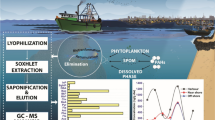
Abstract
Worldwide, studies on polycyclic aromatic hydrocarbons (PAHs) especially focusing on fishing harbours have been found in a limited magnitude only. The characteristics of 16 PAHs and their associated ecological adversities in different environmental compartments of Veraval fishing harbour have been investigated. The concentrations of total PAHs in dissolved, particulate and sediment phases varied from 358.19–42,712 ng L−1, 1450.83–15,291.60 ng g−1 dw and 1263.53–217,560.30 ng g−1 dw, respectively. Two approaches adopted for the source apportionment of PAHs, molecular diagnostic ratio (MDR) method and principal component analysis (PCA), implied the impact of potential anthropogenic PAH sources, including petroleum leakage, pollution from boat repairing, maintenance and burning of coal emissions. The interpretation and integration of indices such as the total toxicity equivalence (TEQcarc), mean risk quotient (M-RQ) and mean effect range-medium quotient (M-ERM-Q) indicated that the harbour areas were under severe threat of ecological risk due to the highest concentration of PAHs, while the offshore region signified a high or moderate level of risk. The present study provides effectual scientific insight into the role of fishing harbours in PAH pollution.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coastal ecosystems undergo significant level of anthropogenic stress greatly from ports, fishing harbours, industries, release of sewage and effluent, tourism activities, etc. globally that eventually lead to the degradation of coastal biodiversity (Yuan et al., 2016; Häder et al., 2020; Gairin et al., 2022). Many places in the west coast of India face the similar situation especially states like Gujarat which covers a coastline area of 1600 km. Gujarat is one of the major fish landing regions in India (MPEDA, 2021), contributing 18.9% of the total marine fish landings of the country (CMFRI, 2021) and has 107 fish landing centres (FLCs) (DoF, 2020). These FLCs are of great importance that they contribute to the economic growth of the country and provide occupations for the local populace. Monitoring the environmental quality of FLCs is important as they can impact the quality of the fish handlings and can affect the health of the people working in these environments. According to the fisheries statistics (Commissioner of fisheries, 2022), 28,355 mechanised boats are operating in the Gujarat state which include trawlers, gill setter, modified bagnet setter and doll net setter. The trawlers and doll net setters operating in the west coast of India use 88- to 118-HP diesel engine (Prajith et al., 2014). The high number of mechanised boats operating in the fishing harbours can make these locations as possible sites for organic pollution, especially with petroleum hydrocarbons (Mora et al., 2022). Emissions like smoke, particulate matter, PAHs and sulphur oxide from these diesel engines cause many environmental problems (Lin et al., 2006: Yang et al., 1998), in which PAHs were tagged as priority pollutants by the United States Environmental Protection Agency (USEPA) and as per International Agency for Research on Cancer (IARC), seven out of which are classified under the potentially carcinogenic pollutants (Zheng et al., 2016).
PAHs are organic compounds having a minimum of two benzene rings with carbon and hydrogen in them (Ravindra et al., 2008). They are considered as a widespread class of environmental pollutants, which are basically toxic in nature and can cause genotoxic, teratogenic and carcinogenic effects in organisms. They can cause potential human health risks which gives them global attention (Duodu et al., 2017; Liang et al., 2017; Wang et al., 2017; Xiang et al., 2018). Several striking epidemiological studies have pointed to the link between cancerous growth on human tissues and PAH exposure (Ali, 2019; Li et al., 2020). PAH contamination is found in various aquatic environmental compartments, including water, suspended particulate matter, sediment and biota. Among these, sediment serves as the primary aquatic repository for PAHs owing to the presence of organic matter. The susceptibility of sediment to re-suspension actions can result in discharge into the water, thereby causing re-pollution. This poses a dual risk, directly impacting benthic organisms and, indirectly, exposing pelagic organisms to the potential adverse effects of PAHs (Liu et al., 2016). Studies on the origin and cycling of PAHs in fishing harbours are important, for pollution management as well as the health improvement of persons associated with the fishing industry.
According to several studies, Veraval Harbour is a highly polluted zone, primarily due to anthropogenic pressure and industrial discharge, particularly with regard to heavy metal accumulation (Majithiya et al., 2018; Sundararajan et al., 2017). According to the Central Pollution Control Board report, Veraval Harbour receives 20,000 m3 and 24,000 m3 wastes discharged from the fish processing industry and municipal sewage system, on a daily basis (CPCB, 2009–2010). The water inside the harbour often remains under anoxic conditions due to the continuous accumulation of such organic and industrial effluents, as well as the hindrances in their proper dilution, together with the formation of NH4+-N and H2S (Majithiya et al., 2018). Despite the abundance of fishing vessels, no research has been done to examine the PAH contamination in this area. Moreover, the distribution of PAHs in water, sediment and suspended particulate matter (SPM) phases holistically in the coastal waters of Indian has not been investigated comprehensively.
Hence, the current study aims to investigate the PAH pollution in Veraval Harbour and its adverse effects on the coastal waters. The main objectives of the present study are (1) to find out the spatial distribution and extent of 16 USEPA priority PAHs in water, sediment and SPM from Veraval Harbour and coastal waters, (2) to find out the source of PAH pollution by using the molecular diagnostic ratios method (MDR) and principal component analysis (PCA) tools and (3) to estimate the potential biological impacts, using indices such as total toxicity equivalence (TEQcarc), mean risk quotient (M-RQ) and mean effect range-median quotient (M-ERM-Q).
2 Materials and Methods
2.1 Study Area and Sample Collections
Veraval Harbour in Gujarat was chosen for the current study due to its significance as one of Asia’s major fishing harbours with a large number of active fishing vessels (Hardikar et al., 2019). Veraval is a port town, situated on the coast of the Arabian Sea, and the chief source of economy for the populace is fishing-related activities. The town has a number of large and small industrial units as well as numerous fish-processing factories that export high-quality seafood globally (Borade et al., 2015; Sundararajan et al., 2017).
Water, SPM and sediments were collected from 17 locations within Veraval Harbour and adjacent coastal areas on March 2019 (Fig. 1). In order to assess and interpret the pollution status in a better way, the study area had been categorized into three zones based on depth at the sampling locations: harbour (S1–S4), the locations consisting of depth less than 10 m as nearshore (S5–S11) and locations within 20–30-m depth, as offshore area (S12—S17). Notably, the offshore stations falls in the active fishing ground. Furthermore, station S17 is situated approximately 10 km away from the harbour area, with a depth exceeding 30 m. The PAH concentration at this location can be compared to that in the harbour area, to assess the extent of PAH pollution in the overall study area. Based on aforementioned considerations, station S17 is deemed as a reference location.
Surface sediment samples (0–5 cm) were collected by van Veen grab sampler and stored in glass containers at − 20 °C. Water samples for studying dissolved and suspended (SPM) forms of PAHs were collected in 1-L amber-coloured glass bottles by using a Niskin water sampler from each sampling location. One litre of sea water collected for SPM was filtered through pre-combusted 0.7-µm glass fibre filter membranes (Whatman GF/F, UK), and filters were kept in aluminium foil. All samples were stored at − 20 °C until extraction.
2.2 Extraction and Analysis
The sediment extraction method was adopted from Singare (2015), with minor modifications. Ten grams of lyophilized (Labconco, USA) sediments was Soxhlet-extracted with dichloromethane for 48 h. The extract was treated with activated copper granules for removing elemental sulphur and concentrated by rotary evaporator (Buchi-R3, Switzerland), re-dissolved in 2 mL n-hexane. Cleanup processes were done by activated 2:1 silica/alumina column and eluted with 20 mL of n-hexane (aliphatic fraction) and 70 mL of 2:1dichloromethane/n-hexane (aromatic fraction). Aromatic fraction was concentrated using rotary evaporator, dried under a stream of nitrogen gas and dissolved in 1 mL n-hexane, kept in − 20 °C before analysis. For SPM extraction, filter paper was lyophilized, weighed and same procedure performed as sediment. Water samples were extracted by solid-phase extraction method by using Strata PAH cartridges (Phenomenex, USA). One hundred millilitres of water sample was passed through preconditioned (with 20 ml dichloromethane-methanol and Milli-Q water) 1.5 g/6 mL SPE cartridge and washed with 5 mL of 1:1 methanol and Milli-Q water, eluted with 6 mL of dichloromethane and dried under a gentile stream of nitrogen and re-dissolved in 1 mL of n-hexane and stored at − 20 °C until analysis.
2.3 GC–MS Analysis
The 16 PAHs such as naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Fle), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fla), pyrene (Pyr), benzo[a] anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), benzo[ghi]perylene (BghiP), dibenzo[a,h]anthracene (DBA) and indeno [1,2,3-cd]pyrene (IND) were determined by using gas chromatography coupled with mass spectrometry (Agilent 7890B-MSD5977B) with HP-5MS Column (30-mm length, 0.25-mm inner diameter, 0.25-µm thickness). Mass-to-charge ratio was identified by selected ion monitoring mode (SIM) with an electron impact ion source (EI) of 70 Ev. The oven temperature was started at 100 °C ramp at 10 °C min−1 to 200 °C (1.5 min), ramp at 25°min−1 to 250 °C (2 min) and then ramp at 5 °C min−1 to a final temperature 300 °C holds at 10 min. Helium was used as carrier gas with a flow rate of 0.8 mL min−1. The ion source and quadruple temperature were 230 °C and 150 °C, respectively.
2.4 Quality Control and Quality Assurance
All analyses were conducted according to the standard quality control procedure including method blank, spiked blank and a sample duplicate which were extracted and analysed with every successive batch. The 5-point calibration method was used for the standardization of the method. The certified reference material (Sigma-Aldrich, CRM-104) used for finding out the extraction efficiency in sediment samples ranged 78–85%, and for water samples, SPE gives 85–92% of total recovery, and suspended sediments showed 73–80% of recovery efficiency for 16 PAHs. The detection limits (LODs) were found to be in a range of 0.01 to 0.3 ng L−1 for water and 0.02 to 1.8 ng g−1 SPM and sediment. Furthermore, the limits of quantification (LOQs) were established at 0.1 to 0.3 ng L−1 for water and 0.2 to 2.1 ng g−1 for SPM and sediment. The measurement uncertainty ranged from 3.85 to 6.46% and 4.13 to 8.22% in solid-phase extraction (water) and Soxhlet extraction methods (sediment), respectively (EURACHEM/CITAC, 2012). GC residue analysis grade solvents were used for all extractions (Sigma-Aldrich, USA). Glassware was muffle furnaced at 350 °C, washed with GC grade organic solvents before use.
2.5 PAH Source Apportionment
Molecular diagnostic ratio (MDR) and principal component analysis (PCA) tools are employed for identifying the sources of PAHs. In MDR analysis, the ratios of PAH isomers play a key role. Specifically, values such as ∑LMW/HMW > 1, Ant/(Ant + Phe) < 0.1, IND/(IND + BghiP) < 0.2 and BaA/(BaA + Chr) < 0.2 are indicative of petrogenic sources according to Magi et al. (2002). Conversely, if Fla/(Fla + Pyr) and IND/(IND + BghiP) exceed 0.5, and BaA/(BaA + Chr) surpasses 0.35, combustion of grass, wood or coal is likely (Li et al., 2006). Ratios falling between 0.4–0.5, 0.2–0.35 and 0.2–0.5 suggest the potential sources as fossil fuel, coal or petroleum combustion, respectively (Wang et al., 2006). PCA serves as a robust tool for source identification, elucidating the total variability within the original data of PAHs. Represented through minimal factors known as principal components, PCA allows for the differentiation of complex characteristics associated with the pollution source (Dudhagara et al., 2016; Zheng et al., 2016). The Kaiser–Meyer–Olkin (KMO) statistic has been employed to affirm the applicability of PCA in the analytical framework (Liu et al., 2009).
2.6 Potential Ecological Risk Assessment
In the present study, different indices such as total toxicity equivalence (TEQcarc), mean risk quotient (M-RQ) and mean effect range-medium quotient (M-ERM-Q) were used to assess the ecological risk of 16 PAHs in sediment, SPM and water from the study area. The total toxicity equivalence (TEQcarc) was determined by the total sum of the multiplied values of individual carcinogenic PAHs (CPAHs) and their corresponding benzo[a]pyrene-based toxic equivalency factors (TEFs). The TEQcarc derivation formula is given in Eq. (1):
where CPAHi is the concentration of carcinogenic PAHs such as bezo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), dibenzo[a,h]anthracene (DBabA) and indeno[1,2,3-cd]pyrene (IP) and 0.1, 0.001, 0.01, 0.01, 1, 1 and 0.1 are the concerned TEF values of the each CPAHs compounds proposed by USEPA (1993).
The mean risk quotient (M-RQ) is used to find out the ecological risk assessed by Eq. (2)
where Ci is the measured value of PAHs in sediment, SPM and water in the study area. CQV is the quality values of concerned PAH compounds, and n is the number of compounds used for deriving RQ. Cao et al. (2010) proposed negligible concentration (NC), which is a hundred times lesser than the maximum permissible concentrations (MPCs), is considered as the CQV in this study. The range of M-RQ values such as < 0.1, 0.1–1.0, 1–10, 10–100 and > 100 is explained as the study area under negligible, low, low to moderate, moderate to high and very high risk, respectively, to the ecological environment (Akhbarizadeh et al., 2016; Wang et al., 2016).
The mean effect range-medium quotient (M-ERM-Q) was estimated by using Eq. (3)
where Csed is the level of PAH concentration in sediment samples, ERMi is the corresponding effect range medium values and n is the total number of PAH compounds which is considered for assessing M-ERM-Q. The M-ERM-Q values are classified according to the level of biological effects; i.e. ≤ 0.10 is considered as no harmful biological effects, 0.11–0.50 indicates possible adverse effects, 0.51–1.50 implies moderate adverse effects and > 1.50 highlights significant adverse biological effects in the sediment compartment (Li et al., 2015).
3 Results and Discussion
3.1 PAHs in Water, SPM and Sediment
The total PAH (∑16PAHs) concentration in water has been found to be in the range of 358.19–42,712.40 ng L−1 (median 4390.08 ng L−1) with the mean concentration of 9712.78 ± 12,184.42 ng L−1 (Fig. 2a, b, c; Table 1). Distribution of ring-wise compounds is as follows: 2–3 rings, the level varies from 314.11 to 3394.2 ng L−1; 4 rings, 8.30–12931.03 ng L−1; and 5–6 rings, 3.85–1290.85 ng L−1. Two to three rings (LMW-PAHs) show high abundance on a range of 51 to 96%, when it comes to the dissolved phase (Fig. 3). Chen (2008) classified the water bodies into four, based on concentration of PAHs, such as low polluted, 10–50 ng L−1; slightly polluted, 50–250 ng L−1; moderately polluted, 250–1000 ng L−1; and highly polluted, ≥ 1000 ng L−1. Depending on this classification, the harbour area, nearshore area and some station in the offshore regions are identified to be highly polluted regions, while stations S16 and S17 which are away from the harbour are found as moderately polluted locations. The low octanol–water partition coefficient, higher water solubility and vapour pressure (Simpson et al., 2005; Sarria-Villa et al., 2016) have been regarded as the responsible factors for the excessive dissolution of low-molecular-weight PAHs (LMW-PAHs) compounds in water.
The ∑16PAH concentration in the particulate phase (SPM) varies over a range of 1450.83–15,291.60 ng g−1 dw (median 6293.98 ng g−1 dw), with the mean value of 6361.72 ± 4166.78 ng g−1 dw. Distribution of ring-wise compounds in SPM showed a variation level of 410.8–2688.92 ng g−1 dw in 2–3-ring compounds; in 4-ring compounds, it is 359.2–4374.86 ng g−1 dw, 5–6-ring compounds, 591.52–8419.40 ng g−1 dw; and 4–6-ring compounds carry utmost abundance in a range of 56–92%. The PAH concentration recorded in SPM (1450.83–15,291.60 ng g−1) as per the present research is more in abundance, in comparison with the PAH concentration (909.9–8222.4 ng g−1) found at the fishing harbour of Tunis (Mzoughi & Chouba, 2011). Data on PAH accumulation in SPM from Pearl estuary and its coastal area (422–1850 ng g−1), Tiber estuary (123.4–1232.5 ng g−1) and Xiamen coast (274.5–2038.9 ng g−1) were also found to be low than Veraval (Luo et al., 2006; Montuori et al., 2016; Tang et al., 2018).
In the sediment phase, the PAH concentration is identified in a range of 1263.53–217,560.30 ng g−1 dw (median 6941.97 ng g−1 dw) with the mean of 23,660.59 ± 52,338.41 ng g−1 dw. As per the sediment PAH level classification of Baumard et al. (1998), 0–100 ng g−1 is low, < 100–1000 ng g−1 dw is high and > 5000 ng g−1 dw is considered to be very high. According to this classification, Veraval Harbour and nearshore regions (except S7 and S11) indicate a very-high-level value, while all the other chosen stations show high level of PAH contamination. The sediments vary: 2–3-ring compounds, 390.80–608,848 ng g−1 dw, 4-ring compounds, 344.74–71,891.97 ng g−1 dw, and 5–6-ring compounds, 527.99–139,579.85 ng g−1 dw, while similar to that of the particulate phase, 4–6-ring compounds show high dominance on a range of 53.9–97.2%. In comparison with LMW compounds, HMW compounds are accumulated in a greater quantity in sediments, because of the high affinity towards the organic matter, as well as the resistance to microbial degradation (Gustafsson et al., 1996; Simpson et al., 2005; Woo et al., 2001).
In summary the total spatial distribution of PAHs in water, SPM and sediments subsides from the harbour to offshore regions. The excessive number of fishing boats, the oil contamination due to boat maintenance activities inside the harbour and the direct discharge of municipal and industrial effluents pollute the harbour region to a greater extent. Moreover, the increased PAH distribution in the harbour is controlled by several factors, especially as a result of the impediment in proper dilution of pollutants, owing to the specialized construction of harbour breakwater system that blocks the wave, tidal and wind action. Nonetheless, in comparison with the near-shore sites, the PAHs’ level in stations 9 and 10, which are situated adjacently to the mouth of the Dewka river, is higher. The observable reasons are the release of industrial and sewage waste into the river and the emission of fly ash from the coal-based thermal power plant, in addition to high boat traffic in the aforementioned zones.
3.2 Comparison of PAH Pollution in Veraval Harbour with Other Coastal Regions in Indian and Fishing Harbours Worldwide
Results of the present study were compared with the PAHs pollution and sources reported in Indian waters and with the PAHs concentration recorded from fishing harbours globally and are presented in Table 2. Comparatively, there is a greater number of research published on the evaluation of PAHs in the sediment matrix of coastal regions of India, than studies in water. Comparison of ∑PAHs concentrations in the water found high ∑PAHs values at Malad-Versova Creek (114,320–347,040 ng L−1) of Mumbai coastal belt (Singare & Shirodkar, 2021a) and Gomti River (600–84200 ng L−1) in the North coastal region of India (Malik et al., 2011) than Veraval Harbour (358.19–42,712.40 ng L−1). Concentration of PAHs in sediments shows locations like Alang-Sosiya, Gulf of Cambay (78,000–719,000 ng g−1), Bhavnagar Coast (5020–981,180 ng g−1) and Gulf of Kutch (118,280–109,9410 ng g−1) in the West coast of India recorded high PAHs than Veraval Harbour (1263.53–217,560.30 ng g−1) (Dudhagara et al., 2016; Rajpara et al., 2017; Reddy et al., 2005).
International studies are scarce on PAH pollution in fishing harbours. PAHs in water at Western Mediterranean harbour (1–60 ng L−1) (Pane et al., 2005) and West coast of the Gulf of Tunis (139.2–1008.3 ng L−1) (Mzoughi & Chouba, 2011) were low. PAH pollutions in sediments of Hsin-ta Harbour (98.1–2048 ng g−1) recorded by Fang et al. (2003), West coast of the Gulf of Tunis (363.3–7026.4 ng g−1) (Mzoughi & Chouba, 2011), Tripoli (243–2965 ng g−1) (Merhaby et al., 2015) and Tema Harbour (2750–5130 ng g−1) by Botwe et al. (2016) were relatively low than Veraval Harbour. The sediments of Auckland Harbour (2000–998,000 ng g−1) (Ahrens & Depree, 2004) and Small Craft Harbour in Nova Scotia (37–336,770 ng g−1) (Davis et al., 2018) were highly polluted. SPM PAH values in Veraval were more or less similar to the West coast of the Gulf of Tunis harbour (909.9–8222.4 ng g−1). It is evident from previous studies that majority of the fishing harbours across the world faces PAH pollution from motor oil, petroleum product leakage and spills (Table 2).
3.3 PAH Source Apportionment
3.3.1 Using Ring Number and MDR Tool
Anthropogenically originated PAH sources can be divided into two: incomplete combustion of petroleum products, coal and biomass (pyrogenic) and the resultant of the direct release of petroleum products (Boonyatumanond et al., 2006). Primary source identification can be carried out based on the concentration of ring-wise distribution of PAHs in environmental samples (Cao et al., 2005; Fang et al., 2003). The composition pattern based on the ring number in the study area is provided in Fig. 3.
In sediments and SPM, 5–6 rings (25–64% and 25–69%, respectively) and 4 rings (19–54% and 23–49%, respectively) dominate. And the distribution of 2–3 rings in the offshore region slightly exceeds that of the harbour region. The high-molecular-weight PAHs (4–6 rings) are usually produced from the high-temperature combustion of petroleum or fossil fuels (Stogiannidis & Laane, 2015). In water, the composition of low-molecular-weight PAHs is found between 51 and 95%. As per the study, in the offshore region, high-molecular-weight PAHs could not be identified; hence, high composition trend of low-molecular-weight PAHs can be seen, which indicates that the source might be of petrogenic origin (Fernandes et al., 1997).
PAHs undergo various changes and transformations due to environmental conditioning, such as photolysis, volatilisation and biodegradation. But this particular method is not always preferable (Wakeham, 1996), since it might lead to several misinterpretations regarding the source apportionment through ring number composition. Therefore, source identification based on isomers that have the same physico-chemical properties can be a more reliable approach (Yunker et al., 2002).
In Fig. 4, the scatter plot represents the distribution of MDR in water, SPM and sediment in the study area. Based on this, BaA/(BaA + Chr) vs Ant/(Ant + Phe), showing coal and petroleum combustions, are found to be the source in all three environmental compartments, except in water samples from the sites S9 and S17. According to Fla/(Fla + Pyr), most of the water and SPM samples from the sites point to the sources of origin as direct petroleum contamination and combustion. Meanwhile, the sources for sediments indicate coal and petroleum combustions. Based on IND/(IND + BghiP) water, SPM and sediment matrices from the sites show petroleum and coal combustions as their origin of source, except in some water samples collected from the stations, such as S5, S8 and S11 that show petrogenic source. The ratio of LMW and HMW shows majority of the sediments and SPM are of pyrogenic origin, while that of water is petrogenic in Veraval.
In conclusion, the resultant MDR ratios indicate a mixed response regarding the sources. Due to that, region-wise-specific source analysis and interpretation seem impossible. So, it can be assumed that the PAH sources in the study area are petroleum combustion, direct petroleum contamination and atmospheric deposition. Meanwhile, PAH inputs from the continuous pyrogenic and petrogenic sources may hinder the process of source identification to an extent (Lubecki & Kowalewska, 2012; Wagener et al., 2010). Under such circumstances, the visible ground-truth information regarding sources based in the study area can be interpreted along with the aforementioned findings (Qi et al., 2019). The activities such as fuelling, boat cleaning, washing, boat maintenance and scrapping works and direct disposal of used cooking oil to harbour waters are mostly centred around the harbour area, and they add to the direct release of engine oil, as well as in the combustion of diesel fuel. The fly ash deposition from a coal-based terminal plant, 2.6 km West of Veraval Harbour, can be considered a strong pyrogenic source, which usually prevents bio- and photo-degradation, when mixed with oil, and may result in contradictory source information (Fang et al., 2007), which may be one among the reasons for the contradictory information regarding source identification.
3.3.2 Principal Component Analysis (PCA)
The PAH loading through varimax rotation factor in water, SPM and sediment is illustrated in Table 3. The Kaiser–Meyer–Olkin (KMO) test had been employed to confirm the adequacy of the test with “df” values such as 66, 120 and 120 were obtained. When 17 samples and 16 PAH components in sediments were used for PCA analysis, 2 principal components were extracted with 96.3% total variance, out of which PC1 showed 93.2% variance, while Nap (2 rings), Fle, Phe and Ant (3 rings) were found to have high loading (values higher than 0.85) and Acy (2 rings), Flu, Pyr, BaA, BbF, BkF, Bap, DBA, BghiP and IND (4–6 rings) were found to be with moderate loading. The 3-ring components such as Fle, Phe and Ant are predominantly produced as a result of coal or biomass combustion (Chen et al., 2005; Zhang et al., 2009; Zheng et al., 2016), and Nap can be considered as a petrogenic origin (Khalili et al., 1995; Dobbins et al., 2006). Meanwhile, the moderate loading of 4–6 rings indicates vehicular emission because of high-temperature combustion of petroleum products as well as coal burning (Harrison et al., 1996; Jiang et al., 2009; Larsen & Baker, 2003). PC2 represents 3.6% variance with the high loading of Ace, which is produced out of the high input of petrogenic sources, such as oil spill or engine oil residue (Dobbins et al., 2006; Marr et al., 1999). Seventeen samples and 16 variables have been used in SPM for PCA analysis, through which two principal components have been extracted with 88.6% total variance, among which PC1 showed 73.3% variance. Nap, Acy and Ace (2 rings) and Fle, Ant and Chr (3 rings) showed heavy loading, and Phe, Fla, Pyr, BaA, BbF, BaP nad IND (4–6 rings) showed moderate loading. Two-ring compounds have been made as a result of the spill or volatilization of petroleum or petroleum products (Larsen & Baker, 2003; Marr et al., 1999; Wang et al., 2007) Meanwhile, Fle, Ant and Chr are produced due to the combustion of coal or natural gas (Khuman et al., 2018; Sofowote et al., 2008). Since DBA and BghiP show high loading with 13.6% variance, it indicates that they are emitted because of the combustion of gasoline or diesel engine (Harrison et al., 1996; Larsen & Baker, 2003; Wang et al., 2007).
Compounds like BkF, DBA, BghiP and IND were not detected in water samples collected from offshore locations. Therefore, these are omitted so as to conduct PCA analysis, making use of 17 samples with 12 PAH compounds and three principal components which have been extracted with 82.5% total variance. In PC1, 73% variance could be seen, and Acy, Ace (2 rings), and Ant (3 rings) showed high loading, and Phe, BaA, Chr and BaP showed moderate loading, Acy and Ace are the products of typical petroleum sources, and Phe, BaA, Chr and BaP are produced out of coal combustion sources (Duval & Friedlander, 1981). In PC2, with 11.5% variance, Fle could be detected as high loading and Nap and Bbf as moderate loading, among which Fle and BbF are prominently produced through the combustion of coal and petroleum products (Sofowote et al., 2008). In PC3, with 8.4% variance, Fla and Pyr are identified with high loading which are considered as the products of coal combustion (Harrison et al., 1996; Stogiannidis & Laane, 2015). Overall, in the study area, petrogenic, combustion of coal and petroleum products have been identified to be the sources of PAH origin. High boat traffic and maintenance works are regarded as the causes for the direct release of diesel oil. The PAHs produced as part of oil combustion are comparatively moderate and are generally found in the sediment, SPM and water samples, as per the study. When MDR and PCA models were considered for source identification, unstable results were found from the present study area which leads to the assumption that the continuous input of HMW and LMW PAHs are responsible for such visible results.
Comparison of PAH sources at Veraval and other coastal regions of India reveals that the major reasons for PAH pollution identified as atmospheric deposition, vehicular emissions, incomplete combustion of wood, coal-fired thermal power plants, grass and fossil fuels, oil and petroleum leakages, etc., and in Veraval, the major PAH sources are petroleum spills and atmospheric deposition.
3.4 Potential Ecological Risk Assessment
The estimated values of total toxicity equivalence (TEQcarc), mean risk quotient (M-RQ) and mean effect range-medium quotient (M-ERM-Q) in the study area are depicted in Table 4. M-ERM-Q value varies between 0.09 (S17) and 9.95 (S1). Sites such as S1, S2 and S3 (harbour area) are under significantly adverse effect on organisms; S4, S9 and S10 showed moderate adverse effect; and the rest of the sites from near- to offshore stations are facing possible adverse effects except S17, which has no harmful biological effect (M-ERM-Q ≤ 0.10). The M-RQ values have been the trend of moderate to high risk with the range of 24.66–262.10 (avg. 313.37) in sediment, 28.51–221.53 (avg. 100.73) in SPM and 22.57–3518.69 (avg. 622.18) in water compartments of the study area. High-risk levels (M-RQ ≥ 100) are evident in the majority of sampling locations for both sediment and SPM. Specifically, stations S7 and S11 within the sediment matrix, along with few SPM locations (S6, S8, S11), and the remaining offshore locations, are classified under the moderate risk level category (M-RQ = 10–100). The dissolved phase indicates high risk levels for stations S1 to S14, while the remaining three stations are characterized by a moderate risk status.
The TEQcarc of seven carcinogenic PAHs in the sediment, SPM and water ranged 0.17–57.78 ng TEQ/g dw, 0.19–3.93 µg TEQ/g dw and 0.0018–0.57 ng TEQ/L, respectively, in the study area. Based on Canadian soil quality guidelines, the level of TEQcarc more than 0.6 ng TEQ/g is considered the threshold level, and it may cause a serious impact on the food web as well as on human health. In the present study, sediments from the harbour and near-shore locations except S7 and S11 are above the threshold value. The ratio of TEQcarc and NC value (TEQQV) which correspond to benzo[a]pyrene in sediment and SPM is 0.027 and 0.0005 for water, considered as the convenient indices tool for risk assessment. According to TEQcarc/TEQQV, values < 0.1 indicate negligible risk; 0.1–1.0 indicate low risk; 1–10 indicate low to moderate risk; 10–100 indicate moderate to high risk; and ≥ 100 represent the high risk. Based on this classification, harbour areas are under high risk, the near-shore is moderate to high and the offshore region showing low to moderate risk in all three environmental compartments.
The integration and interpretation of ecological risk assessment values of different indices from various environmental compartments could be a substantial tool for the comprehensive understanding of the risk status of the study area. The general trend of ecological risk status in the Veraval coastal area, determined through the cumulative interpretation of M-ERM-Q, M-RQ, and TEQcarc values across various environmental compartments, indicates a high level of risk at harbour, and high to moderate risk at near shore to offshore areas. Majority of cancerous PAHs are not detected in the dissolved phase of offshore region causing less ecological risk. Migratory birds spotted during the sampling time within the harbour area consume fish waste generated mainly from boat and auction areas, as part of their dietary needs. Fisherman and local boat workers are exposed to dermal contact, from contaminated water and sediments during their occupational activities, which makes them prone to umpteen health issues. Hence, the ecological risks in harbour regions are at a heightened level, and the concentration of cancerous PAHs is in excessive quantity in water, sediments and SPM, which can act as a serious threat to those workers.
4 Conclusion
PAH pollution in fishing harbours has not been yet explored in the Indian sub-continent. The current study is the first holistic study on the concentration of 16 PAHs in the dissolved, particulate and sediment phases from a major fishing harbour, Veraval. Also, the various sources of PAH contamination were investigated by application of several statistical indices. Results indicated mixed source which was probably due to the continuous loading of petrogenic and pyrogenic pollutants from various routes, such as high boat traffic, combustion of coal and municipal and industrial sewage wastes.
Lighter molecular weight PAHs (2–3 rings) were abundant in water, whereas 4–6 ring fractions were predominant in the sediment and SPM of the Veraval Harbour and nearshore region. The harbour regions were contaminated with PAHs of petroleum origin which could be attributed to direct spill of petroleum products, the high number of fishing craft operations and atmospheric deposition of PAHs from coal and biomass combustions. Suspended sediments also acted as a medium for the disposal of PAHs in the harbour. Source identification of PAHs in sediments at Veraval Harbour was difficult because of influencing factors such as currents, tides and breakwater structure.
Potential ecological risk assessment indicated that the harbour area was at high risk from PAH pollution which may have significant adverse effects on organisms. The PAH pollution and associated potential risks on the coastal ecosystem signified that the harbour areas were under high threat due to the high concentration of cancerous PAHs, which can adversely affect the balance of food web, finally adding to umpteen health issues to humans. The study concludes that the Veraval Harbour and coastal waters require more temporal studies of POPs and other emerging contaminants in different environmental compartments and their effects especially on marine biota. That will give basic insight for policymakers to take appropriate actions on pollution remediation and facilitate sustainable management of fishery harbours.
Data availability
The data used and/or analysed during the current study are available from the corresponding author upon request.
References
Ahrens, M. J., & Depree, C. V. (2004). Inhomogeneous distribution of polycyclic aromatic hydrocarbons in different size and density fractions of contaminated sediment from Auckland Harbour, New Zealand: An opportunity for mitigation. Marine Pollution Bulletin, 48(3–4), 341–350.
Akhbarizadeh, R., Moore, F., Keshavarzi, B., & Moeinpour, A. (2016). Aliphatic and polycyclic aromatic hydrocarbons risk assessment in coastal water and sediments of Khark Island. SW Iran. Marine Pollution Bulletin, 108(1–2), 33–45.
Ali, N. (2019). Polycyclic aromatic hydrocarbons (PAHs) in indoor air and dust samples of different Saudi microenvironments; health and carcinogenic risk assessment for the general population. Science of the Total Environment, 696, 133995.
Ambade, B., Sethi, S. S., Kurwadkar, S., Kumar, A., & Sankar, T. K. (2021). Toxicity and health risk assessment of polycyclic aromatic hydrocarbons in surface water, sediments and groundwater vulnerability in Damodar River Basin. Groundwater for Sustainable Development, 13, 100553.
Ambade, B., Sethi, S. S., Kumar, A., & Sankar, T. K. (2022a). Solvent extraction coupled with gas chromatography for the analysis of polycyclic aromatic hydrocarbons in riverine sediment and surface water of Subarnarekha River and its tributary, India. Miniaturized Analytical Devices: Materials and Technology, 71–89.
Ambade, B., Sethi, S. S., Kurwadkar, S., Mishra, P., & Tripathee, L. (2022b). Accumulation of polycyclic aromatic hydrocarbons (PAHs) in surface sediment residues of Mahanadi River Estuary: Abundance, source, and risk assessment. Marine Pollution Bulletin, 183, 114073.
Bao, K., Zaccone, C., Tao, Y., Wang, J., Shen, J., & Zhang, Y. (2020). Source apportionment of priority PAHs in 11 lake sediment cores from Songnen Plain. Northeast China. Water Research, 168, 115158.
Balu, S., Bhunia, S., Gachhui, R., & Mukherjee, J. (2020). Assessment of polycyclic aromatic hydrocarbon contamination in the Sundarbans, the world’s largest tidal mangrove forest and indigenous microbial mixed biofilm-based removal of the contaminants. Environmental Pollution, 266, 115270.
Basavaiah, N., Mohite, R. D., Singare, P. U., Reddy, A. V. R., Singhal, R. K., & Blaha, U. (2017). Vertical distribution, composition profiles, sources and toxicity assessment of PAH residues in the reclaimed mudflat sediments from the adjacent Thane Creek of Mumbai. Marine Pollution Bulletin, 118(1–2), 112–124.
Baumard, P., Budzinski, H., & Garrigues, P. (1998). Polycyclic aromatic hydrocarbons in sediments and mussels of the western Mediterranean Sea. Environmental Toxicology and Chemistry: An International Journal, 17(5), 765–776.
Boonyatumanond, R., Wattayakorn, G., Togo, A., & Takada, H. (2006). Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine, and marine sediments in Thailand. Marine Pollution Bulletin, 52(8), 942–956.
Borade, S., Dhawde, R., Maloo, A., Gajbhiye, S. N., Ram, A., & Dastager, S. G. (2015). Assessment of enteric bacterial indicators and correlation with physico-chemical parameters in Veraval coast India. Indian Journal of Geo-Marine Sciences, 44(4), 501–507.
Botwe, B. O., Kelderman, P., Nyarko, E., & Lens, P. N. (2017). Assessment of DDT, HCH and PAH contamination and associated ecotoxicological risks in surface sediments of coastal Tema Harbour (Ghana). Marine Pollution Bulletin, 115(1–2), 480–488.
Cao, Z., Liu, J., Luan, Y., Li, Y., Ma, M., Xu, J., & Han, S. (2010). Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons in the Luan River China. Ecotoxicology, 19(5), 827–837.
Cao, Z., Wang, Y., Ma, Y., Xu, Z., Shi, G., Zhuang, Y., & Zhu, T. (2005). Occurrence and distribution of polycyclic aromatic hydrocarbons in reclaimed water and surface water of Tianjin China. Journal of Hazardous Materials, 122(1–2), 51–59.
Chen, Y., Sheng, G., Bi, X., Feng, Y., Mai, B., & Fu, J. (2005). Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China. Environmental Science & Technology, 39(6), 1861–1867.
Chen, Y. Y. (2008). The spatial and temporal distribution, source and bioavailability of PAHs in Qiantang River (pp. 15–16). Zhejiang University, Hangzhou.
CMFRI, K. (2021). CMFRI Annual Report 2020 केंद्रीय समुद्री मात्स्यिकी अनुसंधान संस्थान वार्षिक प्रतिवेदन 2020.
CPCB, 2009–2010. Status of water supply, wastewater generation and treatment in class I cities and class II towns of India. In: Control of Urban Pollution Series: CUPS/70/2009–10.
Davis, E., Walker, T. R., Adams, M., & Willis, R. (2018). Characterization of polycyclic aromatic hydrocarbons (PAHs) in small craft harbour (SCH) sediments in Nova Scotia, Canada. Marine Pollution Bulletin, 137, 285–294.
Department of Fisheries (2020). Handbook of Fisheries Statistics 2020. Ministry of Fisheries, Animal Husbandry and Dairying, Government of India. https://dof.gov.in/sites/default/files/2021-02/Final_Book.pdf
Department of Fisheries (2022). Handbook of Fisheries Statistics 2022. Ministry of Fisheries, Animal Husbandry and Dairying, Government of India. https://dof.gov.in/sites/default/files/2023-01/HandbookFisheriesStatistics19012023.pdf
Dhananjayan, V., S. Muralidharan, & Vinny R. Peter (2012). Occurrence and distribution of polycyclic aromatic hydrocarbons in water and sediment collected along the Harbour Line, Mumbai, India. International Journal of Oceanography 2012.
Dobbins, R. A., Fletcher, R. A., Benner, B. A., Jr., & Hoeft, S. (2006). Polycyclic aromatic hydrocarbons in flames, in diesel fuels, and in diesel emissions. Combustion and Flame, 144(4), 773–781.
Domínguez, C., Sarkar, S. K., Bhattacharya, A., Chatterjee, M., Bhattacharya, B. D., Jover, E., & Satpathy, K. K. (2010). Quantification and source identification of polycyclic aromatic hydrocarbons in core sediments from Sundarban Mangrove Wetland, India. Archives of Environmental Contamination and Toxicology, 59, 49–61.
Dudhagara, D. R., Rajpara, R. K., Bhatt, J. K., Gosai, H. B., Sachaniya, B. K., & Dave, B. P. (2016). Distribution, sources and ecological risk assessment of PAHs in historically contaminated surface sediments at Bhavnagar coast, Gujarat, India. Environmental Pollution, 213, 338–346.
Duodu, G. O., Ogogo, K. N., Mummullage, S., Harden, F., Goonetilleke, A., & Ayoko, G. A. (2017). Source apportionment and risk assessment of PAHs in Brisbane River sediment, Australia. Ecological Indicators, 73, 784–799.
Duval, M. M., & Friedlander, S. K. (1981). Source resolution of polycyclic aromatic hydrocarbons in the Los Angeles atmosphere: Application of a chemical species balance method with first order chemical decay. Final report Jan-Dec 80 (No. PB-82–121336). California Univ., Los Angeles (USA). Dept. of Chemical, Nuclear, and Thermal Engineering.
Ellison, S. L. R., Rosslein, M., & Williams, A. (2012). EURACHEM/CITAC, quantifying uncertainty in analytical measurement. LGC, Teddington. OpenURL.
Fang, M. D., Hsieh, P. C., Ko, F. C., Baker, J. E., & Lee, C. L. (2007). Sources and distribution of polycyclic aromatic hydrocarbons in the sediments of Kaoping river and submarine canyon system Taiwan. Marine Pollution Bulletin, 54(8), 1179–1189.
Fang, M. D., Lee, C. L., & Yu, C. S. (2003). Distribution and source recognition of polycyclic aromatic hydrocarbons in the sediments of Hsin-ta Harbour and adjacent coastal areas Taiwan. Marine Pollution Bulletin, 46(8), 941–953.
Fernandes, M. B., Sicre, M. A., Boireau, A., & Tronczynski, J. (1997). Polyaromatic hydrocarbon (PAH) distributions in the Seine River and its estuary. Marine Pollution Bulletin, 34(11), 857–867.
Fisheries statistics report (2022). Commissioner of Fisheries, Agriculture, Farmers Welfare and Co-operation Department, Government of Gujarat. https://cof.gujarat.gov.in/Images/commissioneroffisheries/pdf/static-information-14-12-2022.pdf
Gairin, E., Dussenne, M., Mercader, M., Berthe, C., Reynaud, M., Metian, M., ... & Lecchini, D. (2022). Harbours as unique environmental sites of multiple anthropogenic stresses on fish hormonal systems. Molecular and Cellular Endocrinology, 111727.
Gosai, H. B., Sachaniya, B. K., Dudhagara, D. R., Rajpara, R. K., & Dave, B. P. (2018). Concentrations, input prediction and probabilistic biological risk assessment of polycyclic aromatic hydrocarbons (PAHs) along Gujarat coastline. Environmental Geochemistry and Health, 40, 653–665.
Goswami, P., Ohura, T., Guruge, K. S., Yoshioka, M., Yamanaka, N., Akiba, M., & Munuswamy, N. (2016). Spatio-temporal distribution, source, and genotoxic potential of polycyclic aromatic hydrocarbons in estuarine and riverine sediments from southern India. Ecotoxicology and Environmental Safety, 130, 113–123.
Gustafsson, Ö., Haghseta, F., Chan, C., MacFarlane, J., & Gschwend, P. M. (1996). Quantification of the dilute sedimentary soot phase: Implications for PAH speciation and bioavailability. Environmental Science & Technology, 31(1), 203–209.
Guzzella, L., Roscioli, C., Vigano, L., Saha, M., Sarkar, S. K., & Bhattacharya, A. (2005). Evaluation of the concentration of HCH, DDT, HCB, PCB and PAH in the sediments along the lower stretch of Hugli estuary, West Bengal, northeast India. Environment International, 31(4), 523–534.
Häder, D. P., Banaszak, A. T., Villafañe, V. E., Narvarte, M. A., González, R. A., & Helbling, E. W. (2020). Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Science of the Total Environment, 713, 136586.
Hardikar, R., Haridevi, C. K., Ram, A., Khandeparker, R., Amberkar, U., & Chauhan, M. (2019). Inter-annual variability of phytoplankton assemblage and Tetraspora gelatinosa bloom from anthropogenically affected harbour, Veraval, India. Environmental Monitoring and Assessment, 191, 1–17.
Harrison, R. M., Smith, D. J. T., & Luhana, L. (1996). Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham UK. Environmental Science & Technology, 30(3), 825–832.
Hong, W. J., Jia, H., Li, Y. F., Sun, Y., Liu, X., & Wang, L. (2016). Polycyclic aromatic hydrocarbons (PAHs) and alkylated PAHs in the coastal seawater, surface sediment and oyster from Dalian, Northeast China. Ecotoxicology and Environmental Safety, 128, 11–20.
Jiang, J. J., Lee, C. L., Fang, M. D., & Liu, J. T. (2009). Polycyclic aromatic hydrocarbons in coastal sediments of southwest Taiwan: An appraisal of diagnostic ratios in source recognition. Marine Pollution Bulletin, 58(5), 752–760.
Khalili, N. R., Scheff, P. A., & Holsen, T. M. (1995). PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmospheric Environment, 29(4), 533–542.
Khuman, S. N., Chakraborty, P., Cincinelli, A., Snow, D., & Kumar, B. (2018). Polycyclic aromatic hydrocarbons in surface waters and riverine sediments of the Hooghly and Brahmaputra Rivers in the Eastern and Northeastern India. Science of the Total Environment, 636, 751–760.
Larsen, R. K., & Baker, J. E. (2003). Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods. Environmental Science & Technology, 37(9), 1873–1881.
Li, F., Wu, S., Wang, Y., Yan, D., Qiu, L., & Xu, Z. (2020). A new spatially explicit model of population risk level grid identification for children and adults to urban soil PAHs. Environmental Pollution, 263, 114547.
Li, G., Xia, X., Yang, Z., Wang, R., & Voulvoulis, N. (2006). Distribution and sources of polycyclic aromatic hydrocarbons in the middle and lower reaches of the Yellow River China. Environmental Pollution, 144(3), 985–993.
Li, P., Cao, J., Diao, X., Wang, B., Zhou, H., Han, Q., & Li, Y. (2015). Spatial distribution, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in surface seawater from Yangpu Bay China. Marine Pollution Bulletin, 93(1–2), 53–60.
Liang, X., Guo, C., Liao, C., Liu, S., Wick, L. Y., Peng, D., & Dang, Z. (2017). Drivers and applications of integrated clean-up technologies for surfactant-enhanced remediation of environments contaminated with polycyclic aromatic hydrocarbons (PAHs). Environmental Pollution, 225, 129–140.
Lin, Y. C., Lee, W. J., Li, H. W., Chen, C. B., Fang, G. C., & Tsai, P. J. (2006). Impact of using fishing boat fuel with high poly aromatic content on the emission of polycyclic aromatic hydrocarbons from the diesel engine. Atmospheric Environment, 40(9), 1601–1609.
Liu, M., Feng, J., Kang, B., Chen, Y., Liu, Q., & Sun, J. (2016). Ecological risk assessment of polycyclic aromatic hydrocarbons in surface sediments from the upper reach of Huaihe River. Polycyclic Aromatic Compounds, 36(5), 817–833.
Liu, Y., Chen, L., Huang, Q. H., Li, W. Y., Tang, Y. J., & Zhao, J. F. (2009). Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Huangpu River, Shanghai China. Science of the Total Environment, 407(8), 2931–2938.
Lubecki, L., & Kowalewska, G. (2012). Indices of PAH origin—A case study of the Gulf of Gdańsk (SE Baltic) sediments. Polycyclic Aromatic Compounds, 32(3), 335–363.
Luo, X. J., Chen, S. J., Mai, B. X., Yang, Q. S., Sheng, G. Y., & Fu, J. M. (2006). Polycyclic aromatic hydrocarbons in suspended particulate matter and sediments from the Pearl River Estuary and adjacent coastal areas China. Environmental Pollution, 139(1), 9–20.
Magi, E., Bianco, R., Ianni, C., & Di Carro, M. (2002). Distribution of polycyclic aromatic hydrocarbons in the sediments of the Adriatic Sea. Environmental Pollution, 119(1), 91–98.
Majithiya, D., Yadav, A., & Ram, A. (2018). Behaviour of trace metals in the anoxic environment of Veraval harbour India. Marine Pollution Bulletin, 129(2), 645–654.
Malik, A., Verma, P., Singh, A. K., & Singh, K. P. (2011). Distribution of polycyclic aromatic hydrocarbons in water and bed sediments of the Gomti River, India. Environmental Monitoring and Assessment, 172, 529–545.
Marr, L. C., Kirchstetter, T. W., Harley, R. A., Miguel, A. H., Hering, S. V., & Hammond, S. K. (1999). Characterization of polycyclic aromatic hydrocarbons in motor vehicle fuels and exhaust emissions. Environmental Science & Technology, 33(18), 3091–3099.
Merhaby, D., Net, S., Halwani, J., & Ouddane, B. (2015). Organic pollution in surficial sediments of Tripoli harbour Lebanon. Marine Pollution Bulletin, 93(1–2), 284–293.
Mitra, S., Corsolini, S., Pozo, K., Audy, O., Sarkar, S. K., & Biswas, J. K. (2019). Characterization, source identification and risk associated with polyaromatic and chlorinated organic contaminants (PAHs, PCBs, PCBzs and OCPs) in the surface sediments of Hooghly estuary, India. Chemosphere, 221, 154–165.
Montuori, P., Aurino, S., Garzonio, F., Sarnacchiaro, P., Nardone, A., & Triassi, M. (2016). Distribution, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in water and sediments from Tiber River and estuary, Italy. Science of the Total Environment, 566, 1254–1267.
Mora, M., Walker, T. R., & Willis, R. (2022). Spatiotemporal characterization of petroleum hydrocarbons and polychlorinated biphenyls in small craft harbour sediments in Nova Scotia, Canada. Marine Pollution Bulletin, 177, 113524.
Mostafa, A. R., Wade, T. L., Sweet, S. T., Al-Alimi, A. K. A., & Barakat, A. O. (2009). Distribution and characteristics of polycyclic aromatic hydrocarbons (PAHs) in sediments of Hadhramout coastal area, Gulf of Aden Yemen. Journal of Marine Systems, 78(1), 1–8.
MPEDA. 2021. Annual Report-2020–21. Government of India. https://www.netfishmpeda.org/publications/pdf/Annual-Report-2021-2022-Final.pdf
Mzoughi, N., & Chouba, L. (2011). Distribution and partitioning of aliphatic hydrocarbons and polycyclic aromatic hydrocarbons between water, suspended particulate matter, and sediment in harbours of the West coastal of the Gulf of Tunis (Tunisia). Journal of Environmental Monitoring, 13(3), 689–698.
Niehus, N. C., Brockmeyer, B., & Witt, G. (2019). Bioavailability and distribution of PAHs and PCBs in the sediment pore water of the German Bight and Wadden Sea. Marine Pollution Bulletin, 138, 421–427.
Pandit, G. G., Sahu, S. K., Puranik, V. D., & Raj, V. V. (2006). Exchange of polycyclic aromatic hydrocarbons across the air–water interface at the creek adjoining Mumbai harbour India. Environment International, 32(2), 259–264.
Pane, L., Boccardo, S., Bonfiglioli, F., Mariottini, G. L., Priano, F., & Conio, O. (2005). Polycyclic aromatic hydrocarbons in water, seston and copepods in a harbour area in the Western Mediterranean (Ligurian Sea). Marine Ecology, 26(2), 89–99.
Pradhap, D., Gandhi, K. S., Krishnakumar, S., Neelavannan, K., Radhakrishnan, K., & Saravanan, P. (2021). Baseline distributions and sources of polycyclic aromatic hydrocarbons (PAHs) in reef-associated sediments of Vembar group of Islands, Gulf of Mannar India. Marine Pollution Bulletin, 171, 112727.
Prajith, K. K., Pravin, P., Remesan, M. P., Madhu, V. R., Leela, E., Rajendra, B., et al. (2014). Fishing fleet of Gujarat. Technical Report, Central Institute of Fishery Technology. https://cift.res.in/publications
Qi, H., Chen, X., Du, Y. E., Niu, X., Guo, F., & Li, W. (2019). Cancer risk assessment of soils contaminated by polycyclic aromatic hydrocarbons in Shanxi China. Ecotoxicology and Environmental Safety, 182, 109381.
Radhakrishnan, K., Rajkumar, A., Akramkhan, N., Prakasheswar, P., Krishnakumar, S., Pradhap, D., & Hussain, S. M. (2021). Distribution of polycyclic aromatic hydrocarbons (PAHs) in surface sediments collected from selected estuaries of Kerala, West Coast of India.
Rajpara, R. K., Dudhagara, D. R., Bhatt, J. K., Gosai, H. B., & Dave, B. P. (2017). Polycyclic aromatic hydrocarbons (PAHs) at the Gulf of Kutch, Gujarat, India: Occurrence, source apportionment, and toxicity of PAHs as an emerging issue. Marine Pollution Bulletin, 119(2), 231–238.
Sarkar, S. K., Binelli, A., Chatterjee, M., Bhattacharya, B. D., Parolini, M., Riva, C., & Jonathan, M. P. (2012). Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons (PAHs) in core sediments of Sundarban mangrove wetland India. Polycyclic Aromatic Compounds, 32(1), 1–26.
Ramzi, A., Rahman, K. H., Gireeshkumar, T. R., Balachandran, K. K., Jacob, C., & Chandramohanakumar, N. (2017). Dynamics of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of Cochin estuary India. Marine Pollution Bulletin, 114(2), 1081–1087.
Ravindra, K., Sokhi, R., & Van Grieken, R. (2008). Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmospheric Environment, 42(13), 2895–2921.
Reddy, M. S., Basha, S., Joshi, H. V., & Ramachandraiah, G. (2005). Seasonal distribution and contamination levels of total PHCs, PAHs and heavy metals in coastal waters of the Alang-Sosiya ship scrapping yard, Gulf of Cambay India. Chemosphere, 61(11), 1587–1593.
Sarria-Villa, R., Ocampo-Duque, W., Páez, M., & Schuhmacher, M. (2016). Presence of PAHs in water and sediments of the Colombian Cauca River during heavy rain episodes, and implications for risk assessment. Science of the Total Environment, 540, 455–465.
Simpson, M. J., Chefetz, B., Deshmukh, A. P., & Hatcher, P. G. (2005). Comparison of polycyclic aromatic hydrocarbon distributions and sedimentary organic matter characteristics in contaminated, coastal sediments from Pensacola Bay Florida. Marine Environmental Research, 59(2), 139–163.
Singare, P. U. (2015). Studies on polycyclic aromatic hydrocarbons in surface sediments of Mithi River near Mumbai, India: Assessment of sources, toxicity risk and biological impact. Marine Pollution Bulletin, 101(1), 232–242.
Singare, P. U. (2016). Carcinogenic and endocrine-disrupting PAHs in the aquatic ecosystem of India. Environmental Monitoring and Assessment, 188(10), 599.
Singare, P. U., & Shirodkar, J. P. (2021a). Persistent and carcinogenic polycyclic aromatic hydrocarbons in the north-western coastal marine environment of India. AIMS Environmental Science, 8(2), 169–189.
Singare, P. U., & Shirodkar, J. P. (2021b). Distribution and fate of PAHs contaminants deposited in the sediments of North-Western Creeks adjoining the Arabian sea of Mumbai (pp. 1–18). Implications for monitoring. Environmental Forensics.
Sofowote, U. M., McCarry, B. E., & Marvin, C. H. (2008). Source apportionment of PAH in Hamilton Harbour suspended sediments: Comparison of two factor analysis methods. Environmental Science & Technology, 42(16), 6007–6014.
Stogiannidis, E., & Laane, R. (2015). Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: An overview of possibilities. Reviews of environmental contamination and toxicology, 49–133
Sukhdhane, K. S., Pandey, P. K., Vennila, A., Purushothaman, C. S., & Ajima, M. N. O. (2015). Sources, distribution and risk assessment of polycyclic aromatic hydrocarbons in the mangrove sediments of Thane Creek, Maharashtra, India. Environmental Monitoring and Assessment, 187, 1–14.
Sundararajan, S., Karthikeyan, R., & Khadanga, M. K. (2016). Spatial distribution of polycyclic aromatic hydrocarbons in Ennore estuary and coastal waters, Chennai India. Asian Journal of Chemistry, 28(1), 35.
Sundararajan, S., Khadanga, M. K., Kumar, J. P. P. J., Raghumaran, S., Vijaya, R., & Jena, B. K. (2017). Ecological risk assessment of trace metal accumulation in sediments of Veraval Harbor, Gujarat Arabian Sea. Marine Pollution Bulletin, 114(1), 592–601.
Tang, G., Liu, M., Zhou, Q., He, H., Chen, K., Zhang, H., & Cai, M. (2018). Microplastics and polycyclic aromatic hydrocarbons (PAHs) in Xiamen coastal areas: Implications for anthropogenic impacts. Science of the Total Environment, 634, 811–820.
Tiwari, M., Sahu, S. K., & Pandit, G. G. (2017). Distribution of PAHs in different compartment of creek ecosystem: Ecotoxicological concern and human health risk. Environmental Toxicology and Pharmacology, 50, 58–66.
Tong, Y., Chen, L., Liu, Y., Wang, Y., & Tian, S. (2019). Distribution, sources and ecological risk assessment of PAHs in surface seawater from coastal Bohai Bay, China. Marine Pollution Bulletin, 142, 520–524.
United States. Environmental Protection Agency. Environmental Criteria, Assessment Office (Cincinnati, & Ohio). (1993). Provisional guidance for quantitative risk assessment of polycyclic aromatic hydrocarbons. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, US Environmental Protection Agency.
Wagener, A., Hamacher, C., Farias, C., Godoy, J. M., & Scofield, A. (2010). Evaluation of tools to identify hydrocarbon sources in recent and historical sediments of a tropical bay. Marine Chemistry, 121(1–4), 67–79.
Wakeham, S. G. (1996). Aliphatic and polycyclic aromatic hydrocarbons in Black Sea sediments. Marine Chemistry, 53(3–4), 187–205.
Wang, C., Zou, X., Li, Y., Zhao, Y., Song, Q., & Yu, W. (2017). Pollution levels and risks of polycyclic aromatic hydrocarbons in surface sediments from two typical estuaries in China. Marine Pollution Bulletin, 114(2), 917–925.
Wang, J. Z., Bai, Y. S., Wu, Y., Zhang, S., Chen, T. H., Peng, S. C., ... & Zhang, X. W. (2016). Occurrence, compositional distribution, and toxicity assessment of pyrethroid insecticides in sediments from the fluvial systems of Chaohu Lake, Eastern China. Environmental Science and Pollution Research, 23(11), 10406–10414.
Wang, X. C., Sun, S., Ma, H. Q., & Liu, Y. (2006). Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Jiaozhou Bay, Qingdao China. Marine Pollution Bulletin, 52(2), 129–138.
Wang, Z., Chen, J., Qiao, X., Yang, P., Tian, F., & Huang, L. (2007). Distribution and sources of polycyclic aromatic hydrocarbons from urban to rural soils: A case study in Dalian China. Chemosphere, 68(5), 965–971.
Woo, S. H., Park, J. M., & Rittmann, B. E. (2001). Evaluation of the interaction between biodegradation and sorption of phenanthrene in soil-slurry systems. Biotechnology and Bioengineering, 73(1), 12–24.
Xiang, N., Jiang, C., Yang, T., Li, P., Wang, H., Xie, Y., & Diao, X. (2018). Occurrence and distribution of Polycyclic aromatic hydrocarbons (PAHs) in seawater, sediments and corals from Hainan Island, China. Ecotoxicology and Environmental Safety, 152, 8–15.
Yahiya, M., & Miranda, M. T. P. (2021). Distribution, sources and potential toxicological significance of polycyclic aromatic hydrocarbons (PAHs) in the south west coast of Kerala, India. Materials Today: Proceedings, 41, 736–743.
Yang, H. H., Lee, W. J., Mi, H. H., Wong, C. H., & Chen, C. B. (1998). PAH emissions influenced by Mn-based additive and turbocharging from a heavy-duty diesel engine. Environment International, 24(4), 389–403.
Yuan, Y., Song, D., Wu, W., Liang, S., Wang, Y., & Ren, Z. (2016). The impact of anthropogenic activities on marine environment in Jiaozhou Bay, Qingdao, China: A review and a case study. Regional Studies in Marine Science, 8, 287–296.
Yunker, M. B., Backus, S. M., Pannatier, E. G., Jeffries, D. S., & Macdonald, R. W. (2002). Sources and significance of alkane and PAH hydrocarbons in Canadian arctic rivers. Estuarine, Coastal and Shelf Science, 55(1), 1–31.
Zanardi-Lamardo, E., Mitra, S., Vieira-Campos, A. A., Cabral, C. B., Yogui, G. T., Sarkar, S. K., & Godhantaraman, N. (2019). Distribution and sources of organic contaminants in surface sediments of Hooghly river estuary and Sundarban mangrove, eastern coast of India. Marine Pollution Bulletin, 146, 39–49.
Zeng, Q., Jeppesen, E., Gu, X., Mao, Z., & Chen, H. (2018). Distribution, fate and risk assessment of PAHs in water and sediments from an aquaculture-and shipping-impacted subtropical lake, China. Chemosphere, 201, 612–620.
Zhang, S., Zhang, W., Wang, K., Shen, Y., Hu, L., & Wang, X. (2009). Concentration, distribution and source apportionment of atmospheric polycyclic aromatic hydrocarbons in the southeast suburb of Beijing China. Environmental Monitoring and Assessment, 151(1), 197–207.
Zheng, B., Wang, L., Lei, K., & Nan, B. (2016). Distribution and ecological risk assessment of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River estuary and the adjacent area, China. Chemosphere, 149, 91–100.
Zuloaga, O., Prieto, A., Ahmed, K., Sarkar, S. K., Bhattacharya, A., Chatterjee, M., & Satpathy, K. K. (2013). Distribution of polycyclic aromatic hydrocarbons in recent sediments of Sundarban mangrove wetland of India and Bangladesh: A comparative approach. Environmental Earth Sciences, 68, 355–367.
Acknowledgements
The authors are grateful to the Director, CSIR-National Institute of Oceanography, Goa, India, and Scientist-in-Charge, CSIR-NIO, Regional Centre, Mumbai, for their constant encouragement and support. This is CSIR-NIO contribution No. 7207.
Funding
This work was funded by an externally sponsored program of CSIR-National Institute of Oceanography under Project No. OLP 2009.
Author information
Authors and Affiliations
Contributions
Midhun M. N.: Investigation, writing (original draft), methodology, visualization, data curation, formal analysis.
Rakesh P. S: Project planning and administration, supervision, methodology, visualization, writing—review and editing.
Pooja Y. K.: Investigation, methodology, formal analysis, writing—review.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nair, M.M., S., R.P. & Kharat, P.Y. Fishing Harbours as a Source of PAHs Pollution: A Case Study from Veraval Harbour, West Coast of India. Water Air Soil Pollut 235, 224 (2024). https://doi.org/10.1007/s11270-024-07004-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07004-9