Abstract
The distribution and ecosystem risk of 16 polycyclic aromatic hydrocarbons (PAHs) in the Luan River, China, has been investigated. ∑PAHs levels ranged from 37.3 to 234 ng L−1 in water, from 20.9 to 287 ng g−1 in sediment and from 36.9 to 378 ng g−1 in bank soil, respectively. A method based on toxic equivalency factors (TEFs) and risk quotient (RQ) which can be used to assess the ecosystem risk of ∑PAHs sensitively and accurately was invented and a new ecosystem risk classification of ∑PAHs was suggested. The results indicated that the PAHs in aquatic environment of the Luan River resulted in low ecosystem risk and at S9 the ecosystem risk of PAHs in water was moderate. Low and moderate molecular PAHs presented much more ecosystem risk than high molecular PAHs in the Luan River basin and the mean ecosystem risk in water was higher than that in sediment and bank soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are an environmental concern due to their carcinogenicity, mutagenicity and toxicity (Liu et al. 2005; Zhang et al. 2004). Four- to seven-ring PAHs are highly mutagenic and carcinogenic, two- or three-ring PAHs are less mutagenic but can be highly toxic (Fernandes et al. 1997). PAHs are compounds, containing typically two to eight aromatic rings, which are produced by high-temperature reactions, such as incomplete combustion and pyrolysis of fossil fuels and other organic material, and release of petroleum and petroleum products (Benner et al. 1990; Harrison et al. 1996). PAHs producing activities include the following: combustion of fuels in vehicular engines, power generation from fossil fuels, coke production, wood burning, incineration of industrial and domestic wastes, oil refinery and chemical engineering processes, etc. (Baek et al. 1991). Byproducts containing PAHs have been dumped on the land, in the water, or buried at subsurface sites, and they can also undergo long-range transport, find their final destination in soil and sediment of aquatic systems and exist for a long time in the environment (Christensen et al. 1997). PAHs can be introduced into the aquatic environment through various routes, such as wastewater discharge, atmospheric deposition, surface runoff, and oil leaking, etc. (Heemken et al. 2000). For the reasons above, PAHs’ behavior, transport, fate, sources and environmental risk to ecological systems have been extensively studied.
The Luan River begins in the Mongolia Plateau, snakes through the Yan mountains and the eastern Hebei plain, and empties into the Bohai sea. This 888 km long river, together with over 10 tributaries, irrigates a land area of 45,000 Km2. The Panjiakou reservoir and the Daheiting reservoir, the most important water sources of Tianjin and Tangshan, were constructed in the 1980s for supplying water to 6.5 million people of these two cities in order to alleviate the water shortages. At present, the two reservoirs export 1.5 billion m3 water to these two cities every year. However, since 1988, the runoff volume in this region has decreased by 30.8%, which led to low flow conditions in the lower reaches of the Luan River down Daheiting reservoir in the previous years, and the contamination of the Luan River and its tributaries has been aggravated by the fast growth of industrialization and urbanization. For these reasons, it is important to monitor contaminants, including PAH, of the Luan River. Safe drinking water resources affect 10.5 million people’s daily life.
In aquatic systems, PAHs in water is closely related to the safety of drinking water, and that in bank soil and sediment are potential sources of PAHs, which may all cause potential eco-toxicity to the ecosystem. So water, bank soil and sediment were sampled and analysed in this study. The objectives of the present work were to determine the contamination status, environmental risk and possible sources of PAHs in the aquatic environments of the Luan River in order to assess the environmental quality of aquatic system of drinking water resource, to assess the effect on PAHs contamination caused by rapid industrialization and urbanization, to provide data for comparison with other rivers and suggestions for organic pollution control to protect the safety of drinking water.
Methods
Sampling
In this study twenty sampling sites were investigated. 13 stations along the Luan River and 7 stations at its 7 tributaries were selected to find which region makes more contribution to the contamination of the Luan River. Among these 20 stations, 10 stations were located at suburban areas which were not under direct impact of industrial and traffic activities, and 10 stations were located close to 6 cities including the famous tourist city, Chengde. Thus, concentrations obtained in this study reflected both the ‘background’ level and the ‘maximum’ value of the PAH in the Luan River watershed.
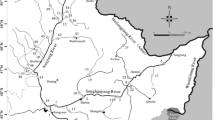
Samples were collected during January 2009. Locations of the sampling sites are shown in Fig. 1. Global positioning system (GPS) was used to locate the sampling positions. Surface sediment samples (1–5 cm deep) were collected with a grab sampler, and the top 1 cm surface layer was carefully removed with a stainless steel spoon (Zhou et al. 2000) and stored in aluminum jars, which had been prewashed with methylene chloride, and then properly cooled with ice during transportation to the laboratory where they had been stored at −20°C before being freeze-dried. The dried sediment samples were sieved to obtain particles less than 180 μm (Mai et al. 2001). At each site three samples were collected within a distance of 5 m. Samples in triplicates were mixed thoroughly to make a composite sample. Mixing of samples helped to minimize within-site variability, and ensured that the sediment samples collected were truly representative of each site (Koh et al. 2004). Bank soil samples (1–5 cm deep) were collected on the river bank about 10 m away from the watercourse with a stainless steel spoon, and they were kept, transported, pretreated, extracted and analysed together with the sediment samples during the same process. At each location, 5 L of water was collected and stored in amber glass bottles. Water samples were pretreated within 24 h of sampling. The samples were filtered using 0.45 μm glass fiber filters under vacuum to obtain dissolved samples (Khim et al. 2001).
Location of the sampling sites. (S1: Goutaizi; S2: Guojiatun; S3: Jiutun; S4: Boluonuo; S5: Zhangbaiwan; S6: Sandaohezi; S7: Hanjiaying; S8: Luanhedaqiao; S9: Chengde; S10: Shangbancheng; S11: Xiabancheng; S12: Wulongji; S13: Dazhangzi; S14: Kuancheng; S15: Baohekou; S16: Yanziyu; S17: Daheiting; S18: Luanxian; S19: Jianggezhuang; S20: Estuary)
Chemicals
Before using, anhydrous sodium sulphate and silica gel were heated at 650°C for 4 h to remove moisture and organic impurities. The silica gel was then activated at 130°C for 10 h; anhydrous sodium sulfate were kept in desiccators. Mixed standards of sixteen PAHs (PAH-Mixture 610/525/550), each at 100 mg mL−1, were obtained from ChemService Inc. (West Chester, PA). All the solvents used for sample processing (n-hexane, dichloromethane) were of chromatographic grade. The solvents used for GC/MS analysis were Pesticide Residue grade. Active copper sheets were prepared by washing with HCl (1 N) and pure methanol. The silica gel was deactivated with 1% ultra-pure water prior to cleanup.
Sample extraction for organic pollutants
Concentrations of water samples involved passing the filtered waters through solid-phase extraction (SPE) mini-columns (Supelco C18 Plus (500 mg) cartridges) at the maximum flow rate of 5 mL min−1. A Supelco Visiprep Solid Phase Extraction Vacuum Manifold was used to allow simultaneous processing of up to 12 water samples. Prior to concentration, the C18 cartridges were pre-conditioned with 10 mL of dichloromethane followed by 5 mL of methanol and then 10 mL of ultra-pure water. Following concentration, the cartridges were wrapped up using aluminium foil and stored at −20°C before elution. Then, the cartridges were blowed dry under a stream of purified N2. PAH were eluted from the cartridge using 10 mL of dichloromethane to KD vessels. The eluate was then evaporated under a stream of purified N2 in a water bath to 2–3 mL and was passed through a silica gel column packed with anhydrous sodium sulphate to remove water content. The solution was then evaporated with a rotary evaporator to almost dryness at a water-bath temperature of 35°C to avoid PAH losses. The residue was redissolved in hexane and the volume was reduced to 100 μL for GC-MS analysis.
Frozen bank soil and sediment samples were dried using a freeze-drying apparatus. Prior to extraction, these samples were ground into granular powder that were passed through a 100-mesh sieve and homogenized. Extraction was based on the modified procedure of US EPA method 3545 (pressurized fluid extraction), 3630C (silica gel cleanup), and 3660B (sulphur cleanup). In brief, mashed samples were first mixed with anhydrous sodium sulphate in a weight ratio of 5:1 and with activated copper sheets in a ratio of about 1:1, to desulfurize the extract. The mixtures were extracted by accelerated solvent extraction (ASE300, Dionex, Sunnyvale, CA) with n-hexane/dichloromethane (1:1 by volume) for 10 min (two static cycles). Extraction temperature was set at 125°C and pressure at 1,500 psi.
Extracts of bank soil and sediment samples were reduced to about 2 mL using a rotary evaporator and purified by a silica gel column packed with anhydrous sodium sulphate. The column was first rinsed with 10 mL n-hexane solvent to remove non-PAHs impurities, such as aliphatic hydrocarbons. Then the column was rinsed with 15 mL mixed solvent of dichloromethane and n-hexane (1:1, v/v). The second eluate contained PAH and was collected and concentrated using a rotary evaporator. The effluent is then reduced to about 0.5 mL under a stream of purified N2 in KD vessels and adjusted to 1.0 mL by hexane for GC-MS analysis.
Sample analyses
All the extracted samples were analyzed using a Hewlett-Packard 6890 Gas Chromatograph (GC) connected to a Hewlett-Packard 5973 N Mass Selective Detector (MSD) (Agilent, Wilmington, DE). The chromatographic column was a 30 m, 0.25 mm inner diameter, 0.25 μm film thickness HP-5MS capillary column (#19091s-433, Agilent, J and W Scientific, Folsom, CA). Helium was used as the carrier gas at a constant flow rate of 1 mL min−1. The oven temperature was ramped from 60 to 260°C at 6°C min−1, and then held for 15 min. The MSD was operated in the electron impact mode at 70 eV. Ion source temperature was 280°C. The mass spectra were recorded at a scan mode covering the range of 45–400 mass units for sediment samples, and at a selected ion mode (SIM) when analyzing the 16 US EPA priority PAHs in water samples. Quantification was performed by the external standard method with a 16 PAHs standard mixture (PPH-10JM, Chem Service Inc., West Chester, PA). Retention time and identity of PAHs were also confirmed by this standard. GC/MS data were acquired and processed using an Enhanced ChemStation (HPG1701CA, Agilent, Wilmington, DE). The 16 PAHs detected in sequence were: Naphthalene (Na), Ace-naphthylene (Ayl), Acenaphthene (Aen), Fluorene (F), Phenanthrene (Ph), Anthracene (An), Fluoranthene (Fl), Pyrene (Py), Benz[a]anthracene (BaA), Chrysene (Ch), Benzo[b]fluoranthene (BbF), Benzo[k]fluoranthene (BkF), Benzo[a]pyrene (BaP), Indeno[1,2,3-cd]pyrene (IP), Dibenzo[a,h]anthracene (DA), Benzo[ghi]perylene (BghiP).
Analytical quality controls
All data were subject to strict quality control procedures. Deuterated internal standards were used to compensate for losses involved in the sample extraction and work-up. Six internal standards in water, bank soil and sediment samples were determined with good precision, and recoveries ranged from 76 ± 9 to 118 ± 8% for water samples, from 71 ± 9 to 110 ± 11% for bank soil samples and from 72 ± 7 to 108 ± 10% for sediment samples. The Limit of Detection was 1.2–18 ng L−1 for water sample and 1.3–15 ng g−1 for bank soil and sediment samples, respectively. The results showed that the precision was generally satisfactory.
Results
PAHs concentrations and composition profiles
The concentrations of PAHs in water from the Luan River at different sampling sites were listed in Table 1. It could be seen from Table 1 that 16 US EPA priority PAHs were present in majority water samples. The concentrations of ∑PAHs in water ranged from 37.3 (S1) to 234 ng L−1 (S9) with the mean value of 99.4 ng L−1 (Fig. 2). The mean concentration was 15.8 ng L−1 for 2-ring PAHs, 64.9 ng L−1 for 3-ring PAHs, 17.7 ng L−1 for 4-ring PAHs, 0.8 ng L−1 for 5-ring PAHs, and 0.1 ng L−1 for 6-ring PAHs, respectively. The mean compositional profiles of PAHs in water are showed in Fig. 3 which indicates that PAHs in water were dominated by 2-, 3- and 4-ring PAHs, which accounted for 15.9, 65.3 and 17.8% of ∑PAHs, respectively. This was attributed to the relatively high vapor pressure and water solubility of these PAHs (Mackay et al. 1992) and the lower aqueous solubility and hydrophobic nature of the high molecular weight PAHs (Li et al. 2006).
The concentrations of PAHs in sediment at different sampling sites were illustrated in Table 1. As shown in Table 1, the concentrations of ∑PAHs in sediment ranged from 20.9 (S1) to 287 ng g−1 (S9) dry weight with the mean value of 115.3 ng g−1 (Fig. 2). The mean compositional profiles of PAHs in sediment are showed in Fig. 3 and it could be seen from the figure that 3- and 4-ring PAHs were abundant at most sampling sites, accounting for 33.6 and 36.6% of ∑PAHs, respectively. The proportion of high molecular weight (5- and 6-ring) PAHs increased to 19.1% in sediment, 19 times than that in water.
Table 1 shows the concentrations of PAHs in bank soil from the Luan River at different sampling sites. The concentrations of ∑PAHs in bank soil ranged from 36.9 (S1) to 377.6 ng g−1 (S8) dry weight with the mean value of 141.4 ng g−1 (Fig. 2). The mean compositional profiles of PAHs are showed in Fig. 3 which shows that 3- and 4-ring PAHs were the most abundant components at most sampling sites, accounting for 27.7 and 51.0% of ∑PAHs, respectively. Low molecular weight PAHs (2- and 3-ring) were still abundant in bank soil, and the proportion of high molecular weight PAHs (5- and 6-ring) was 6.0%, much higher than that in water but lower than that in sediment samples.
The median concentrations of individual PAHs were basically lower than the corresponding average concentrations, indicating that majority of the sites contained relatively low PAHs concentrations. Coefficients of variation (CV) of all PAHs were over 50.0% and even some of them exceeded 200%, suggesting great variation in the concentrations of PAHs in different sampling sites. The ∑PAHs concentrations also varied to some extent in water (CV = 59%, n = 20), sediment (CV = 69%, n = 20) and bank soil (CV = 76%, n = 17).
Spatial distribution pattern
The spatial distribution of PAHs in water, sediment and bank soil were studied by comparing the concentrations of ∑PAHs among different sampling sites from the upper reaches to the estuary of the Luan River. The results were summarized in Fig. 2. It could be observed from Fig. 2 that relatively high concentrations of ∑PAHs were detected from sampling sites S7, S8, S9, S10, S18, and S19 (>150 ng L−1) in water, S7, S8, S9, S10, S14, and S18 (>190 ng g−1) in sediment, and S7, S8, S9, S14, and S18 (>200 ng g−1) in bank soil, respectively. Among these sites, it could be seen from Fig. 2 that concentrations of ∑PAHs were all higher both in water, sediment and bank soil at sites S7, S8, S9, and S18. For spatial distribution pattern, it’s obvious that these most polluted sites all gathered in the middle and lower reaches of the region, are in accordance with the distribution pattern of cities in the Luan River basin. The high concentrations of ∑PAHs were closely related to discharging of industrial waste water, urban runoffs and emission of atmospheric particles of the cities such as Chengde, Longhua, Kuancheng and Luanxian, and it could be seen that these areas were the main contribution sources of PAHs to the Luan River.
From Fig. 4, it’s more obvious that PAHs were much higher in water, sediment and bank soil, in city zones than in rural areas or the estuary where they were similar. What’s more, this distribution was different from the situations in Yangtze River and Pearl River where the concentrations of ∑PAHs in the estuaries were the highest compared with other regions in the whole basins (Mai et al. 2001; Feng et al. 2007). This could be explained from the following reasons. First, there is little direct PAHs discharge of industrial, traffic and combustion in the Luan River estuary because there were no cities or strong interferential human activities. Second, the Luan River Basin has large population density and a huge burden of enormous industrial and agricultural water consumption. Third, the two major reservoirs (Panjiakou and Daheiting) export 1.5 billion m3 water to Tianjin and Tangshan every year, which caused, in recent years, a very small or even no volume of water entering into the sea. So, there was very little indirect input of PAHs into the estuary from the upper, middle and lower reaches of the river, and most of the pollutants were accumulated in the Luan River basin itself and not exported into the sea. Great gaps exited between estuary, rural areas and city zones on the mean concentrations of PAHs in sediment and bank soil, while the gap on the mean concentrations of PAHs in water between estuary, rural areas and city zones was relatively small. However, there was no significant difference between the main river and it’s tributaries on PAHs contamination in water, sediment and bank soil.
Discussion
Kunte and Borneff pointed out that PAHs contamination in dissolved phases could be divided into four grades, micro-polluted (10–50 ng L−1), light-polluted (50–250 ng L−1), moderately polluted (250–1,000 ng L−1) and heavily polluted (>1,000 ng L−1) (Chen 2008). The concentrations of ∑PAHs in water from the Luan River ranged from 37.3 to 234 ng L−1, showing that five of the sampling sites could be classified as ‘micro-polluted’ and other fifteen sites were light-polluted.
The pollution levels of sediment are assigned (Baumard et al. 1998) as low (0–100 ng g−1), moderate (100–1,000 ng g−1), high (1,000–5,000 ng g−1) and very high (>5,000 ng g−1). The concentrations of ∑PAHs in sediment ranged from 20.9 to 287 ng g−1 in the Luan River basin, thus nine of the sampling sites could be classified as ‘Low level’ and other eleven sites were moderately contaminated.
According to the classification established by Maliszewska-Kordybach, the PAHs pollution levels of soil were divided into not contaminated (<200 ng g−1), weakly contaminated (200–600 ng g−1), contaminated (600–1,000 ng g−1) and heavily contaminated (>1,000 ng g−1) (Maliszewska-Kordybach 1996). The threshold values of this classification (200, 600 and 1,000 ng g−1) were derived from the results of determinations of PAHs concentrations in European soil, as well as from an estimation of risks of human exposure. As the concentrations of ∑PAHs in bank soil ranged from 36.9 to 377.6 ng g−1 dry weight, five of our sampling sites are under the ‘Weakly contaminated’ classification, and the other sites can be considered as ‘Not contaminated’. However, according to other references, Edwards estimated that an endogenous PAHs concentration, resulting from the biosynthesis and forest fires, would be 1–10 ng g−1, and the typical ∑PAH levels in the Luan River basin were all higher than the target value set by Dutch government for unpolluted soil (20 ± 50 mg/kg dry weight), showing the soil were all contaminated with PAHs (Van Brummelen et al. 1996).
In order to assess potential ecosystem risk of PAHs in the Luan River, PAHs levels detected in water, sediment and bank soil were compared against their corresponding quality values in the present study. The reference values reported in the literature for other areas (Kalf et al. 1997) were used for the investigated area. The levels of risk posed by certain PAHs were characterized by risk quotient (RQ), which was calculated as follows:
where C PAHs was the concentration of certain PAHs in the medium and C QV was the corresponding quality values of certain PAHs in the medium. In the present study, the negligible concentrations (NCs) and the maximum permissible concentrations (MPCs) of PAHs in water, sediment and bank soil reported by Kalf et al. (1997) were used as the quality values in the medium. Therefore, RQNCs and RQMPCs were defined as follows:
where C QV(NCs) was the quality values of the NCs of PAHs in the medium and C QV(MPCs) was the quality values of the MPCs of PAHs in the medium.
However, this could only be used to assess the ecosystem risk of 10 individual PAHs. In order to evaluate the ecosystem risk of other 6 individual PAHs, the toxic equivalency factors (TEFs) for individual PAHs (Nisbet and LaGoy 1992) was used to infer NCs and MPCs of Ayl, Aen, F, Py, BbF and DA (Table 2). The TEFs were used because they represented the toxic equivalency factors of 16 individual PAHs (Table 2), and if different individual PAHs showed the same TEFs, it was true that their toxicity was similar to each other. So it could be concluded that the toxicity of Ayl, Aen, F and Py (TEFs = 0.001) was similar to An (TEFs = 0.001), that of BbF (TEFs = 0.1) similar to BaA (TEFs = 0.1), and that of DA (TEFs = 1) similar to BaP (TEFs = 1). As C QV(NCs) and C QV(MPCs) of An, BaA and BaP were known, it is reasonable to use the values of An, BaA and BaP for that of Ayl, Aen, F, Py, BbF and DA (Table 2). According to this, C QV(NCs) and C QV(MPCs) of 16 PAHs were got and the ecosystem risk of 16 individual PAHs could be assessed, which greatly improved the previous method.
Furthermore, according to the previous method, RQ∑PAHs, RQ∑PAHs(NCs) and RQ∑PAHs(MPCs) were defined as follows:
This calculation method neglected the ecosystem risk of individual PAHs when calculate the ecosystem risk of ∑PAHs. For example, in term of a given PAHs concentration from one sampling site, if several individual PAHs did not show ecosystem risk (RQ of these PAHs less than 1) while other PAHs did (RQ not less than 1), it is probably that the RQ∑PAHs was less than 1, which showed that there was no ecosystem risk. Therefore, according to this method, contradiction may exit between the risk assessment of individual PAHs and that of ∑PAHs.
However, the new method proposed here could remedy the problem showed above. RQ∑PAHs, RQ∑PAHs(NCs) and RQ∑PAHs(MPCs) were defined as follows:
Based on the ecosystem risk assessment of 16 individual PAHs, RQ(NCs) and RQ(MPCs) of individual PAHs which were not less than 1 were summated to calculate the RQ∑PAHs(NCs) and RQ∑PAHs(MPCs) of ∑PAHs. Thus, with this new calculation method, the ecosystem risk of ∑PAHs could be assessed with the ecosystem risk of individual PAHs fully considered. As a result, this new method was more accurate and scientific than the old one.
Besides, a new ecosystem risk classification of ∑PAHs (Table 3) was suggested based on the new method, which can be used to assess ecosystem risk of PAHs in any mediums, and the ecosystem risk levels of PAHs in different mediums can be compared with each other.
The mean values of RQ(NCs) and RQ(MPCs) in different mediums were listed in Table 2. In principle, RQ(NCs) < 1.0 indicated that the single PAHs might be probably negligible concern, while RQ(MPCs) > 1.0 would indicate that contamination of the single PAHs was much severe and some control measures and remedial actions must be undertaken at once. In case of RQ(NCs) > 1.0 and RQ(MPCs) < 1.0, which indicated that contamination of the single PAHs might list as middle levels and some control measures or remedial actions need be undertaken. RQ(MPCs) of individual PAHs were all less than 1.0 except for BaA in water sample at S9 (Wuliehe, RQ(MPCs) = 1.53), which indicated that some severe toxicity might be caused to the aquatic organisms by BaA. The mean values of RQ(NCs) of Na, Ayl, Aen, F, Ph, An, Fl, Py, BaA, BbF in water, Na, Ayl, Aen, F, Ph, An, Py, BaA, BbF in sediment, Na, Ayl, Aen, F, Ph, An, Fl, Py, BaA, BbF in bank soil were higher than 1.0, indicating that Na, Ayl, Aen, F, Ph, An, Fl, Py, BaA, BbF widely showed middle level ecosystem risk in the Luan River basin and Ch, BkF, BaP, DA and IP only showed ecosystem risk at particular sampling sites, with BghiP showing no ecosystem risk at any site (Table 4). The median RQ(NCs) of individual PAHs were basically lower than the corresponding average RQ(NCs), indicating that majority of the sites contained relatively low ecosystem risk. Coefficients of variation (CV) of all PAHs were over 50.0% and even some of them exceeded 200%, suggesting great variation in the ecosystem risk of individual PAHs in different sampling sites. The CV of ∑PAHs also varied to some extent in water (CV = 88%, n = 20), sediment (CV = 64%, n = 20) and bank soil (CV = 72%, n = 17), which was different from the variation of PAHs concentrations. The probability of risk (PR) of Ayl, Ph in water, Na, F in sediment, and Na, F, Ph, An, Py in bank soil were all 100%, showing these PAHs presented moderate ecosystem risk in all sampling sites. And PR of high molecular PAHs were basically very low (Table 4).
It could be observed from Fig. 5 that 3- and 4-ring PAHs contributed to the main ecosystem risk burden, and Na caused a certain degree of ecosystem risk in water and sediment. Comparing Fig. 5 with 3, it can be found that the value of concentrations of PAHs in water is the lowest (Fig. 5) while the value of RQ(NCs) of PAHs in water is the highest (Fig. 3), and high molecular PAHs accounts for certain proportions on concentrations of PAHs (Fig. 5), but they accounts for much lower proportions on RQ(NCs) of PAHs (Fig. 3). Though high molecular PAHs are highly mutagenic and carcinogenic and low molecular PAHs are less mutagenic, low and moderate molecular PAHs presented much more ecosystem risk than high molecular PAHs in the Luan River basin. Therefore, more deeply investigation of sources of these PAHs, some control measures and remedial actions should be conducted to decrease the contamination of 2-, 3- and 4-ring PAHs.
RQ(MPCs) of ∑PAHs in water, sediment and bank soil in the Luan River basin were all zero except that in water at S9 (1.53), and RQ(NCs) of ∑PAHs in water, sediment and bank soil in the Luan River basin were all higher than 1.0 (Fig. 6). On the whole, the results indicated that the PAHs in aquatic environment of the Luan River resulted in low ecosystem risk and at S9 the ecosystem risk of PAHs in water was moderate (Table 3). Comparing Fig. 6 with 2, it can be concluded that the changing trend of PAHs concentrations in water, sediment and bank soil was a little different from that of ecosystem risk, which was caused by the different composition profiles for PAHs at different sampling sites. The mean ecosystem risk in water (RQ∑PAHs(NCs) = 91.6) was more 1.5 times than that in bank soil (RQ∑PAHs(NCs) = 57.5) and more 2 times than that in sediment (RQ∑PAHs(NCs) = 40.3) (Table 4). According to Fig. 7, it’s obvious that the ecosystem risk of ∑PAHs in city zones were much higher in water, sediment and bank soil, and the ecosystem risk of ∑PAHs in water, sediment and bank soil were similar in rural areas and the estuary. Great gaps of ecosystem risk for PAHs exited between estuary, rural areas and city zones in all the three mediums. However, there was no significant difference between the main river and it’s tributaries on ecosystem risk in water, sediment and bank soil.
Conclusions
In this study, the comprehensive survey of 16 PAHs in water, sediment, and bank soil in the Luan River basin, China, has been conducted. The profile of PAHs showed that low molecular (2- to 3-ring) weight PAHs were predominant in dissolved phase, and 4-ring and higher molecular (5- to 6-ring) weight PAHs were abundant in bank soil and sediment. The contamination of PAHs in city zones were heavier than that in the rural areas and the estuary and there was no significant difference between the main river and it’s tributaries on PAHs contamination in water, sediment and bank soil.
Ecosystem risk assessment indicated that Na, Ayl, Aen, F, Ph, An, Fl, Py, BaA, BbF widely showed middle level ecosystem risk in the Luan River basin and Ch, BkF, BaP, DA and IP only showed ecosystem risk at particular sampling sites, with BghiP showing no ecosystem risk at any site.Though high molecular PAHs are highly mutagenic and carcinogenic and low molecular PAHs are less mutagenic, low and moderate molecular PAHs presented much more ecosystem risk than high molecular PAHs in the Luan River basin. In this study, a method which can be used to assess the ecosystem risk of ∑PAHs was proposed and the results indicated that the ∑PAHs in aquatic environment of the Luan River were of low ecosystem risk and at S9 the ecosystem risk of PAHs in water was moderate. The mean ecosystem risk in water was more 1.5 times than that in bank soil and more 2 times than that in sediment. Some control measures and remedial actions should be conducted to decrease the contamination of PAHs. If the values of NCs and MPCs of PAHs in other medias, or that of other pollutants which show ecosystem risk were tested, this risk assessment method could also be used. And it is suggested that analysis of RQ∑PAHs(NCs) and RQ∑PAHs(MPCs) could replace that of the concentration of ∑PAHs in studies of PAHs because they could better reflect the contamination level.
References
Baek SO, Field RA, Goldstone ME (1991) A review of atmospheric polycyclic aromatic hydrocarbons: sources, fate and behavior. Water Air Soil Pollut 60:279–300
Baumard P, Budinski H, Michon Q, Garrigues P, Burgeot T, Bellocq J (1998) Origin and bioavailability of PAHs in the Mediterranean Sea from mussel and sediment records. Estuar Coast Shelf S 47:77–90
Benner BA, Nelson PB, Stephen AW, George WM, Robert CL, Mervin FF (1990) Polycyclic aromatic hydrocarbon emissions from the combustion of crude oil on water. Environ Sci Technol 24:1418–1428
Chen YY (2008) The spatial and temporal distribution, source and bioavailability of PAHs in Qiantang River. College of Environmental and Resource Science, Zhejiang University, Hangzhou, 15–16 (in Chinese)
Christensen ER, Irwan AL, Razak A, Rachdawong P, Karls JF (1997) Sources of polycyclic aromatic hydrocarbons in sediment of the Kinnickinnic River, Wisconsin. J Great Lakes Res 23:61–73
Feng CL, Xia XH, Shen ZY, Zhou Z (2007) Distribution and sources of polycyclic aromatic hydrocarbons in Wuhan section of the Yangtze River, China. Environ Monit Assess 133:447–458
Fernandes MB, Sicre MA, Boireau A, Tronszynski J (1997) Polycyclic aromatic hydrocarbon (PAHs) distributions in the Seine River and its estuary. Mar Pollut Bull 34:857–867
Harrison RM, Smith DJT, Luhana L (1996) Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environ Sci Technol l30:825–832
Heemken OP, Stachel B, Theobald N, Wenclawiak BW (2000) Temporal variability of organic micropollutants in suspended particulate matter of the River Elbe at Hamburg and the River Mulde at Dessau, Germany. Arch Environ Contam Toxicol 38:11–31
Kalf DF, Crommentuijn T, Vandeplassche EJ (1997) Environment quality objectives for l0 Polycyclic aromatic hydrocarbons (PAHs). Ecotoxicol Environ Saf 36:89–97
Khim JS, Lee KT, Kannan K, Villeneuve DL, Giesy JP, Koh CH (2001) Trace organic contaminants in sediment and water from Ulsan bay and its vicinity, Korea. Arch Environ Contam Toxicol 40:141–155
Koh CH, Khim JS, Kannan K, Villeneuve DL (2004) Polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), biphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAHs) and 2, 3, 7, 8-TCDD equivalents (TEQs) in sediment from the Hyeongsan River, Korea. Environ Pollut 132:489–501
Li GC, Xia XH, Yang ZF (2006) Distribution and sources of polycyclic aromatic hydrocarbons in the middle and lower reaches of the Yellow River, China. Environ Pollut 144:985–993
Liu X, Zhang G, Jones KC, Li X, Peng X, Qi S (2005) Compositional fractionation of polycyclic aromatic hydrocarbons (PAHs) in mosses (Hypnum plumaeformae WILS) from the northern slope of Nanling Mountains, South China. Atmos Environ 39:5490–5499
Mackay D, Shi WY, Ma KC (1992) Illustrated handbook of physical-chemical properties and environmental fate for organic chemicals, vol II. Lewis Publishers, Chelsea, MI
Mai BX, Fu JM, Zhang G, Lin Z (2001) Polycyclic aromatic hydrocarbons in sediment from the Pearl river and estuary, China, spatial and temporal distribution and sources. Appl Geochem 16:1429–1445
Maliszewska-Kordybach B (1996) Polycyclic aromatic hydrocarbons in agricultural soil in Poland, preliminary proposals for criteria to evaluate the level of soil contamination. Appl Geochem 11:121–127
Nisbet C, LaGoy P (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16:290–300
Van Brummelen TC, Verweij RA, Wedzinga SA, Van Gestel CAM (1996) Enrichment of polycyclic aromatic hydrocarbons in forest soil near a blast furnace plant. Chemosphere 32:293–314
Zhang ZL, Huang J, Yu G, Hong HS (2004) Occurrence of PAHs, PCBs and organochlorine pesticides in the Tonghui River of Beijing, China. Environ Pollut 130:249–261
Zhou JL, Hong HS, Zhang ZL, Maskaoui K, Chen WQ (2000) Multi-phase distribution of organic micropollutants in Xiamen Harbour, China. Water Res 34:2132–2150
Acknowledgments
We wish to thank Ministry of Science and Technology, China for supporting scientists of China working on the National Basic Research Program of China (No. 2006CB403403) and the National Water Pollution Control Major Project of China (No. 2008ZX07209009). And we acknowledge Lin Chao from the Hai River Water Conservancy Commission and Cao Jinling from Beijing Normal University for help in sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, Z., Liu, J., Luan, Y. et al. Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons in the Luan River, China. Ecotoxicology 19, 827–837 (2010). https://doi.org/10.1007/s10646-010-0464-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0464-5