Abstract
The study investigates the pollution characteristics of 16 priority PAHs, accumulated in copepods from a major fishing harbour and its adjacent coastal waters of Veraval, west coast of India. The total PAH accumulation is in the range of 922.16–27,807.49 ng g−1 dw, with the mean concentration of 5776.59 ng g−1 dw. High concentrations of PAHs were present in the copepod samples from inside the harbour. Notably, there was no significant correlation between the lipid content of copepods and the accumulation of PAHs. The molecular diagnostic ratio method (MDR) indicates that the PAH sources are petrogenic in origin, while principal component analysis (PCA) points to petroleum, coal combustion and vehicular emission sources. Total cancerous PAHs (C-PAHs) in the study area dominate by 40% of the total PAHs identified; moreover, the bioaccumulation factor (BAF) is very high in the offshore area, which is also a fishing ground. The global relevance and magnitude of the present study in the Veraval, one of the prime seafood exporting hubs in India, should be dealt with utmost avidity as the accumulation status of PAHs in the zooplankton has never been explored in the Indian coastal waters. Moreover, the current study gives the foremost data on the bioaccumulation status of PAHs in copepods from the tropical waters of India.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copepods are planktonic crustaceans and form the major group of organisms in the zooplankton community (Longhurst, 1985; Ohtsuka and Nishida, 2016; Jyothibabu et al., 2018). Within the marine food web, copepods serve as primary consumers that feed on phytoplankton and serve as a crucial food source for higher trophic levels (Turner, 2004). Copepods hold a pivotal position in the pelagic food web and contribute significantly to the transport and fate of persistent organic pollutants (POPs) in marine ecosystems (Cailleaud et al., 2007; Steinberg & Landry, 2017).
Persistent organic pollutants (POPs) encompass a group of compounds, such as polycyclic aromatic hydrocarbons (PAHs), which are designated as priority pollutants by the United States Environmental Protection Agency (USEPA). PAHs have garnered worldwide attention due to their potential risks to human health through biomagnification, given their genotoxic, teratogenic and carcinogenic properties (Honda & Suzuki, 2020; Poulsen et al., 2021). PAH compounds are characterized by their lipophilic and hydrophobic nature, exhibiting a high octanol–water partition coefficient (Kow). Consequently, they tend to accumulate substantially in organic-rich sediments and biota within the environment (Zhang et al., 2019).
Copepod species are widely recognized for their remarkable ability to accumulate substantial reserves of energy-rich lipids, making them some of the highest lipid-containing organisms in marine ecosystems (Kattner & Hagen, 2009). According to Lee (1975), lipid molecules constitute approximately 42 to 64% of the dry weight of Arctic copepods. This elevated lipid content, coupled with the planktonic nature of copepods and their feeding habits, predisposes them to accumulate polycyclic aromatic hydrocarbons (PAHs) within their lipid and fat tissues (Berrojalbiz et al., 2011; Harris et al., 1977). Notably, PAHs are known for their adverse effects on organisms and their potential to disrupt the cellular membrane function and enzymatic systems of zooplankton (Almeda et al., 2013).
Bioaccumulation of hydrophobic pollutants like pesticides and PAHs in marine organisms has been the subject of extensive research facilitated by the use of a bioaccumulation factor (BAF) as a critical evaluation tool (Connell, 1988; Borgå, 2013). The BAF is a proxy that quantifies the ratio of PAH concentrations in the ambient water to their concentration in zooplankton, measured on a wet-weight basis (Barron, 1994). However, it is worth noting that studies investigating the accumulation of PAHs in copepods are mainly carried out in temperate waters, whereas Indian coastal waters remain unaddressed. Moreover, environmental data on the accumulation of PAHs in mesozooplankton is minimal (Hsieh et al., 2019). The primary aim of this research is to provide an understanding of PAH accumulation in marine copepods from Indian waters, particularly in the context of quantity of accumulation. Such insights can have far-reaching implications, not only for marine ecology but also for human health, considering the role of seafood consumption as a prominent route of PAH exposure. The current study aims to shed light on the dynamics of PAHs in marine primary consumers and their potential impacts on the environment and human populations. Recognizing this significant knowledge gap, the current study is designed with the following objectives: (1) to assess the bioaccumulation of PAHs in copepods from the coastal waters of Veraval, (2) to assess the relationship between PAH accumulation with biomass and lipid content in copepods and (3) to find out the source apportionment of accumulated PAHs in copepods.
Materials and methods
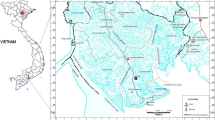
Study area
The state of Gujarat on the west coast of India claims itself to be one of the most industrial-saturated states in the country, housing the world’s largest petroleum refinery complex, rayon manufacturing company and Asia’s most oversized ship-breaking yard. This industrial advancement and urbanized anthropogenic activities have a detrimental effect on the coastal belt, turning it into a highly polluted territory (Dudhagara et al., 2016; Gosai et al., 2018; Mahajan et al., 2021; Rajpara et al., 2017). The harbour city of Veraval sits along the 1600 km coastline of Gujarat and is one of the largest fishing harbours in Asia. The primary livelihood of the local populace in the region is fishing-related activities with a large facility of fish processing units, and the products are exported to several countries, including the USA, Japan, South Asian countries, the Middle East and Europe (Hardikar et al., 2019; Sundararajan et al., 2017). Various studies on pollution status have been conducted, specifically about the PAHs and heavy metals accumulation in sediment and water from this location, which revealed that Veraval harbour and its coastal areas are exceedingly polluted zones (Nair et al., 2024b; Majithiya et al., 2018; Sundararajan et al., 2017).
Water quality analysis
Sea water samples collected from 15 locations from Veraval were analyzed for salinity; dissolved oxygen (DO), suspended solids (SS), dissolved organic carbon (DOC) and dissolved PAHs were collected with the help of a Niskin sampler (Hydro-Bios, Germany). Salinity and DO were analyzed by following Mohr titration and Winkler’s method according to Grasshoff (1983). The gravimetric method (APHA, 2005) was used for finding SS in the water column. The total lipid analysis of copepods was carried out according to Folch et al. (1957). The total organic carbon in the sample is determined by a process known as high-temperature catalytic oxidation (HTCO), using TOC Analyzer (Shimadzu-TOC-L, Japan). Standardization of the protocol was done using potassium hydrogen phthalate as the total carbon (TC) standard and sodium carbonate (Na2CO3 – 5–500 ppm) as the inorganic carbon (IC) standard. The sample is fed to the instrument with a nebulizer, and their signals are measured using a non-dispersive infra-red (NDIR) detector (Krishna et al., 2015).
Zooplankton sample collection and taxonomic identification
The mesozooplankton samples were collected from 15 locations within Veraval harbour and adjacent coastal areas in March 2019 (Fig. 1). To assess and interpret the pollution of PAH status in a better way, the study area had been divided into three zones, as Harbour (H1–H4), the locations consisting of depth less than 10 m as nearshore (N5–N9) and within 20–30 m depth as offshore area (S10–S15). The offshore region is part of an active fishing ground on the west coast of India. From each location, mesozooplankton samples were collected with the help of a Heron Tranter Net (HT net) having a mouth area of 0.25 m2 and mesh size of 200 µm towed with a boat horizontally just below the water surface for a duration of ~ 4–5 min with a boat speed of ~ 1.5 knots. The net was fitted with a calibrated flow meter (General Oceanics, Model 2030, USA) to quantify the volume of water filtered. Each sample collected was divided into two subsamples by a Folsom plankton splitter (Hydro-Bios, Germany); one portion was preserved in 5% buffered formalin solution for group-level taxonomic identification according to Conway et al. (2003). The second portion was washed with ultrapure water and filtered through precombusted (450 °C, 6 h) GF/F (Whatman, UK) filter paper and stored in amber-coloured glass vials at − 20 °C for PAH analysis.
PAH extraction and analysis
Copepods were picked with hexane-cleaned forceps from mesozooplankton samples kept at − 20 °C and freeze-dried (Labconco, USA) for 24 h, homogenized and made into fine powder. The powdered samples were soxhlet extracted with dichloromethane for 48 h. Saponification process (methanolic KOH method) was performed according to Jafarabadi et al. (2018) to remove the lipid content from the sample. The saponified solution was concentrated to 1 ml by using a rotary evaporator (BUCHI, R-3) and eluted by 1:2 alumina/silica gel column with 70 ml of DCM/hexane (2:3), concentrated and dissolved in n-hexane and dried under a gentle stream of nitrogen to 0.2 ml in an amber vial and kept at − 20 °C until analysis (Singare, 2015).
GC–MS analysis
Sixteen EPA priority PAHs include naphthalene (Nap), acenaphthylene (Acy), acenaphthene(Ace), fluorene (Fle), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fla), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), benzo[g, hi]perylene (BgP), dibenzo[a,h]anthracene (DBA) and indeno[1,2,3-cd]pyrene (InP), and its accumulation in mesozooplankton in the study area was assessed. PAH concentration in the sample was determined by using GC–MS (Agilent 7890B-5977B) with HP-5MS column (30 m length, 0.250 mm dia). The oven temperature was set at 100 to 200 °C ramp at 10 °C min−1 holds 1.5 min, ramp at 25 to 250 °C (2 min) and the final temperature 300 °C and ramp at 5 °C min−1 (10 min) (Nair et al., 2024a). A selected ion monitoring mode was used to identify the mass-to-charge ratio. High-purity helium was used as the carrier gas with a flow rate of 0.8 ml min−1.
Quality assurance and control
All samples were analyzed by following quality control procedures such as method blank, spiked blank and sample duplicates. The percentage of extraction recovery was assessed by 16 PAH congeners (Sigma-Aldrich, CRM-104) spiked with each sample, which varied at 81–89% (Spl. Table S1). The detection limits (LODs) showed in an arrange of 0.06–0.15 ng g−1 dw, and the limits of quantification (LOQs) were established at 0.3 to 2.3 ng g−1. The measurement uncertainty ranged from 4.13 to 7.54% Soxhlet extraction method. GC residue analysis grade solvents were used for all extractions (Sigma-Aldrich, USA). Glassware was muffle furnaced at 350 °C, washed with GC-grade organic solvents before use.
Bioaccumulation factor (BAF)
The bioconcentration factor means the ratio between the accumulated PAH concentration in copepods and PAHs dissolved in the coastal waters. The formula is given below in Eq. (1).
Of these, PAHs cop is the concentration of PAHs in the copepods (ng kg−1), and PAHswater is the concentration of PAHs dissolved in the water (Almeda et al., 2013).
Bioaccumulation involves the absorption of a chemical by an organism through various exposure pathways. Bioaccumulation factor (BAF) calculation is in Eq. (2).
In this context, ku represents the rate constant for diffusive uptake (m3 kg−1 d−1), while kfood denotes the rate constant for uptake through feeding on phytoplankton (m3 kg−1 d−1). The variable kd stands for the rate constant for diffusive depuration (d−1), while keg represents the depuration rate resulting from the egestion of faecal pellets and laying of eggs (d−1) (Berrojalbiz et al., 2009). Indeed, the BAF and BCF values of PAHs for copepods exhibit no statistically significant difference (p < 0.01), as determined by t-tests comparing the BAF and BCF values of each studied PAH (Ziyaadini et al., 2016; Magnusson et al., 2007; Fisk et al, 2001). Therefore, in this study, the BCF equation was used to determine the bioaccumulation of PAHs instead of the BAF equation.
Results and discussion
Physico-chemical parameters, mesozooplankton biomass and lipid content
Physico-chemical parameters were analyzed and represented in supplementary table S2. Dissolved oxygen (DO) was found high in offshore region (6.1–7.3 ± 0.4 mg/L) and recorded very low at inside harbour (0.02–1.1 ± 0.5 mg/L). Salinity plays a major role in the distribution of mesozooplankton and was ranged between 24.2–36.5 ± 3.5 PSU in the study area. Relatively low salinity was observed inside the harbour (24.2–33.1 ± 4.1 PSU) because of the effluents and sewage mixing happening in the region. Suspended solids (SS) in the water column were showing a decreasing trend with high values inside the harbour followed by nearshore and offshore stations with a range of 214.6–350.4 ± 66.3 mg/L, 28.6–128.8 ± 46.5 mg/L and 28.2–44.2 ± 5.9 mg/L, respectively. Dissolved organic carbon (DOC) varied between 2.1–16.1 ± 4.2 ppm in the study area. High DOC were observed inside the harbour (9.9–16.1 ± 2.5 ppm) followed by the nearshore (5.3–6.4 ± 0.4 ppm) and offshore waters (2.1–4.1 ± 0.7 ppm). Low DO, especially inside the Veraval harbour evolved with the sewage and waste release from fish processing units, were recorded by Majithiya et al (2018). The relatively high DOC values inside the harbour area may be due to the organic-rich waste received from fish processing plants and sewage from the surrounding regions (Figueroa‐Nieves et al., 2014).
The mesozooplankton biomass (Spl. Table S3) in the waters of Veraval ranged between 0.003 and 0.042 mL/m3 (Av. 0.02 ± 0.014) with higher biomass in the offshore region (Av. 0.03 ± 0.01 mL/m3) and lower values found inside the harbour (Av. 0.008 ± 0.002 mL/m3). Copepods (Av. 89.84%) were found as a dominant taxa among mesozooplankton groups, order Calanoida contributed maximum (67.4%) to copepod, followed by order Cyclopoida (25.8%) and Harpaticoida (6.8%) (Spl. Table S3). Similar observations were made by Padmavati & Goswami (1996), Gaonkar et al. (2010) and Venkataramana et al. (2017) in their studies on zooplankton from the coastal waters of India. Based on rapid species identification conducted in the copepod taxa found, Undinula vulgaris (48.3%), Paracalanus parvus (11.8%) and Acartia danae (7.6%) species were dominated among calanoid copepods in the study area. In comparison to the adjacent nearshore and offshore regions, the copepod abundance in the Veraval harbour zone is notably lower. This can be the instinctive mixing of water in the harbour area through wind and tidal action which has been partially inhibited by the harbour breakwater system, resulting in the agglomeration of organic pollutants and the area becomes anoxic (Majithiya et al., 2018 ), which in turn decreases the copepod biomass inside the harbour.
Lipids are considered the main energy reserve in copepods. Lipid content in the copepods from Veraval was in the range between 6.6 and 17.7 ± 3.1% dry w. during the present study period. The lipid content present in the copepods at Veraval was found to be lower than the lipid content present in the temperate water copepods (Kattner & Hagen, 2009; Lee, 1975; Lee et al., 1971) (Spl Table S2). Our data was correlating with the lipid content in copepods from the west coast of India (Madhupratap, 1979; Goswami et al., 2000; Jagadeesan et al., 2010). Bhat and Wagh (1992) found high lipid content in zooplankton samples with > 90% copepod population from the Bombay High region, but the lipid reserve found was less than from Veraval waters.
PAH accumulation in copepods
The accumulation of 16 priority PAHs in copepods at Veraval coastal waters is depicted in Table 1. Total PAHs vary in a range of 922.16–27,807.49 ng g−1 dw, and individual PAHs are distributed in an order of Phe > Pyr > BaP > Fla > Chr > BaA > Fle > BkF > BbF > Nap > InP> Ant > BgP > DBA > Acy. Inside the harbour area, Phe, Ant and BaP compounds contribute a higher concentration of ƩPAHs than the other two zones. A detailed literature survey has been carried out to compare our results with the ƩPAH accumulation in mesozooplankton and copepods reported globally and presented in Table 2. The literature collection indicated that studies on PAH accumulation in Indian coastal waters are not explored and recorded a high accumulation of ƩPAHs in the copepods at Veraval coast. High concentrations of PAHs (6570.39–27,807.49 ng g−1 dw) were present in the samples collected from inside the harbour region. The nearshore (3788.58–5706.07 ng g−1 dw) and offshore stations (922.06–4638.31 ng g−1 dw) recorded less concentrations of PAHs (Fig. 2).
Table 2 describes the accumulation of PAHs in copepods and mesozooplankton across the globe, and by comparing the data from Qatar, Gulf of Genova and Port Valdez, the concentration of PAHs in copepods from Veraval was found to be very high. The PAH accumulation status in mesozooplankton from locations like Black and Marmara seas (560–2486 ng g−1 dw), Eastern Mediterranean (548–1903 ng g−1 dw), East China sea (29–5384 ng g−1 dw) and Gaoping coast (5–5440 ng g−1 dw) was comparable for nearshore and offshore locations at Veraval. Hsieh et al. (2019) describes substantial accumulation of PAHs in zooplankton by direct sorption and by diet in regions with mixed sources of PAH pollution. However, it is imperative to note that a sole focus on copepods or zooplankton may not provide a comprehensive understanding of PAH pollution at a specific location. To delve into the intricacies of this phenomenon, we must explore the multifaceted exposure pathways of persistent organic pollutants (POPs) in these myriad organisms. Copepods experience continuous immersion in water, which facilitates the potential accumulation of dissolved PAHs within their body. Moreover, the ingestion of food particles, including microalgae and various suspended particulate matter, serves as an additional avenue for copepods to encounter PAHs (Almeda et al., 2013; Berrojalbiz et al., 2009; Lotufo, 1998). These food particles, often adsorbed and accumulated with PAHs, play a vital role in transferring these pollutants up the food chain. The bioavailability and subsequent uptake of PAHs by copepods through this dietary route underscore the intricate nature of pollution dynamics within aquatic ecosystems. This knowledge is crucial for assessing the potential consequences of PAH bioaccumulation in copepods and its repercussions on the overall health and stability of marine ecosystems. The feeding habit of copepods from Veraval coastal waters also plays the role for the high accumulation of PAHs, for example gut content studies revealed the presence of natural particulate matter in U. vulgaris (Gerber & Marshall, 1974) and P. parvus and A. danae are known to their diet on detritus matter and other non-phytoplankton particles (Ezhilarasan et al., 2020; Kleppel, 1993).
In our current investigation, we sought to explore the potential association between an organism’s total lipid content and the accumulation of organic pollutants. Contrary to previous studies, we did not observe a statistically significant correlation (Fig. 3) between the lipid content and the concentrations of individual and total PAHs in copepods. Remarkably, our study revealed a positive correlation between dissolved and accumulated PAHs (r = 0.85). Additionally, strong correlations were evident between environmental parameters such as total suspended solids (TSS) and dissolved organic carbon (DOC) (r = 0.96). Furthermore, correlations were observed between TSS and dissolved PAHs (r = 0.82) as well as between TSS and copepod PAHs (r = 0.59), both indicating substantial associations. Similarly, total organic carbon (TOC) exhibited positive correlations with PAHs in the dissolved phase (r = 0.87) and within copepod bodies (r = 0.85). These findings underscore the intricate interplay between environmental parameters and the dynamics of PAHs, shedding light on the complex pathways through which these pollutants are transported and metabolized in copepod populations. The high correlations observed emphasize the need for a comprehensive understanding of the intricate relationships between environmental factors and pollutant accumulation in aquatic organisms.
In the ring-wise distribution of PAH compound, the 2–3-ring compound varied in 35.60–827.83 ng g−1 dw, and the 4-ring compounds vary at an average concentration of 0.80–890.25 ng g−1 dw (Fig. 4; Spl. Table S4). The accumulation percentage of 2-ring compounds is 2.6–37.6%, 11.1–32.0% for 3-ring compounds, 6.7–61.7% for 4-ring compounds, 12.1–54.6% for 5-ring compounds, and 0.1–16.2% for 6-ring compounds in the copepods. Conforming to the composition pattern of PAH ring number, source determining is considered a primary identification tool (Fang et al., 2003). PAHs with 4 rings were showing high accumulation in copepods followed by 5 rings and 3 rings. High molecular weight (HMW) PAH compounds were found to have increased concentrations in the zooplankton body (Berrojalbiz et al., 2009). Lotufo (1998) and Mitra et al. (2012) recorded HMW PAHs such as fluoranthene and pyrene that once adsorbed remain in zooplankton for longer periods. These HMW PAHs mainly originate from the high-temperature combustion of pyrogenic sources such as coal or vehicular exhaust; however, low molecular weight (LMW) compounds are originated due to the low / moderate-temperature combustion of coal/biomass (Mai et al., 2003; Stogiannidis and Laane, 2014. The total concentration of carcinogenic PAHs (ƩCPAHs) copepods is in the range of 287.93–11,528.22 ng g−1 dw (Av. 2233.85 ng g−1 dw), distributed in an order of Bap > Chr > BaA > BKF > BbF > InP > DBA. BaP, which dominates 38% of the rest of the cancerous PAHs, varies in the range of 27.67–1517.89 ng g−1 dw and has a higher cancer causing potential than the others (Spl. Fig. S1). The risk of bioaccumulation through the food web in Veraval is very high as the offshore area is an active fishing ground (Zhao et al., 2014).
Source apportionment of PAHs in copepods
Molecular diagnostic ratios (MDR) proxies such as Ant / Phe, Fla / Pyr, BaA / Chr and LMW / HMW were used for the source apportionment (Fig. 5) of PAHs in copepods. Source identification based on an isometric ratio is considered a more reliable approach to track the origin of PAHs (Yunker et al., 2002). In this technique, the values Phe / Ant > 10, Fla / Pyr < 1, LMW / HMW < 1 and Chr / BaA > 10 are considered to be of petrogenic origin, whereas the pyrogenic source has a condition of Phe/Ant < 10, Fla / Pyr > 1, LMW / HMW > 1 and Chr / BaA < 10 (Hasanati et al., 2011). Based on the Phe / Ant values, aside from two stations (H1 and F10), the cumulation of PAHs in all copepod samples was of petrogenic origin. The Chr / BaA ratio from sites F10, N8, N7 and F14 are shown as pyrogenic and all others are of petrogenic origin. The Fla / Pyr ratio indicates all the stations except H4 are petrogenic. The LMW / HMW ratio indicates that all the stations other than F10 are petrogenic. In brief, based on different MDR ratios, the PAHs accumulated in the copepods are mainly from petrogenic source, which leads to the high number of fishing boats present in the harbour.
Another approach for source identification is by representing the variability minimum factors (principal components) of the individual PAHs in each sample using the principal component analysis (PCA) tool (Dudhagara et al., 2016; Zheng et al., 2016). The Kaiser–Meyer–Olkin (KMO) test was used to confirm the applicability of PCA, and a df value of 91 was obtained. The compounds such as DBA and BgP were not detected in copepod samples from most of the offshore stations. Subsequently, the PCA test was conducted with 15 samples and 14 PAH compounds, excluding previously undetected compounds, resulting in extracting 2 principal components with 83.81% total variability. The varimax rotated factor loading of PAH concentration in mesozooplankton is plotted in Fig. 1. PC1 shows 69.85% of the variance with high molecular compounds such as InP, BkFF, BaA, Chr, Pyr, and Fla, and low molecular compound Ant show higher loading (> 0.9) (Fig. 6). It can be assumed that the high loading of the HMW compound is mainly from the vehicular sources (Larsen & Baker., 2003; Jiang et al., 2009), and InP and BnF are generally originated from diesel/gasoline engine emissions (Harrison et al., 1996). The distribution of high Pyr and BaA loading also leads to the possibility of coal combustion (Larsen & Baker., 2003; Sofowote et al., 2008). Low molecular weight compounds Phe, Ale and Acy indicate moderate loading (0.7–0.8) giving mixed source response of coal combustion and vehicular emission (Chen et al., 2005), while Fle, BaP and BbF show weak loading (0.2–0.4). Nap has a high loading with a 13.96% variance in PC2 which indicates the presence of oil leakage or spill as the source (Bao et al., 2021; Dobbins et al., 2006; Marr et al., 1999).
Concisely, most molecular isomeric ratio values demonstrate petrogenic origin at most stations, while in PCA, it changes into a mixed source response. PAH accumulation in copepods is mainly through passive exposure and dietary intake (phytoplankton and suspended organic-rich particles); however, PAH compounds, which are subjected to biotransformation and elimination process in the copepod body, affect the source identification. Furthermore, copepods are the floating and drifting organisms, and their movements greatly depend on water current, and tide making site-specific source identification impossible, unlike the sediment. In such a case, the result obtained from the visible sources (ground truth data) along with the molecular diagnostic ratio and PCA in the study area can be interpreted (Qi et al., 2019). The presence of engine oil, diesel and kerosene from fishing vessel operations and boat maintenance area in Veraval harbour can be considered a strong petrogenic source of PAHs, while the influence of fly ash from a coal-based thermal power plant functioning 2.6 km west of the harbour area is considered a strong pyrogenic source. Apart from this, municipal sewage discharged into the inner harbour region is also considered a pyrogenic source of PAHs in the study area.
Bioaccumulation factor (BAF) of PAHs in copepods
The bioaccumulation factor in this study means the ratio between the accumulated PAH concentration in copepods and PAHs dissolved in the coastal waters. The formula is given below.
BAF = PAHscop X 1000/ PAHswater.
Of these, PAHscop is the concentration of PAHs in the copepods (ng kg−1), and PAHswater is the concentration of PAHs dissolved in the water (Almeda et al., 2013). The BAF value of PAHs in copepods at Veraval harbour and its adjacent area is given in Fig. 7. According to this, the BAF value of BaP can be seen to dominate all the areas. The BAF value in the harbour area is mainly varied in the order of BaP > IND > Phe > BkF and Bap > InP> Chr > BaA in the nearshore region and BaP > Fla > Pyr > BbF in the offshore region, respectively. The BAF values of Nap, Acy and Ace, which have low molecular weight, are found to be low at all stations. In general, the BAF values were found higher in the offshore region than nearshore and harbour region. However, since PAH compounds with higher molecular weight could not be traced in some of the offshore stations, BAF values in these areas have not been derived. The log BAF and log Kow values in the study area imply a linear relationship (Spl. Fig. S2) (Almeda et al., 2013; Meador et al., 1995). The lower availability of PAHs and their lipophilic and hydrophobic nature indicate an association with bioaccumulation. Not only that, the metabolism and depuration rate of individual PAHs depends upon its chemical properties (Cailleaud et al., 2009; Juhasz & Naidu, 2000). Therefore, in some copepod species, the “Nap” excretes instantly, while flu, Pyr and BaP sustain for a prolonged period in the zooplankton body (Harris et al., 1977 ; Lotufo, 1998; Mitra et al., 2012).
Conclusion
Research concerning the accumulation of PAHs in copepod taxa within tropical waters has been conspicuously scarce, with a particular dearth of data for Indian coastal waters. This study significantly contributes to this knowledge gap by recording the concentration of PAHs in copepods from the Veraval coastal area. The findings, as determined through molecular diagnostic ratio (MDR) and principal component analysis (PCA), elucidate the pivotal role of emissions stemming from fishing vessels, operations at fishing crafts repair centres, and the influence of a coal-based thermal power plant located in close proximity to the harbour in driving the accumulation of PAHs within the Veraval region. Furthermore, the noteworthy bioaccumulation factor (BAF) of benzo(a)pyrene, a known carcinogenic PAHs, was discovered to exhibit alarming levels in offshore copepods. This discovery underscores the pressing concern of the trophic transfer of these carcinogenic PAHs through the food web. The present study clearly establishes copepods as a valuable test organism for evaluating PAH accumulation within the primary consumer trophic level within marine ecosystems. Therefore, further research on copepods is warranted to comprehensively assess their role in accumulating persistent organic pollutants and other emerging contaminants in coastal environments. Such investigations establish a direct link to the cycling of these persistent pollutants within higher trophic levels, offering critical insights into the ecological impact of PAH accumulation in this fishing ground and nearby coastal ecosystem.
Data availability
The data used and/or analyzed during the current study are available in the supplementary table.
References
Almeda, R., Wambaugh, Z., Wang, Z., Hyatt, C., Liu, Z., & Buskey, E. J. (2013). Interactions between zooplankton and crude oil: Toxic effects and bioaccumulation of polycyclic aromatic hydrocarbons. PLoS ONE,8(6), e67212.
American Public Health Association. (1926). Standard methods for the examination of water and wastewater (Vol. 6). American Public Health Association.
Bao, K., Zhang, Y., Zaccone, C., & Meadows, M. E. (2021). Human impact on C/N/P accumulation in lake sediments from northeast China during the last 150 years. Environmental Pollution,271, 116345.
Barron, M. G. (1994). Bioaccumulation and biomagnification. Handbook of Ecotoxicology, Lewis Publishers.
Berrojalbiz, N., Dachs, J., Ojeda, M. J., Valle, M. C., Castro-Jiménez, J., Wollgast, J., & Zaldivar, J. M. (2011). Biogeochemical and physical controls on concentrations of polycyclic aromatic hydrocarbons in water and plankton of the Mediterranean and Black Seas. Global Biogeochemical Cycles, 25(4).
Berrojalbiz, N., Lacorte, S., Calbet, A., Saiz, E., Barata, C., & Dachs, J. (2009). Accumulation and cycling of polycyclic aromatic hydrocarbons in zooplankton. Environmental Science & Technology,43(7), 2295–2301.
Bhat, K. L., & Wagh, A. B. (1992). Biochemical composition of zooplankton of Bombay High. Indian Journal of Marine Sciences,21, 220–223.
Borgå, K. (2013). Ecotoxicology: bioaccumulation. In Elsevier eBooks. https://doi.org/10.1016/b978-0-12-409548-9.00765-x
Cailleaud, K., Budzinski, H., Menach, K. L., Souissi, S., & Forget-Leray, J. (2009). Uptake and elimination of hydrophobic organic contaminants in estuarine copepods: An experimental study. Environmental Toxicology and Chemistry: An International Journal,28(2), 239–246.
Cailleaud, K., Forget-Leray, J., Souissi, S., Hilde, D., LeMenach, K., & Budzinski, H. (2007). Seasonal variations of hydrophobic organic contaminant concentrations in the water-column of the Seine Estuary and their transfer to a planktonic species Eurytemora affinis (Calanoïda, copepoda). Part 1: PCBs and PAHs. Chemosphere,70(2), 270–280.
Chen, Y., Sheng, G., Bi, X., Feng, Y., Mai, B., & Fu, J. (2005). Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China. Environmental Science & Technology,39(6), 1861–1867.
Connell, D. W. (1988). Bioaccumulation Behavior of Persistent Organic Chemicals with Aquatic Organisms. In Reviews of environmental contamination and toxicology (pp. 117–154). https://doi.org/10.1007/978-1-4612-3810-2_3
Carls, M. G., Short, J. W., & Payne, J. (2006). Accumulation of polycyclic aromatic hydrocarbons by Neocalanus copepods in Port Valdez. Alaska. Marine Pollution Bulletin, 52(11), 1480–1489.
Conway, D. V., White, R. G., Hugues-Dit-Ciles, J., Gallienne, C. P., & Robins, D. B. (2003). Guide to the coastal and surface zooplankton of the south-western Indian Ocean. Occasional Publication of the Marine Biological Association of the United Kingdom, 15, 1–354.
Dobbins, R. A., Fletcher, R. A., Benner, B. A., Jr., & Hoeft, S. (2006). Polycyclic aromatic hydrocarbons in flames, in diesel fuels, and in diesel emissions. Combustion and Flame,144(4), 773–781.
Dudhagara, D. R., Rajpara, R. K., Bhatt, J. K., Gosai, H. B., Sachaniya, B. K., & Dave, B. P. (2016). Distribution, sources and ecological risk assessment of PAHs in historically contaminated surface sediments at Bhavnagar coast, Gujarat, India. Environmental Pollution,213, 338–346.
Ezhilarasan, P., Kanuri, V. V., Kumar, P. S., Kumaraswami, M., Rao, G. D., Patra, S., ... and Murthy, M. R. (2020). Influence of environmental variables on the distribution and community structure of mesozooplankton in the coastal waters of the eastern Arabian Sea. Regional Studies in Marine Science, 39, 101480.
Fang, M. D., Lee, C. L., & Yu, C. S. (2003). Distribution and source recognition of polycyclic aromatic hydrocarbons in the sediments of Hsin-ta Harbour and adjacent coastal areas, Taiwan. Marine Pollution Bulletin,46(8), 941–953.
Figueroa-Nieves, D., McDowell, W. H., Potter, J. D., Martínez, G., & Ortiz-Zayas, J. R. (2014). Effects of sewage effluents on water quality in tropical streams. Journal of Environmental Quality,43(6), 2053–2063.
Fisk, A. T., Hobson, K. A., & Norstrom, R. J. (2001). Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the Northwater Polynya marine food web. Environmental Science & Technology,35(4), 732–738.
Folch, J., Lees, M., & Sloane Stanley, G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry,226(1), 497–509.
Gaonkar, C. A., Krishnamurthy, V., & Anil, A. C. (2010). Changes in the abundance and composition of zooplankton from the ports of Mumbai, India. Environmental Monitoring and Assessment,168, 179–194.
Gerber, R. P., & Marshall, N. (1974). Ingestion of detritus by the lagoon pelagic community at Eniwetok Atoll. Limnology and Oceanography,19(5), 815–824.
Gosai, H. B., Sachaniya, B. K., Dudhagara, D. R., Rajpara, R. K., & Dave, B. P. (2018). Concentrations, input prediction and probabilistic biological risk assessment of polycyclic aromatic hydrocarbons (PAHs) along Gujarat coastline. Environmental Geochemistry and Health,40, 653–665.
Grasshoff, K. (1983). Methods of sea water analysis (2nd ed.). Verlag Chemie.
Goswami, S. C., Kumari, L. K., & Shivastava, Y. (2000). Diel variation in zooplankton and their biochemical composition from Vengurla to Ratnagiri, west coast of India. Indian Journal of Marine Sciences,20, 277–280.
Hardikar, R., Haridevi, C. K., Ram, A., Khandeparker, R., Amberkar, U., & Chauhan, M. (2019). Inter-annual variability of phytoplankton assemblage and Tetraspora gelatinosa bloom from anthropogenically affected harbour, Veraval, India. Environmental Monitoring and Assessment,191, 1–17.
Harrison, R. M., Smith, D. J. T., & Luhana, L. J. E. S. (1996). Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environmental Science & Technology, 30(3), 825–832.
Harris, R., Berdugo, V., Corner, E., Kilvington, C., & OfHara, S. (1977). Factors affecting the retention of a petroleum hydrocarbon by marine planktonic copepods. In Elsevier eBooks (pp. 286–304). https://doi.org/10.1016/b978-0-08-021613-3.50035-8
Hasanati, M., Savari, A., Nikpour, Y., & Ghanemi, K. (2011). Assessment of the sources of polycyclic aromatic hydrocarbons in Mousa inlet by molecular ratios. J. Environ. Stud., 37, 1–6.
Honda, M., & Suzuki, N. (2020). Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. International Journal of Environmental Research and Public Health,17(4), 1363.
Hsieh, H. Y., Huang, K. C., Cheng, J. O., Lo, W. T., Meng, P. J., & Ko, F. C. (2019). Environmental effects on the bioaccumulation of PAHs in marine zooplankton in Gaoping coastal waters, Taiwan: Concentration, distribution, profile, and sources. Marine Pollution Bulletin, 144, 68–78.
Hung, C. C., Ko, F. C., Gong, G. C., Chen, K. S., Wu, J. M., Chiang, H. L., & Santschi, P. H. (2014). Increased zooplankton PAH concentrations across hydrographic fronts in the East China Sea. Marine pollution bulletin, 83(1), 248–257.
Jafarabadi, A. R., Bakhtiari, A. R., Maisano, M., Pereira, P., & Cappello, T. (2018). First record of bioaccumulation and bioconcentration of metals in Scleractinian corals and their algal symbionts from Kharg and Lark coral reefs (Persian Gulf, Iran). Science of the Total Environment,640, 1500–1511.
Jagadeesan, L., Arivuselvan, N., Thirumaran, G., Anantharaman, P., & Balasubramanian, T. (2010). Biomass and biochemical composition of zooplankton along the Arabian Sea, west coast of India. Advance Journal of Food Science and Technology,2(2), 96–99.
Jiang, J. J., Lee, C. L., Fang, M. D., & Liu, J. T. (2009). Polycyclic aromatic hydrocarbons in coastal sediments of southwest Taiwan: An appraisal of diagnostic ratios in source recognition. Marine Pollution Bulletin,58(5), 752–760.
Juhasz, A. L., & Naidu, R. (2000). Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo [a] pyrene. International Biodeterioration & Biodegradation,45(1–2), 57–88.
Jyothibabu, R., Jagadeesan, L., Karnan, C., Arunpandi, N., Pandiyarajan, R. S., & Balachandran, K. K. (2018). Ecological indications of copepods to oxygen-deficient near-shore waters. Ecological Indicators,93, 76–90.
Kattner, G., & Hagen, W. (2009). Lipids in marine copepods: latitudinal characteristics and perspective to global warming. In Springer eBooks (pp. 257–280). https://doi.org/10.1007/978-0-387-89366-2_11
Kleppel, G. S. (1993). On the diets of calanoid copepods. Marine Ecology-Progress Series,99, 183–183.
Krishna, M. S., Prasad, V. R., Sarma, V. V. S. S., Reddy, N. P. C., Hemalatha, K. P. J., & Rao, Y. V. (2015). Fluxes of dissolved organic carbon and nitrogen to the northern Indian Ocean from the Indian monsoonal rivers. Journal of Geophysical Research: Biogeosciences,120(10), 2067–2080.
Larsen, R. K., & Baker, J. E. (2003). Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods. Environmental Science & Technology,37(9), 1873–1881.
Lee, R. F., Hirota, J., & Barnett, A. M. (1971). Distribution and importance of wax esters in marine copepods and other zooplankton. Deep-sea Research and Oceanographic Abstracts/Deep Sea Research and Oceanographic Abstracts, 18(12), 1147–1165. https://doi.org/10.1016/0011-7471(71)90023-4
Lee, R. F. (1975). Lipids of Arctic zooplankton. Comparative Biochemistry and Physiology Part b: Comparative Biochemistry,51(3), 263–266.
Longhurst, A. R. (1985). Relationship between diversity and the vertical structure of the upper ocean. Deep Sea Research Part A. Oceanographic Research Papers, 32(12), 1535–1570.
Lotufo, G. R. (1998). Bioaccumulation of sediment-associated fluoranthene in benthic copepods: Uptake, elimination and biotransformation. Aquatic Toxicology,44(1–2), 1–15.
Madhupratap, M. (1979). Distribution, community structure and species succession of copepods from Cochin backwaters. Indian J. Mar. Sci., 8, 1–8.
Magnusson, K., Magnusson, M., Östberg, P., Granberg, M., & Tiselius, P. (2007). Bioaccumulation of 14C-PCB 101 and 14C-PBDE 99 in the marine planktonic copepod Calanus finmarchicus under different food regimes. Marine Environmental Research,63(1), 67–81.
Mahajan, M., Manek, D., Vora, N., Kothari, R. K., Mootapally, C., & Nathani, N. M. (2021). Fungi with high ability to crunch multiple polycyclic aromatic hydrocarbons (PAHs) from the pelagic sediments of Gulfs of Gujarat. Marine Pollution Bulletin,167, 112293.
Mai, Qi, S., Zeng, E. Y., Yang, Zhang, G., Fu, J., & Wang. (2003). Distribution of polycyclic aromatic hydrocarbons in the coastal region off Macao, China: Assessment of input sources and transport pathways using compositional analysis. Environmental Science & Technology, 37(21), 4855–4863.
Majithiya, D., Yadav, A., & Ram, A. (2018). Behaviour of trace metals in the anoxic environment of Veraval harbour, India. Marine Pollution Bulletin,129(2), 645–654.
Marr, L. C., Kirchstetter, T. W., Harley, R. A., Miguel, A. H., Hering, S. V., & Hammond, S. K. (1999). Characterization of polycyclic aromatic hydrocarbons in motor vehicle fuels and exhaust emissions. Environmental Science & Technology,33(18), 3091–3099.
Meador, J. P., Stein, J. E., Reichert, W. L., & Varanasi, U. (1995). Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. Reviews of environmental contamination and toxicology, Springer, , 143, 79–165.
Mitra, S., Kimmel, D. G., Snyder, J., Scalise, K., McGlaughon, B. D., Roman, M. R., & Campbell, P. L. (2012). Macondo-1 well oil-derived polycyclic aromatic hydrocarbons in mesozooplankton from the northern Gulf of Mexico. Geophysical Research Letters, 39(1).
Nair, M. M., Rakesh, P. S., & Kharat, P. Y. (2024b). Fishing harbours as a source of PAHs pollution: A case study from Veraval harbour, west coast of India. Water, Air, Soil Pollution, 235(4), 1–20.
Nair, M. M., Sreeraj, M. K., Rakesh, P. S., Thomas, J., Kharat, P. Y., & Sukumaran, S. (2024a). Distribution, source and potential biological impacts of polycyclic aromatic hydrocarbons in the core sediments of a networked aquatic system in the northwest coast of India–A special focus on Thane Creek Flamingo Sanctuary (Ramsar site). Regional Studies in Marine Science, 70, 103377.
Nour El-Din, N. M., & Abdel-Moati, M. A. R. (2001). Accumulation of Trace Metals, Petroleum Hydrocarbons, and Polycyclic Aromatic Hydrocarbons in Marine Copepods from the Arabian Gulf. Bulletin of Environmental Contamination & Toxicology, 66(1).
Ohtsuka, S., & Nishida, S. (2016). Copepod biodiversity in Japan: Recent advances in Japanese Copepodology. In Diversity and commonality in animals (pp. 565–602). https://doi.org/10.1007/978-4-431-56432-4_22
Padmavati, G., & Goswami, S. C. (1996). Zooplankton distribution in neuston and water column along west coast of India from Goa to Gujarat. Indian Journal of Marine Sciences,25, 85–90.
Pane, L., Boccardo, S., Bonfiglioli, F., Mariottini, G. L., Priano, F., & Conio, O. (2005). Polycyclic aromatic hydrocarbons in water, seston and copepods in a harbour area in the Western Mediterranean (Ligurian Sea). Marine Ecology, 26(2), 89–99.
Poulsen, R., Gravert, T. K. O., Tartara, A., Bensen, H. K., Gunnarsen, K. C., Dicová, K., ... & Christensen, J. H. (2021). A case study of PAH contamination using blue mussels as a bioindicator in a small Greenlandic fishing harbor. Marine Pollution Bulletin, 171, 112688.
Qi, H., Chen, X., Du, Y. E., Niu, X., Guo, F., & Li, W. (2019). Cancer risk assessment of soils contaminated by polycyclic aromatic hydrocarbons in Shanxi, China. Ecotoxicology and Environmental Safety,182, 109381.
Rajpara, R. K., Dudhagara, D. R., Bhatt, J. K., Gosai, H. B., & Dave, B. P. (2017). Polycyclic aromatic hydrocarbons (PAHs) at the Gulf of Kutch, Gujarat, India: Occurrence, source apportionment, and toxicity of PAHs as an emerging issue. Marine Pollution Bulletin,119(2), 231–238.
Singare, P. U. (2015). Studies on polycyclic aromatic hydrocarbons in surface sediments of Mithi River near Mumbai, India: Assessment of sources, toxicity risk and biological impact. Marine Pollution Bulletin,101(1), 232–242.
Sofowote, U. M., McCarry, B. E., & Marvin, C. H. (2008). Source apportionment of PAH in Hamilton Harbour suspended sediments: Comparison of two factor analysis methods. Environmental Science & Technology,42(16), 6007–6014.
Steinberg, D. K., & Landry, M. R. (2017). Zooplankton and the ocean carbon cycle. Annual Review of Marine Science,9, 413–444.
Stogiannidis, E., & Laane, R. (2014). Source Characterization of polycyclic aromatic hydrocarbons by using their molecular indices: An overview of possibilities. In Reviews of environmental contamination and toxicology (pp. 49–133). https://doi.org/10.1007/978-3-319-10638-0_2
Sundararajan, S., Khadanga, M. K., Kumar, J. P. P. J., Raghumaran, S., Vijaya, R., & Jena, B. K. (2017). Ecological risk assessment of trace metal accumulation in sediments of Veraval Harbor, Gujarat. Arabian Sea Marine Pollution Bulletin,114(1), 592–601.
Turner, J. T. (2004). The importance of small planktonic copepods and their roles in pelagic marine food webs. Zoological Studies,43(2), 255–266.
Venkataramana, V., Sarma, V. V. S. S., & Reddy, A. M. (2017). Impact of river discharge on distribution of zooplankton biomass, community structure and food web dynamics in the Western coastal Bay of Bengal. Regional Studies in Marine Science,16, 267–278.
Wan, Y., Jin, X., Hu, J., & Jin, F. (2007). Trophic dilution of polycyclic aromatic hydrocarbons (PAHs) in a marine food web from Bohai Bay. North China. Environmental science & technology, 41(9), 3109–3114.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry,33(4), 489–515.
Zhang, M., He, P., Qiao, G., Wang, J., Huang, J., Yuan, X., & Li, Q. (2019). Distribution, sources, and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Subei Shoal, China. Marine Pollution Bulletin,149, 110640.
Zhao, Z., Zhang, L., Cai, Y., & Chen, Y. (2014). Distribution of polycyclic aromatic hydrocarbon (PAH) residues in several tissues of edible fishes from the largest freshwater lake in China, Poyang Lake, and associated human health risk assessment. Ecotoxicology and Environmental Safety,104, 323–331.
Zheng, B., Wang, L., Lei, K., & Nan, B. (2016). Distribution and ecological risk assessment of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River estuary and the adjacent area, China. Chemosphere,149, 91–100.
Ziyaadini, M., Mehdinia, A., Khaleghi, L., & Nassiri, M. (2016). Assessment of concentration, bioaccumulation and sources of polycyclic aromatic hydrocarbons in zooplankton of Chabahar Bay. Marine Pollution Bulletin, 107(1), 408–412.
Acknowledgements
The authors thank the Director, CSIR-National Institute of Oceanography, Goa, India, and Scientist-in-Charge, CSIR-NIO, Regional Centre, Mumbai, for their constant encouragement and support. The authors also acknowledge the suggestions and comments given by the unknown reviewers. The funding for this work was provided by Project OLP2009. This is CSIR - NIO contribution No.7249.
Funding
This work was funded by an externally sponsored program of CSIR-National Institute of Oceanography under Project No. OLP 2009.
Author information
Authors and Affiliations
Contributions
Pooja Yuvraj Kharat: investigation, writing—original draft, formal analysis. Midhun M. Nair.: investigation, formal analysis, writing—original draft, methodology, data curation. Rakesh P. S: project planning and administration, supervision, methodology, visualization, writing review and editing. C. K. Haridevi: reviewing manuscript and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kharat, P.Y., Nair, M.M., Rakesh, P.S. et al. Distribution and bioaccumulation status of polycyclic aromatic hydrocarbons (PAHs) in Veraval coastal waters using copepods as bio-indicators. Environ Monit Assess 196, 711 (2024). https://doi.org/10.1007/s10661-024-12805-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-12805-w