Abstract
This study assessed soil pollution in the Varanasi environs of Uttar Pradesh in India. Assessing the concentration of potentially harmful heavy metals in the soils is imperative in order to evaluate the potential risks to human. To identify the concentration and sources of heavy metals and assess the soil environmental quality, 23 samples were collected from different locations covering dumping, road and agricultural area. The average concentrations of the heavy metals were all below the permissible limits according to soil quality guidelines except Cu (copper) and Pb (lead) in dumping and road soils. Soil heavy metal contamination was assessed on the basis of geoaccumulation index (Igeo), pollution index (PI) and integrated pollution index (IPI). The IPI of the metals ranged from 0.59 to 9.94, with the highest IPI observed in the dumping and road soils. A very significant correlation was found between Pb and Cu. The result of principal component analysis suggested that PC1 was mainly affected by the use of agrochemicals, PC2 was affected by vehicular emission and PC3 was affected by dumping waste. Meanwhile, PC4 was mainly controlled by parent material along with anthropogenic activities. Appropriate measures should be taken to minimize the heavy metal levels in soils and thus protect human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil contamination with heavy metals and toxic elements due to parent materials or point sources often occurs in a limited area and can easily be to identify. The use of agrochemicals, fertilizers, liming materials, atmospheric deposition and organic amendments such as sewage sludge and wastewater may cause soil contamination on a large scale (Senesi et al. 1999; Singh and Agrawal 2010). Disposal of municipal solid waste is also a major environmental problem affecting soil quality. As landfills are used to dispose municipal solid waste (MSW) due to lack of proper engineering facilities, these landfills have always been the source of pollution in the soil and water environment (Sharholy et al. 2008). In recent years a great deal of concern has been expressed over problems of soil contamination with heavy metals due to rapid industrialization and urbanization. Heavy metal pollution in agricultural soils and road dusts has become a serious problem during the last two decades (Wei and Yang 2010). Pesticides, fertilizer, sewage sludge, waste water and surface runoff are some of the sources of heavy metal contamination in agricultural soil. The environmental risk of heavy metal pollution in agricultural soils is of great concern. As heavy metals would be accumulated into the crops grown in these soils and eventually get transferred to the human body (Cheng et al. 2011; Liu et al. 2011). Heavy metals can lead to adverse health effects on animals and humans due to their toxicity (Dong et al. 2010; EI Hamiani et al. 2010; Raju 2012). High Mn content in the human body can cause skeletal abnormalities and brain damage (Kalembkiewicz et al. 2008), and Cd exposure can increase the chance of renal tubular damage, osteoporosis and cancer (Järup et al. 1998; Godt et al. 2006).
The contamination of road soil by vehicular emission and dust is a major source of dumping soil contamination. Previous studies have reported Pb and Cu pollution in road dust due to heavy traffic (Wei et al. 2009). Limited information is available on heavy metal pollution of dumping soil for developing countries (Khoshgoftarmanesh and Kalbasi 2002; Sarwar et al. 2008; Rawat et al. 2009; Panahpour et al. 2010; Roghanian et al. 2012). Dumping soil receives varying inputs of heavy metals from several sources such as domestic waste, industrial waste, construction and demolition waste that could be significant source of soil pollution. Therefore, the study of dumping soil is important for determining the origin, distribution and level of heavy metal in soil surface environments. This study is the first report on heavy metal contamination in dumping, road and agricultural soils in the city of Varanasi. The main objective of the study was to assess the environmental risk of heavy metal pollution in the soils and source of contamination using multivariate analysis.
Study area and geology
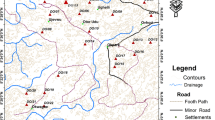
The study area lies between latitude 25° 19.5′ N–25° 14′ 48.5″ N and longitude 83° 0′ E–83° 7.7′ E (Fig. 1) and is located in the middle of Ganga. As per census 2011, Varanasi city has a population of 1,586,821 in an area of 80 km2. Varanasi environs belong to the middle Ganga plain with an average height of about 76.19 m above the mean sea level with even topography. The Ganga is the principal river of Varanasi environs flowing incised into its narrow valley from south to north direction. The study area falls in the subtropical climate region, with three distinct seasons namely summer, rainy and winter. The maximum temperature recorded is 48 and 24 °C and minimum 32 and 8 °C in summer and winter seasons, respectively. The study area receives greater part of annual rainfall through south-west monsoon between June and September. The average annual rainfall of the study area is around 1020 mm.
Geologically, the study area is underlain by the Quaternary alluvial sediments of Pleistocene to recent times. The Quaternary alluvial deposits are divided into older and newer alluviums (Shukla and Raju 2008). In the study area, the older alluvial deposit consists of fairly consolidated clay with kankar and polycyclic sequence of fine to medium sand with little gravel. The thick unit of the older alluvium is developed throughout the study area and is predominated by gray micaceous sand in the west and yellowish concretized clay in the east side. The gray sands consist of clear angular quartz with small amounts of altered alkali feldspar, micas, hornblende, garnet, kyanite, hypersthene, tourmaline and zircon indicating Himalayan sources (Shukla and Raju 2008). The sandy horizons indicate profound fluvial activity in the region, and thick clay horizons indicate the low-energy fluvial phase. Clay found associated with kankar indicates a dry spell of nondepositional phase. The newer alluvium occurs adjacent to the drainage courses of the river Ganga, and the area is subjected to flood during rainy season each year, which deposits a mat of fresh silt, clay and loam. It occurs in mainly narrow belts and is affected by the action of water currents.
Materials and methods
The dumping (sites 1–9), road (sites 10–17) and agricultural (sites 18–23) soil samples (Table 1) were collected from 23 locations with vertical depths of 0–10, 10–20 and 30–60 cm across Varanasi environs. The sampling points are shown in Fig. 1. The exact location (longitudes and latitudes) of each sample points was measured by Garmin Global Positioning System (GPS) (Oregon 600). All soil samples were collected using a hand auger and stored in transparent polyethylene bags. The collected soil samples were air-dried and sieved through a 2-mm polyethylene sieve to remove large debris, stones and pebbles. Soil pH was measured in a 1:5 soil-to-water suspension after stirring for 2 h by glass electrode pH meter (EUTECH instruments) standardized with pH 4, 7 and 9.2 reference buffers. Electrical conductivity (EC) of the soil samples was determined from saturation extract by conductivity electrode (IARI 2011). Organic carbon (OC) in the soil samples was determined by Walkley and Black’s rapid titration method (Allison 1973). Available phosphorous (PO4 3−) in the samples was quantified by the NaHCO3 extraction method given by Olsen and Sommers (1982). Nitrate (NO3 −) and sulphate (SO4 2−) in the soil can be estimated by phenoldisulfonic acid method and barium sulphate precipitation method (IARI 2011). Sulphate, nitrate and phosphate content were analyzed using UV-3200 double-beam spectrophotometer. For analysis of heavy metals in the soil samples, one gram of sample was digested in 20 ml of tri-acid mixture (HNO3/H2SO4/HClO4 5:1:1) for 8 h at 80 °C following the method described by Allen et al. (1986). The resulting solution was filtered, and the filtrate was analyzed for heavy metals. The total metal concentrations were determined by atomic absorption spectrophotometer (M series AAS, Thermo scientific, Cambridge, UK). Calibration was performed every ten sets of samples using prepared internal standards (i.e. standards curve approach). Care was taken in the preparation of analytical standards with high-grade chemicals and pre-acid cleaned glassware. Double-distilled water was used throughout the study. Statistical parameters such as correlation, box whisker plot and principal component analyses were carried out by IBM SPSS Statistics 19.
Methods of heavy metal pollution assessment
A number of calculation methods have been put forward for quantifying the degree of metal pollution in soils (Sun et al. 2010; Wei and Yang 2010; Kong et al. 2011). In this study, geoaccumulation index (Igeo), pollution index (PI) and integrated pollution index (IPI) were used to assess the soil environmental quality.
The pollution levels of heavy metals in soils were assessed by using Igeo introduced by Muller (1969). Igeo enables the assessment of contamination by comparing current and pre-industrial concentration levels; it can also be applied for the assessment of soil contamination levels. Researchers have elsewhere used this index for the heavy metal pollution of urban road dust (Faiz et al. 2009; Lu et al. 2009; Wei et al. 2009; Sun et al. 2010; Wei and Yang 2010; Kong et al. 2011; Yang et al. 2013) and agricultural soils (Bhuiyan et al. 2010; Wei and Yang 2010). Igeo is computed using the following equation (Ji et al. 2008; Muller 1969).
where Cn is the concentration of a given element in the soil tested and Bn is the local natural background value of corresponding element in the earth’s crust. The constant 1.5 allows us to analyze natural fluctuations in the content of a given substance in the environment and to detect very small anthropogenic influences (Ji et al. 2008). According to Muller (1969), the Igeo for each metal is calculated and classified as follows: uncontaminated (Igeo ≤0), uncontaminated to moderately contaminated (0 < Igeo ≤ 1), moderately contaminated (1 < Igeo ≤ 2), moderately to heavily contaminated (2 < Igeo ≤ 3), heavily contaminated (3 < Igeo ≤ 4), heavily to extremely contaminated (4 < Igeo ≤ 5) and extremely contaminated (Igeo ≥ 5).
To further assess the soil quality of the study area, pollution index (PI) of each metal and integrated pollution index (IPI) of the metals were calculated in this study. PI and IPI are also commonly used to assess the environment quality. The PI of each element was calculated and classified as either low (PI ≤ 1), middle (1 < PI ≤ 3) or high (PI >3). The IPI of all measured elements for each sample was defined as the mean value of the element’s PI and was then classified as low (IPI ≤ 1), middle (1 < IPI ≤ 2) or high (IPI > 2) (Chen et al. 2005).
The PI of each element is defined as the ratio of the heavy metal concentration in the soil to the local background soil concentration of the corresponding metal and can be calculated using the following formula (Lu et al. 2009; Wei and Yang 2010).
where Cn and Bn are the measured and background concentrations of element n, respectively, in study area.
Background (control) data
Geochemical baseline maps are not yet available in India though work is initiated under global geochemical baseline programme. Six soil samples were collected and analyzed as a background (control) sample where there were no anthropogenic activities like traffic emission, agricultural practice and dumping influence (Fig. 1). The mean metal concentrations of these samples were taken as local natural background data (Table 1). For the soils, the Igeo, PI and IPI were calculated with respect to local natural background concentrations.
Result and discussion
Soil characteristics
Soil quality determination is important in assessing their nutrient availability and soil environmental quality. Analytical results of physico-chemical characteristics of soil samples are shown in Table 1. pH is a good measure of acidity and alkalinity of soil and provides a good identification of soil chemical nature. The pH values of soil samples ranged between 6.9 and 7.8 with a mean of 7.3. pH of soil samples was slightly alkaline in nature due to presence of basic salts, i.e. Na, Ca, Mg and K salt. Most nutrients can dissolve easily when the pH of the soil solution ranges from 6 to 7.5 (IARI 2011). The EC level of the soil water is a good indication of the amount of available nutrients. The range of EC in soil sample was 0.18 to 1.98 mS/cm with a mean of 0.81 mS/cm. All the major and minor nutrients important for plant growth take the form of either cations or anions. These ions that are dissolved in the soil water carry electrical charge and thus determine the EC level in soil. If the soil EC is too high, it can be indicative of excess nitrogen-based fertilizer or a high level of exchangeable sodium (Raju et al 1991; Raju et al. 2009). Soils with an accumulation of exchangeable sodium are often characterized by poor tilth and low permeability, making them unfavourable for plant growth. High EC contents in dumping soil might be due to higher concentration of dissociated ions leached from the dumping site (Rawat et al. 2009). Conductivity value of less than 0.5 mS/cm is perfectly safe, and it does not have any negative effect on plant growth. High value of EC can be toxic to plants and may prevent them from obtaining water from soil. The OC content of soil samples ranged between 0.21 and 2.37 with a mean value of 1.23. Maximum concentration of organic content was observed in the dumping soils. The main source of organic content in the biodegradable solid wastes is food scraps, lawn waste, cow dung waste, fallen leaves, etc. (Goswami and Sarma 2008). On the basis of available nutrient status in the soils, OC content is generally classified as low (<0.5), medium (0.5–0.75) and high (>0.75) soil nutrient fertility ratings. Out of 23 soil sample, 87 % represented high soil nutrient and remaining 13 % showed low soil nutrient. This might have been due to open dumping of MSW, effective agricultural activities, anthropogenic activities and direct discharge of effluents in the soil of the study area.
Nitrogen is originally fixed from the atmosphere and mineralized by soil bacteria into ammonium. The determination of inorganic N, mainly NH4 + and NO3 − in soil, is often useful, because, despite their usually low levels, these inorganic forms are readily available for plant uptake (Zhang and Bai 2003). The concentration of nitrate in the soil sample ranges between 15 and 331 mg/kg (mean of 88 mg/kg), and maximum nitrate concentration was observed in the agricultural soils. Urea and ammonium nitrate are most commonly used fertilizers contributing nitrates to the soil. N occurs in soils in soluble mineral forms such as ammonium, nitrate and nitrous oxide (gas) and soluble organic compounds such as urea and amino acids form (IARI 2011). Sulphate concentration in soil sample ranged between 40 and 181 mg/kg (mean of 90 mg/kg). The highest SO4 − concentrations were found in agricultural soils due to irrigation water (mostly untreated wastewater). Phosphorous level in soil samples ranged between 41 and 128 mg/kg (mean 81 mg/kg). Phosphorus occurs almost entirely as phosphate, and both organic and inorganic forms are of major importance in plant-soil-water interaction. Plants generally absorb most of their phosphorous as H2PO4 − and smaller amount as HPO4 2− depending on the pH; i.e. lower pH values will increase the absorption of H2PO4 − ion, whereas higher pH values will increase the absorption of HPO4 2− ion (Tisdale 1970). The highest amounts of phosphate and sulphate are found in agricultural soils, and these amounts decrease with increasing the depths of the soil.
Heavy metal content in soil
The average concentrations of Fe, Zn, Cu, Cd, Cr, Pb and Mn in soil collected from dumping, road and agriculture soils are shown in Table 1. Heavy metals of the study area are compared with the Canadian soil environmental quality guidelines (2002) and Indian standard of potential toxic element for agricultural soils (Awashthi 2000) (Table 1). It was observed that majority of the heavy metals fell within the permissible limits except Cu and Pb in dumping and roadside soils. The high Pb and Cu content in roadside soils could be attributed to its proximity to highway. One of the most important activities, which cause high concentrations of Pb and Cu in road soils, is due to atmospheric deposition of toxic fumes and considerable traffic movement. In general, influences between air and soil pollution are mutual. The atmosphere can transfer a large amount of heavy metals into urban soils through precipitation (Patel et al. 2001) while soil dust can also contribute to the concentrations of heavy metals in the air. Consequently, airborne particles and soil dust containing elevated heavy metal concentrations may enter and harm the human body through inhalation and ingestion (Chen et al. 1997). High concentration of Cu and Pb in the dumping soil which may be due to plastics, batteries, leather, paint products, metallic items, fluorescents lamps, wood preservatives and metal scrap was dumped in the solid waste disposal site. It was observed that in all the soil samples, the concentration of Fe, Zn, Cu, Cd, Cr, Pb and Mn showed decreasing trend with depth. The depth of penetration may give some indication of the mobility of heavy metals in soil. The heavy metal concentration in soils in the study area may be significantly influenced by anthropogenic activities.
Heavy metal pollution assessment
Geoaccumulation index
The minimum, maximum and mean values of Igeo for each metal are shown in Table 2. The mean values of Igeo decrease in the order of Pb > Fe > Cu > Zn > Cd > Cr > Mn. The Muller Igeo values are shown in Table 2, and the mean Igeo value for all the metals was lower than zero except for Pb (1.05) and Fe (0.13) which indicated that soil in the study area was uncontaminated with Cu (−0.13), Zn (−0.69), Cd (−1.22), Cr (−1.41) and Mn (−2.11). The highest Igeo value for Fe (1.04), Zn (1.4), Cd (1.8), Cr (0.18) and Mn (1.18) showed that the soils were unpolluted to moderately polluted by these metals. The highest Igeo values for dumping soil (site 3) and roadside soil (sites 17) were 4.16 and 4.2 for Cu and 4.19 and 4.46 for Pb, respectively, which indicated that contamination level of Cu and Pb was higher in dumping and roadside soils. This indicated that the dumping and road soils in the study area were contaminated by the metals mostly derived from anthropogenic sources. The sources of Pb and Cu in roadside soil were mainly derived from high traffic density and were, probably, affected by vehicle fumes. The highest Igeo value for Pb and Cu in dumping soil could possibly be due to solid waste being randomly dumped on open land and along the roads. This kind of disposal introduces the spreading of heavy metals on land. These heavy metals can infiltrate into the soil causing groundwater pollution.
Pollution index
The PIs are shown in Table 3 and varied greatly across the different metals. The mean values of PI decrease in the order of Pb > Cu > Fe > Zn > Cd > Mn > Cr. The mean PIs for Fe (1.76) and Zn (1.3) were higher than 1, which indicated medium pollution with these metals, while the mean PIs for Cd (0.96), Cr (0.66) and Mn (0.75) were less than 1, which indicated low pollution with these metals in soils of Varanasi environs. The PIs of Cu ranging from 0.09 to 27.55 (mean 3.88) and all of the samples had low to high PIs, indicating the Cu pollution in soils of Varanasi environs. The PIs of Pb ranging from 0.22 to 33.12 (6.77) were much higher since all of the dumping soils (sites 1–9) and roadside soils (sites 10–17) contained medium to high PIs. The maximal PIs for dumping soil (site 3) and road soil (site 17) were 26.7 and 27.5 for Cu and 27 and 33 for Pb, respectively. Thus, it is found that dumping and road soils of the study area were highly polluted with Cu and Pb. These results indicated that the Cu and Pb pollution was higher in dumping and roadside soils than in agricultural soils.
The calculated IPI values in the study area ranging from 0.59 to 9.94 with an average of 2.30 are shown in Table 4. Approximately 39 % of all samples had extremely high pollution levels with IPI higher than 2 while 35 % of all samples had low to medium pollution levels with IPI values between 1 and 2. Twenty-six per cent of samples showed the IPI <1, indicating the presence of no pollution or low-level pollution in the study area. In particular, site 3 and site 17 demonstrated an IPI of 8.79 and 9.94, respectively, indicating the presence of serious heavy metal pollution. These sample sites with extremely high pollution levels were located in the areas close to highways and dumping site. Thus, the soil quality of Varanasi environs has clearly been polluted by the heavy metal derived from anthropogenic activities. In the busy road areas, most of the road dust samples were at high levels of pollution, which can be attributed to traffic emission and long-term accumulation of heavy metals. Moreover, the areas close to dumping were also at high levels of pollution due to spreading of dumping materials in the surrounding places. The sources of metals in agricultural soils were mainly influenced by parent materials, sewage irrigation, sewage sludge, pesticide and fertilizer.
Multivariate statistical analysis
Multivariate analysis (correlation analysis, principal component analysis) has been widely used for the interpretation of environmental data (Raju 2006; Verma and Singh 2013; Tiwari and Singh 2014). Correlation analysis was used to identify the relationships between elements. Principal component analysis was performed to identify a common source for elements and distinguish the natural and anthropogenic inputs of element (Garcia et al. 1996; Facchinelli et al. 2001; Lucho-Constantino et al. 2005). All data analyses were performed with IBM SPSS Statistics 19, and the statistical significance level was P < 0.05.
Correlation analysis
The correlation analysis was applied to describe the degree of relation between two variables. The Pearson correlation analysis results for heavy metals and soil characteristics in the soils of study area are shown in Table 5. Most of the heavy metals were positively correlated with each other in concentration, and the correlations between Zn and Cr (P < 0.05), Zn and Pb (P < 0.05) and Cu and Pb (P < 0.01) were significant or very significant. These results suggested that Pb and Cu could be associated with each other and might originate from common sources. Mn and Cd were positively correlated with NO3 −, PO4 3− and SO4 2−, thus showing that all of them have same origin.
Principal component analysis
Principal component analysis (PCA) was applied for the identification of source of pollutants. To reduce the high dimensionality of variable space and better understand the relationships among the analyzed parameters, principal component was applied to the transformed data matrices. It is widely used to reduce data (Loska and Wiechuya 2003) and to extract a small number of latent factors (principal components (PCs)) for analyzing relationships among the observed variables. The number of components to keep was based on the Kaiser normalization, for which only components with eigenvalues greater than unity are retained. Contribution of a component is said to be significant when the corresponding eigenvalue is greater than unity. Four PCs were obtained with eigenvalues greater than unity accounting almost 76 % of total variance in the available dataset (Table 6). Each component is characterized by a few high loadings and many near zero loadings. Maximizing the variance implies maximizing range of loadings, which tends to produce either extreme positive or negative or near zero loadings. The first components explain 26 % of the variance and thus accounts for the majority of the variance in the original dataset. Components 2–4 show less percentage of variance, and each of these three components explains about the variance between 14 and 18 %. Loadings, which represent the importance of the variables for the components, are in italics for values greater than 0.4.
PC1 included Cd, Mn, SO4 2−, NO3 − and PO4 3− and can be defined as an agricultural component due to their high-level presence in agricultural soil samples. High Cd and Mn value can come from agrochemicals that contain them, such as phosphatic fertilizers, organic fertilizers, nitrogen fertilizers and some kinds of pesticides and germicides. Wastewater used to irrigate some agricultural area could also be the source for Mn and Cd. Phosphate fertilizers are an important source of heavy metals entering agricultural soils, especially Cd (Chen et al. 2008). The anthropogenic inputs of Cd into soil may be attributed to commercial activities, application of organic manures and phosphate fertilizer. Other sources of Cd may include other inorganic fertilizers (e.g. nitrogen or potash), atmospheric deposition or anthropic wastes such as wastewater or waste materials (Micó et al. 2006).
PC2 which included Zn, Cu and Pb could be attributed to anthropogenic component due to the presence of high levels in some soils. Zn, Cu and Pb could be related to the deposition of aerosol particles emitted by vehicular traffic and also from building construction, fossil fuel combustion and street dust resuspension (Friedlander 1973; Kowalczyk et al. 1982; DeMiguel et al. 1997; Zheng et al. 2002; Cyrys et al. 2003; Gray et al. 2003). The normal activity and deterioration of vehicles on the roads can emit heavy metals into the air, especially Cu (Ritter and Rinefierd 1983; Martin et al. 1998). Zn is used as a vulcanization agent in vehicle tyres. Cu is often a component in car lubricants, while leaded gasoline is the major source of Pb in the roadside soil. This points out that traffic is most possibly the major source for the enrichment of heavy metals in the soils of Varanasi environs. MSW and leachate also containing high amount of heavy metals were also another source for the heavy metals in the study area.
PC3 included Fe, OC and EC and could also be defined as an anthropogenic component due to their high-level presence in dumping soils. The major anthropogenic source of iron and other iron-containing alloys in dumping soils is steel industry waste, which is dumped in the landfill without prior treatment. Over period of time, the iron seeps into groundwater from landfills with rainwater in monsoon period.
PC4 can be considered to be a lithogenic component, as the variability of the elements seems to be controlled by parent rocks. Cr concentrations in soil samples were comparable with the background values of soils in Varanasi environs, and there was no obvious pollution of Cr in the soils. The parent material present in alluvial soil determines Cr and Zn content. Zn has a lithogenic source as it forms a number of soluble salts (e.g. chlorides, sulphates and nitrates) or insoluble salts (e.g. silicates, carbonates, phosphates, oxides and sulphides) according to the prevailing pedogenic processes (Adriano 2001). In this study, soil concentration of Zn was also found to be correlated with the level of anthropogenic activities. Although the Zn concentration in the study area may be influenced by anthropogenic activities, it appears to be largely related to the parent materials of the soils since Zn was distributed in PC2 and PC4 in the results of PCA.
Conclusions
Soil quality determination is very important for nutrient supply in plant growth. Nearly all the concentrations of the analyzed metals from 23 locations in Varanasi environs were below the permissible values except for the concentration of Cu and Pb in dumping and roadside soils, which were in close to highway. The Igeo values suggested that soils of Varanasi environs were contaminated with Cu and Pb at two locations. All of the dumping and roadside soils had medium to high PIs of Pb, indicating that there was a considerable Pb contamination, which mainly originated from anthropogenic activities. The IPIs of soil sample suggested that the 39 % of all samples had extremely high pollution levels with IPI higher than 2. The PCA results indicated that the Mn and Cd primarily were derived from agrochemicals, such as phosphate fertilizers, organic fertilizers and nitrogen fertilizers. Zn, Cu and Pb were affected by anthropogenic activities such as vehicular emission and MSW while distributions of Zn and Cr were controlled by the parent material. In conclusion, the agricultural soils studied can be considered to be unpolluted as their heavy metal contents were within typical ranges, but the higher value of Cu and Pb in road and dumping soils indicated their polluted status. Such information is necessary for promoting environmental sustainability of soil systems around the city and reducing health risks caused by heavy metal accumulation. Appropriate measures, such as reducing the use of agrochemicals, avoiding wastewater irrigation, proper dumping of waste and preventing atmospheric deposition in soils should be performed to effectively control the heavy metal levels in soils and thus protect human health.
References
Adriano, D. C. (2001). Trace elements in terrestrial environments. Biogeochemistry, bioavailability and risks of metals. New York: Springer-Verlag.
Allen, S. E., Grimshaw, H. M., & Rowland, A. P. (1986). Chemical analysis. In Moore, P. D., & Chapman, S. B. (Eds.), Method in plant ecology (pp. 285–344). Oxford, London: Blackwell Scientific publication.
Allison, F. A. (1973). Soil organic matter and its role in crop production. Amsterdam: Elsevier.
Awashthi, S. K. (2000). Prevention of Food Adulteration Act no 37 of 1954. Central and State rules as amended for 1999 (3rd ed.). New Delhi: Ashoka Law House.
Bhuiyan, M. A. H., Parvez, L., Islam, M. A., Dampare, S. B., & Suzuki, S. (2010). Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. Journal of Hazardous Materials, 173, 384–392. doi:10.1016/j.jhazmat.2009.08.085.
Chen, T. B., Wong, W. J. C., Zhou, H. Y., & Wong, M. H. (1997). Assessment of trace metal distribution and contamination in surface soil of Hong Kong. Environmental Pollution, 96(1), 61–68.
Chen, T. B., Zheng, Y. M., Lei, M., Huang, Z. C., Wu, H. T., Chen, H., Fan, K. K., Yu, K., Wu, X., & Tian, Q. Z. (2005). Assessment of heavy metal pollution in surface soils of urban parks in Beijing, China. Chemosphere, 60(4), 542–551.
Chen, T., Liu, X., Zhu, M., Zhao, K., Wu, J., Xu, J., & Huang, P. (2008). Identification of trace element sources and associated risk assessment in vegetable soils of the urban-rural transitional area of Hangzhou, China. Environmental Pollution, 151(1), 67–78.
Cheng, Z., Lee, L., Dayan, S., Grinshtein, M., & Shaw, R. (2011). Speciation of heavy metals in garden soils: evidences from selective and sequential chemical leaching. Journal of Soils and Sediments, 11, 628–638.
Cyrys, J., Stolzel, M., Heinrich, J., Kreyling, W. G., Menzel, N., Wittmaack, K., Tuch, T., & Wichmann, H. E. (2003). Elemental composition and sources of fine and ultrafine ambient particles in Erfurt, Germany. Science of the Total Environment, 305(1-3), 143–156.
DeMiguel, E., Llamas, J. F., Chacon, E., Berg, T., Larssen, S., Royset, O., & Vadset, M. (1997). Origin and patterns of distribution of trace elements in street dust: unleaded petrol and urban lead. Atmospheric Environment, 31(17), 2733–2740.
Dong, X., Li, C., Li, J., Wang, J., Liu, S., & Ye, B. (2010). A novel approach for soil contamination assessment from heavy metal pollution: a linkage between discharge and adsorption. Journal of Hazardous Materials, 175(1-3), 1022–1030. doi:10.1016/j.jhazmat.2009.10.112.
EI Hamiani, O., EI Khalil, H., Lounate, K., Sirguey, C., Hafidi, M., Bitton, G., Schwartz, C., & Boularbah, A. (2010). Toxicity assessment of garden soils in the vicinity of mining areas in Southern Morocco. Journal of Hazardous Materials, 177(1-3), 755–761. doi:10.1016/j.jhazmat.2009.12.096.
Canadian soil Environmental Quality Guidelines (2002) Summary table for soil quality guidelines.
Facchinelli, A., Sacchi, E., & Mallen, L. (2001). Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environmental Pollution, 114(3), 313–324.
Faiz, Y., Tufail, M., Javed, M. T., Chaudhry, M. M., & Siddique, N. (2009). Road dust pollution of Cd, Cu, Ni, Pb and Zn along Islamabad Expressway, Pakistan. Microchemical Journal, 92, 186–192. doi:10.1016/j.microc.2009.03.009.
Friedlander, S. K. (1973). Chemical element balances and identification of air pollution sources. Environmental Science and Technology, 7(3), 235–240. doi:10.1021/es60075a005.
Garcia, R., Maiz, I., & Millan, E. (1996). Heavy metal contamination analysis of road soils and grasses from Gipuzkoa (Spain). Environmental Technology, 17, 763–770.
Godt, J., Scheidig, F., Grosse-Siestrup, C., Esche, V., Brandenburg, P., Reich, A., & Groneberg, D. A. (2006). The toxicity of cadmium and resulting hazards for human health. Journal of Occupational Medicine and Toxicology, 1, 22.
Goswami, U., & Sarma, H. P. (2008). Study of the impact of municipal solid waste dumping on soil quality in Guwahati City. Pollution Research, 27(2), 327–330.
Gray, C. W., McLaren, R. G., & Roberts, A. H. C. (2003). Atmospheric accessions of heavy metals to some New Zealand pastoral soils. Science of the Total Environment, 305(1-3), 105–115.
IARI (2011) Methods manual soil testing in India, New Delhi.
Järup, L., Berglund, M., Elinder, C. G., Nordberg, G., & Vahter, M. (1998). Health effects of cadmium exposure—a review of the literature and a risk estimate. Scandinavian Journal of Work, Environment and Health, 24, 1–51.
Ji, Y., Feng, Y., Wu, J., Zhu, T., Bai, Z., & Duan, C. (2008). Using geoaccumulation index to study source profiles of soil dust in China. Journal of Environmental Sciences, 20(5), 571–578.
Kalembkiewicz, J., Sitarz-Palczak, E., & Zapała, L. (2008). A study of the chemical forms or species of manganese found in coal fly ash and soil. Microchemical Journal, 90, 37–43. doi:10.1016/j.microc.2008.03.003.
Khoshgoftarmanesh, A. H., & Kalbasi, M. (2002). Effect of municipal waste leachate on soil properties and growth and yield of rice. Communications in Soil Science and Plant Analysis, 33, 13–14. doi:10.1081/ CSS-120005745.
Kong, S. F., Lu, B., Ji, Y. Q., Zhao, X. Y., Chen, L., Li, Z. Y., Han, B., & Bai, Z. P. (2011). Levels, risk assessment and sources of PM10 fraction heavy metals in four types dust from a coal based city. Microchemical Journal, 98, 280–290. doi:10.1016/j.microc.2011.02.012.
Kowalczyk, G. S., Gordon, G. E., & Rheingrover, S. W. (1982). Identification of atmospheric particulate sources in Washington, D.C., using chemical element balances. Environmental Science and Technology, 16, 79–90.
Liu, P., Zhao, H., Wang, L., Liu, Z., Wei, J., Wang, Y., Jiang, L., Dong, L., & Zhang, Y. (2011). Analysis of heavy metal sources for vegetable soils from Shandong Province, China. Agricultural Sciences in China, 10(1), 109–119.
Loska, K., & Wiechuya, D. (2003). Application of principle component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere, 51(8), 723–733.
Lu, X., Wang, L., Lei, K., Huang, J., & Zhai, Y. (2009). Contamination assessment of copper, lead, zinc, manganese and nickel in street dust of Baoji, NW China. Journal of Hazardous Materials, 161(2-3), 1058–1062. doi:10.1016/j.jhazmat.2008.04.052.
Lucho-Constantino, C. A., Alvarez-Suárez, M., Beltrán-Hernández, R. I., Prieto-García, F., & Poggi-Varaldo, H. M. (2005). A multivariate analysis of the accumulation and fractionation of major and trace elements in agricultural soils in Hidalgo State, Mexico irrigated with raw wastewater. Environmental International, 31(3), 313–323.
Martin, A. C., Rivero, V. C., & Marin, M. T. L. (1998). Contamination by heavy metals in soils in the neighborhood of a scrapyard of discarded vehicles. Science of the Total Environment, 212, 145–152.
Micó, C., Recatalá, L., Peris, M., & Sánchez, J. (2006). Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere, 65, 863–872.
Muller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal, 2, 108–118.
Olsen, S. R., & Sommers, L. E. (1982). Phosphorus. In A. L. Page et al. (Eds.), Methods of soil analysis. Part 2 (Agronomy monograph, Vol. 9, pp. 403–430). Madison: ASA and SSSA.
Panahpour, E., Gholami, A., & Beni, Z. H. M. (2010). Effect of leachate on plant nutrition and reduction of environmental pollution. International Journal of Agronomy and Plant Production, 1(2), 68–72.
Patel, K. S., Shukla, A., Tripathi, A. N., & Hoffmann, P. (2001). Heavy metal concentrations of precipitation in east Madhya Pradesh of India. Water, Air, and Soil Pollution, 130, 463–468.
Raju, N. J. (2006). Seasonal evaluation of hydro-geochemical parameters using correlation and regression analysis. Current Science, 91(6), 820–826.
Raju, N. J. (2012). Arsenic exposure through groundwater in the middle Ganga plain in the Varanasi environs, India: a future threat. Journal of Geological Society of India, 79, 302–314.
Raju, N. J., Kotaiah, B., & Reddy, T. V. K. (1991). Biogeochemical aspects in and around a sewage farm at Tirupati, Andhra Pradesh, India. Environmental Conservation, 18(3), 267–269.
Raju, N. J., Ram, P., & Dey, S. (2009). Groundwater quality in the lower Varuna River basin, Varanasi district, Uttar Pradesh, India. Journal of Geological Society of India, 73, 178–192.
Rawat, M., Ramanathan, A. L., & Subramanian, V. (2009). Quantification and distribution of heavy metals from small-scale industrial areas of Kanpur city, India. Journal of Hazardous Materials, 172(2-3), 1145–1149. doi:10.1016/j.jhazmat.2009.07.115.
Ritter, C. J., & Rinefierd, S. M. (1983). Natural background and pollution levels of some heavy metals in soils from the area of Dayton, OH. Environmental Geology, 5, 73–78.
Roghanian, S., Hosseini, H. M., Savaghebi, G., Halajian, L., Jamei, M., & Etesami, H. (2012). Effects of composted municipal waste and its leachate on some soil chemical properties and corn plant responses. International Journal of Agriculture, 2(6), 801–814.
Sarwar, G., Schmeisky, H., Hussain, N., Muhammad, S., Ibrahim, M., & Safdar, E. (2008). Improvement of soil physical and chemical properties with compost application in rice-wheat cropping system. Pakistan Journal of Botany, 40(1), 275–282.
Senesi, G. S., Baldassarre, G., Senesi, N., & Radina, B. (1999). Trace element inputs into soils by anthropogenic activities and implications for human health. Chemosphere, 39(2), 343–377.
Sharholy, M., Ahmad, K., Mahmood, G., & Trivedi, R. C. (2008). Municipal solid waste management in Indian cities – a review. Waste Management, 28, 459–467.
Shukla, U. K., & Raju, N. J. (2008). Migration of Ganga River and its implication on hydro-geological potential of Varanasi area. U. P. Journal of Earth System Sciences, 117(4), 489–498.
Singh, R. P., & Agrawal, M. (2010). Variations in heavy metal accumulation, growth and yield of rice plants grown at different sewage sludge amendment rates. Ecotoxicology and Environmental Safety, 73, 632–641.
Sun, Y. B., Zhou, Q. X., Xie, X. K., & Liu, R. (2010). Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang, China. Journal of Hazardous Materials, 174(1-3), 455–462. doi:10.1016/j.jhazmat.2009.09.074.
Tisdale, S. L. (1970). Soil fertility and fertilizers (2nd ed.). New York: The Macmillan.
Tiwari, A. K., & Singh, A. K. (2014). Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh. Journal of Geological Society of India, 83(3), 329–343.
Verma, A. K., & Singh, T. N. (2013). Prediction of water quality from simple field parameters. Environmental Earth Sciences, 69(3), 821–829.
Wei, B. G., & Yang, L. S. (2010). A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchemical Journal, 94, 99–107.
Wei, B., Jiang, F., Li, X., & Mu, S. (2009). Spatial distribution and contamination assessment of heavy metals in urban road dusts from Urumqi, NW China. Microchemical Journal, 93, 147–152.
Yang, L., Huang, B., Hua, W., Chen, Y., & Mao, M. (2013). Assessment and source identification of trace metals in the soils of greenhouse vegetable production in eastern China. Ecotoxicology and Environmental Safety, 97, 204–209. doi:10.1016/j.ecoenv.2013.08.002.
Zhang, Y., & Bai, S. (2003). Effects of nitrogen forms on nutrient uptake and growth of trees. Ying Yong Sheng Tai Xue Bao, 14(11), 2044–2048.
Zheng, Y. M., Yu, K., Wu, H. T., Huang, Z. C., Chen, H., Wu, X., Tian, Q. Z., Fan, K. K., & Chen, T. B. (2002). Lead concentrations of soils in Beijing urban parks and their pollution assessment. Geographical Research, 21(4), 418–424.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Raju, N.J. & Nazneen, S. Environmental risk of heavy metal pollution and contamination sources using multivariate analysis in the soils of Varanasi environs, India. Environ Monit Assess 187, 345 (2015). https://doi.org/10.1007/s10661-015-4577-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4577-4