. Blackie, London
Aspila I, Agemian H, Chau ASY (1976) Semi-automated method for the determination of inorganic, organic and total phosphate in sediments. Analyst 101:187–197
Basha SKM, Rajya Laksmi E, Ratneswara Rao B, Murthy CVN, Savithramma N (2012) Biodiversity and conservation of Pulicat lake—Andhra pradesh. Int J Geol Earth Environ Sci 2:129–135
Batvari BPD, Kamala-Kannan S, Shanthi K, Krishnamoorthy R, Lee KJ, Jayaprakash M (2007) Heavy metals in two fish species (Carangoidel malabaricus and Belone stronglurus) from Pulicat Lake, North of Chennai, Southeast Coast of India. Environ Monit Assess 145:167–175
Berner RA (1980) Early diagenesis a theoretical approach. Princeton University Press, Princeton
Blomqvist S, Larsson U, Borg H (1992) Heavy metal decrease in the sediments of a Baltic Bay following tertiary sewage treatment. Mar Pollut Bull 24:258–266
Boxall ABA, Comber SD, Conrad AU, Howcroft J, Zaman N (2000) Inputs, monitoring and fate modeling and antifouling biocides in UK estuaries. Mar Pollut Bull 40:898–905
Carver RE (1971) Procedure in sedimentary petrology. Wiley-Intersciences New York, p 637
Chakravarthy M, Patgiri AD (2009) Metal pollution and assessment in sediments of the Dikrong River N.E. India. J Hum Ecol 27:63–67
Cook ME, Morrow H (1995) Anthropogenic Sources of Cadmium in Canada, National Workshop on Cadmium Transport Into Plants. Canadian Network of Toxicology Centres Ottawa Ontario, Canada, pp 20–21
Deepthi K, Natesan U, Muthulakshmi AL, Ferrer VA, Venugopalan VP, Narasimhan SV (2014) Heavy metals in nearshore sediments of Kalpakkam, southeast coast of India. Environ Earth Sci 72(3):717–729
Dhanakumar S, Murthy KR, Solaraj G, Mohanraj R (2013) Heavy-metal fractionation in surface sediments of the Cauvery River Estuarine Region, Southeastern Coast of India. Arch Environ Contam Toxicol 65:14–23
El Nemr A, Khaled A, El Sikaly A (2006) Distribution and statistical analysis of leachable and total heavy metals in the sediments of Suez Gulf. Environ Monit Assess 118:89–112
Forster U, Wittman GTW (1983) Metal pollution in aquatic environment. Springer Verlag, New York
Franz C, Abbt-Braun G, Lorz C, Roig HL, Makeschin F (2014) Assessment and evaluation of metal contents in sediment and water samples within an urban watershed: an analysis of anthropogenic impacts on sediment and water quality in Central Brazil. Environ Earth Sci 72(12):4873–4890
Guan QY, Wang LJ, Wang L, Pan BT, Zhao SL, Zheng Y (2014) Analysis of trace elements (heavy metal based) in the surface soils of a desert-loess transitional zone in the south of the Tengger Desert. Environ Earth Sci 72(8):3015–3023
Jackson ML (1958) Soil chemical analysis. Prentice-Hall Englewood Cliffs N J, p 498
Jayaraju N, Surya Kumar I, Reddy KR (2007) Foraminiferal species densities and Environmental variables of Pulicat Lake, South East coast of India. J Geol Soc India 70:829–836
Jonathan MP, Roy PD, Thangadurai N, Srinivasalu S, Rodríguez-Espinosa PF, Sarkar SK, Knoppers B, Lacerda LD, Patchineelam SR (1990) Nutrients, heavy metals and organic micro pollutants in an Eutrophic Brazilian Lagoon. Mar Poll Bull 21:381–384
Kamala-Kannan S, Krishnamoorthy R (2006) Isolation of mercury resistant bacteria and influence of abiotic factors on bioavailability of mercury—a case study in Pulicat Lake north of Chennai, south east India. Sci of the Total Environ 367:341–363
Kim KW, Myung JH, Ahn JS, Chon HT (1998) Heavy metal contamination in dusts and stream sediments in the Taejon area, Korea. J Geochem Explor 64:409–419
Knoppers B, Lacerda LD, Patchineelam SR (1990) Nutrients, Heavy Metals and Organic Micro pollutants in an Eutrophic Brazilian Lagoon. Mar Pollut Bull 21:381–384
Krumbein WC, Pettijohn FJ (1938) Manual of sedimentary petrography. Appleton-Century, New York
Laxmi Priya S, Senthilkumar B, Hariharan G, Paneer Selvam A, Purvaja R and Ramesh R (2011) Bioaccumulation of heavy metals in mullet (Mugil cephalus) and oyster (Crassostrea madrasensis) from Pulicat lake, south east coast of India. Oxicol Ind Health 27:117–126
Li JG, Pu LJ, Liao QL, Zhu M, Dai XQ, Xu Y, Zhang LF, Hua M, Jin Y (2014) How anthropogenic activities affect soil heavy metal concentration on a broad scale: a geochemistry survey in Yangtze River Delta, Eastern China. Environ Earth Sci 73(4):1823–1835
Loring DH, Rantala RTT (1992) Manual for the geochemical analyses of marine sediments and suspended matter. Earth Sci Rev 32:235–283
Martin JM, Meybeck M (1979) Elemental mass balance of materials carried by major world rivers. Mar Chem 7:173–206
Mehotra MN, Singh SN, Srivastava A, Singh KM (1991) Lead in bank sediments of Ganga and its role in water pollution. Bull Indian Geolo Assoc 24:61–66
Millward GE, Moore RM (1982) The adsorption of Cu, Mn and Zn byiron oxyhydroxides in model estuarine solutions. Water Res 6:981–985
Muller G (1967) Methods in sedimentary petrology. J Sed Petrol Part 1Gebruder Ran2 Dietenheim
Muller G (1969) Index of geoaccumulation in sediments of the Rhine river. GeoJournal 2:108–118
Muller G (1979) Schwermetalle in den sediment des Hiens, Verandrungem Seit, 1971. Umschau 79:778–783
Nanda Kumar NV, Nagarjuna A, Reddy DC (2010) Ecology of pulicat lake and conservation strategies. Bioscan Spec Issue 2:461–478
Natesan U, Rajalakshmi PR, Ferrer VA (2014a) Shorline dynamics and littoral transport around the tidal inlet at Pulicat south east coast of India. Cont Shelf Res 80:49–56
Natesan U, Kumar MM, Deepthi K (2014b) Mangrove sediments a sink for heavy metals? An assessment of Muthupet mangroves of Tamil Nadu, southeast coast of India. Environ Earth Sci 72(4):1255–1270
Prema D, Leelabhai KS, Valsala KK, Nandakumar A, Khambedkar LR, Pillai VK, Krishnakumar PK, Kaladharan P (2012) Database on trace metal levels in the Indian marine ecosystem: decadal trends of lead and cadmium in sediment off Cochin. Central Marine Fisheries Research Institute Kochi Service T&E Ser. No. 213
Rajendran A, Dileep KM, Baker JF (1992) Control of manganese and iron in Skagerrak sediments northeastern North Sea. Chem Geol 98:111–129
Ramesh R, Purvaja R, Ramesh S, James RA (2002) Historical pollution trends in coastal environments of India. Environ Monit Assess 79:151–176
Ramessur RT, Boodhoo K (2013) Trace metal contamination in urban sediments in port Louis, Mauritius. Ecol Environ Res 3:155–160
Rath P, Panda UC, Bhatta D, Sahoo BN (2005) Environmental qualification of heavy metals in the sediments of the Brahmani and Nandira rivers, Orissa. J Geol Soc India 65:487–492
Reddy SR, Jayaraju N, Reddy KR (2012) Anthropogenic impact on the pulicat lagoon monitoring with foraminifera, East coast of India. Mar Sci 2:66–76
Rubio B, Nombela MA, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria deVigo (NW Spain): an assessment of metal pollution. Mar Pollut Bull 40:968–980
Salomons W, Forstner U (1984) Metals in the hydrocycle. Springer, Berlin
Sanjeevraj PJ (2006) Macrofauna of Pulicat lake NBA Scientific Bulletin no.6 Natioanal Biodiversity Authority Chennai, India
Schuler LJ, Hoang TC, Rand GM (2008) Aquatic risk assessment of Cu in fresh water and salt water ecosystems of South Florida. Ecotoxicology 17:642–659
Silva JD, Srinivasalu S, Roy PD, Jonathan MP (2014) Environmental conditions inferred from multi-element concentrations in sediments off Cauvery delta, Southeast India. Environ Earth Sci 71(5):2043–2058
Sundaray SK, Nayak BB, Lee BG, Bhatta D (2014) Spatio-temporal dynamics of heavy metals in sediments of the river estuarine system: Mahanadi basin (India). Environ Earth Sci 71(4):1893–1909
Sutherland RA, Tolosa CA, Tack FMG, Verloo MG (2000) Characterisation of selected element concentration and enrichment ratios in background and anthropogenically impacted roadside areas. Arch Environ Contam Toxicol 38:428–438
Tomilson DC, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in assessment of heavy metals in estuaries and the formation of pollution index. Helgol Meeresuntlers 33:566–575
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Bull Geol Soc Am 72:175–192
Van Andel JH, Postma H (1954) Recent sediments of Gulf of Paria, reports of Orinco shelf Expedition. North Holland Publishing Co Amsterdam 245
Wang Y, Van Cappellen (1996) A multicomponent reactive transport model of early diagenesis: application to redox cycling in coastal marine sediments. Geochem Acta 60(16):2993–3014
Zhao JT, Hu BQ, Li J, Yang J, Bai FL, Dou YG, Yin XB (2014) One hundred-year sedimentary record of heavy metal accumulation in the southeastern Liaodong Bay of China. Environ Earth Sci 71(3):1073–1082
Acknowledgments
One of the authors (NK) is thankful to University Grants Commission (UGC), Delhi, Centre with Potential for Excellence in Environmental Sciences (CPEES), Anna University, Chennai and to Institute for Ocean Management (IOM), Anna University, Chennai for providing funding and other necessary facility for conducting this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
K, N., Ramesh, R., Ambujam, N.K. et al. Geochemistry of surface sediments of a tropical brackish water lake in South Asia. Environ Earth Sci 75, 247 (2016). https://doi.org/10.1007/s12665-015-4964-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-4964-8