Abstract
Nitric oxide (NO) is a mediator and biomarker of pro- and anti-inflammatory processes. Excessive levels of NO for long periods have been associated with inflammation and tissue damage. The metabolism and synthesis of NO is usually measured indirectly, as metabolites and enzymes involved in reactions, often as the nitrite/nitrate (NOx) level. The aim of the present study was to measure the NOx levels in vital organs of juvenile silver catfish (Rhamdia quelen) exposed to various levels of eprinomectin in the water. The fish were exposed for 24 and 48 h to start concentration (0 h) of eprinomectin in water (0.0, 1.12, 1.80, and 3.97 μg/L). The eprinomectin concentrations in water were lower at 24 h (0.0, 0.85, 1.14, and 1.15 μg/L) and 48 h (0.0, 0.39, 0.69, and 1.28 μg/L), due to the process of eprinomectin metabolization. Subsequently, the fish were left for 48 h of recovery in eprinomectin-free water. NO levels were measured indirectly, as NOx levels in brain, liver, and gill tissue. Within 24 h of exposure, there was no significant increase in NOx levels in the organs evaluated at any of the concentrations tested. However, increases in NOx levels did occur at 48 h of exposure in all organs, particularly at the two highest concentrations of eprinomectin (1.80 and 3.97 μg/L). The transfer of fish to eprinomectin-free water did not result in reversal of NOx levels after 48 h of recovery, especially in fish that had been exposed to the two highest concentrations in the brain and liver tissues, and for the highest concentration in the gills. We conclude that silver catfish exposed to eprinomectin for up to 48 h present possible cerebral, hepatic, and branchial inflammatory process associated with increased tissue NOx levels, and that recovery for 48 h in water without antiparasitic is insufficient for the fish to recover from the poisoning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitric oxide (NO) is a simple, small, versatile gas molecule with high diffusibility; however, it is highly toxic by virtue of the presence of a free radical (an extra electron) that causes it to become highly reactive. NO is produced by enzymes iNOS (nitric oxide synthase) and eNOx (endothelial oxide synthase) (Thomas et al. 2008). When diluted, NO has a half-life of less than 10 s due to its rapid oxidation to nitrite and nitrate, which gives rise to the variable used to measure NO, the nitrite/nitrate level (NOx) (Tatsch et al. 2011). NO functions as a neurotransmitter, in addition to endocrine, autocrine, and paracrine activity. It is also recognized as intercellular messenger or NO-messenger in the cardiovascular, bronchopulmonary, renal, and nervous systems (Bahadoran et al. 2020). NO can act beneficially or as a toxin, depending on its concentration and the tissue in question, as well as on the tissue clearance capacity for NO. Among its cytotoxic effects, NO can bind to hemoglobin or other proteins that contain a heme nucleus and can induce cell death, compromising tissues and organs (Flora Filho and Zilberstein 2000).
Eprinomectin is a veterinary antiparasitic drug from the group of macrocyclic lactones and is a member of the avermectin family. It is administered primarily in topical or injectable form. Its main indication is the prevention or treatment of infections by endoparasitic organisms, mainly nematodes, and ectoparasites such as flies. Veterinary drugs that contain eprinomectin are intended for ruminants, especially cattle (Merck 1996), and also can be used for treatment of cats (Kvaternick et al. 2014) and dogs (Kozan et al. 2008). Contamination of terrestrial and aquatic biotas by eprinomectin can occur through direct or indirect human activity, inadequate disposal of residual drugs or packaging, leaching, through the feces of animals treated during grazing or organic fertilization, or through contact of these animals with water during baths or rain exposure (when the animal is treated topically) (Serafini et al. 2019a, 2019b, 2019c). According to the Environment Agency of England, the predicted eprinomectin concentration in surface water and groundwater used to treat pasture animals are 0.94 μg/L and 0.001 μg/L, respectively, or 1.11 μg/L in surface water when used to treat intensively reared livestock. The pollution of the aquatic environment with eprinomectin, as well as its effects on non-target aquatic organisms, has attracted particular attention because of the lack of scientific data on its toxicological aspects.
Recent studies have focused on demonstrating that eprinomectin has adverse and toxic effects on non-target terrestrial (Serafini et al. 2019a) and aquatic (Alak et al. 2017; Serafini et al. 2019b, 2019c) organisms, and that these effects can cause concerning impacts on the environment, even directly or indirectly in aquaculture production, especially in freshwater fish. Recently, Serafini et al. (2019b) found that silver catfish (Rhamdia quelen) exposed to 1.124, 1.809, and 3.976 μg eprinomectin/L water for 24 and 48 h gave rise to hepatic oxidative stress caused by increased levels of oxidation biomarkers (reactive oxygen species (ROS) and lipid peroxidation) and impaired activity of antioxidant enzymes (glutathione peroxidase, superoxide dismutase, and glutathione-S transferase), contributing to hepatic damage and toxicity. The same authors reported an impairment of essential enzymes involved in maintenance of bioenergetic homeostasis in silver catfish exposed to eprinomectin, as adenylate kinase and pyruvate kinase (Serafini et al. 2019b), which reveals the negative effects of this drug on non-target organisms. Serafini et al. (2019c) also found that these same concentrations of eprinomectin affect brain tissue, manifesting as behavioral changes caused by increased levels of ROS and inhibition of important neurotransmitters, including acetylcholine and the sodium-potassium ATPase. It is important to note that the recovery periods proposed by these studies (i.e., 48 h in eprinomectin-free water) were insufficient to reverse the effects of the drug on the broadest range of biochemical and behavioral effects, especially those caused by exposure to the highest concentrations of eprinomectin in water (Serafini et al. 2019b, c). These data indicate impairment of fish health in environments contaminated by eprinomectin, even if in the presence of low concentrations over short exposure periods.
The findings also suggest that NO is an important mediator and biomarker molecule in pro- and anti-inflammatory processes; NOx exerts cytotoxic and tissue effects, contributing to the impairment of organs and their physiological functions (Souza et al. 2017; Baldissera et al. 2017). Although NO can be considered a defense molecule that participates in the anti-inflammatory pathway when generated in low concentrations and for short periods of time, at high concentrations, it acts as an inflammatory agent that contributes to tissue injury (Souza et al. 2017; Baldissera et al. 2018b). Therefore, the aim of this study was to measure levels of NOx levels in the brain, liver, and gills of silver catfish exposed to eprinomectin and to determine its toxic effects.
Materials and methods
Eprinomectin
Eprinomectin (C49H73NO14) (3.6%; molecular weight of 900.1 g/mol) was purchased commercially (Eprinex®; Merial, Brazil) and was directly applied in the water to evaluate its possible toxic effects on silver catfish exposed to 1.12, 1.80, and 3.97 μg/L.
Fish maintenance and experimental design
Juvenile R. quelen (males; 7 months old; 60.99 ± 4.14 g; 14.10 ± 1.11 cm) were collected from a fish farm located in Southern Brazil. The animals were transported alive and were maintained for acclimation in 250-L fiberglass tanks with continuous aeration and controlled water variables (temperature 21 ± 0.2 °C; pH 6.41 ± 0.1; ammonia 0.84 ± 0.06 mg/L; non-ionized ammonia 0.0046 ± 0.00031 mg/L; dissolved oxygen 6.71 ± 0.59 mg/L) for 10 days, which was monitored once per day. The fish were fed to apparent satiation with commercial pellets (Supra®; Alisul, RS, Brazil), 2.5-mm in size, once a day, in the proportion of 10% of the biomass of the group and with continuous feeding during the experimental period. Any uneaten feed, feces, and other residues were removed daily 30 min after feeding.
The fish were randomly allocated in aerated tanks (static water system) with the same characteristics used in the acclimation period, with three replicates per concentration and six fish per replicate, a total of 72 fish. Water quality variables remained unaltered throughout the entire experimental period. Four treatments were applied: 0.0 μg/L of eprinomectin (control group) and three actual concentrations of eprinomectin in water—1.12, 1.80, and 3.97 μg/L—as recommended by Serafini et al. (2019b), (2019c) who observed negative impacts on silver catfish.
Actual water eprinomectin concentration
Actual eprinomectin concentration in water was quantified using ultra-high-performance liquid chromatography coupled to mass spectrophotometry (UHPLC-MS), as published in detail by Serafini et al. (2019b), (2019c). The actual concentrations at each time period of collection (0, 24, and 48 h) were as follows: at 0 h, eprinomectin concentrations in water were 1.12, 1.80, and 3.97 μg/L in the three groups, respectively. At 24 h, eprinomectin concentrations in water were 0.85, 1.14, and 1.15 μg/L in the three groups, respectively. At 48 h, eprinomectin concentrations in water were 0.39, 0.69, and 1.28 μg/L in the three groups, respectively (Serafini et al. 2019b, 2019c). It is important to note that the concentration of eprinomectin decreases in water over time due to metabolism of eprinomectin molecule; no additional water was added to the boxes between 0 and 48 h.
Sample collection
After 24 and 48 h of exposure and after 48 h of recovery in eprinomectin-free water, two fish from each tank (six fish per treatment at each given time) were anesthetized using 50 mg/L eugenol (Odontofarma®, RS, Brazil) (Da Cunha et al. 2010), followed by spinal cord section according to ethics committee recommendations. Subsequently, brain, liver, and gills were removed and dissected in glass dishes over ice to evaluate the parameters detailed below. Cerebral, hepatic, and branchial tissues were homogenized (1:10 w/v) in glass Potter tubes with 10 mM Tris-HCl buffer pH 7.4 and centrifuged at 2000×g for 10 min. The supernatants were collected and stored at − 20 °C.
Nitrite/nitrate (NOx) concentration
Cerebral, hepatic, and branchial NOx levels were evaluated indirectly using nitrite/nitrate quantification according to the Griess method (Tatsch et al. 2011) as described by Baldissera et al. (2018b). Results were expressed as μmol/mg of protein.
Statistical analysis
Data were tested for normality and homoscedasticity using Kolmogorov–Smirnov and Levene tests, respectively. Subsequently, statistical analysis was performed using bilateral two-way analysis of variance (ANOVA), followed by the Tukey post hoc test. Significance was determined when p < 0.05. The results were expressed as mean and standard deviation.
Results
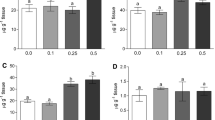
No significant differences were observed between groups with respect to brain, hepatic, or branchial NOx levels after 24 h of exposure at all eprinomectin concentrations from those of the control group (0.0 μg/L) (Figs. 1, 2, and 3).
Nitric oxide (NOx) levels in brain of juvenile silver catfish (Rhamdia quelen) after 24 and 48 h of eprinomectin exposure in the water experimentally contaminated, and after 48 h of recovery in eprinomectin-free water. Data are expressed as mean and standard deviation (┬) in six animals for each group. Different letters (a, b) under the bar indicate statistically significant differences between treatments using analysis of variance (ANOVA) and Tukey’s test (p < 0.05; n = 6 per group), at each evaluation time point
Nitric oxide (NOx) levels in liver of juvenile silver catfish (Rhamdia quelen) after 24 and 48 h of eprinomectin exposure in the water experimentally contaminated, and after 48 h of recovery in eprinomectin-free water. Data are expressed as mean and standard deviation (┬) in six animals for each group. Different letters (a, b) under the bar indicate statistically significant differences between treatments using analysis of variance (ANOVA) and Tukey’s test (p < 0.05; n = 6 per group), at each evaluation time point
Nitric oxide (NOx) levels in gills of juvenile silver catfish (Rhamdia quelen) after 24 and 48 h of eprinomectin exposure in the water experimentally contaminated, and after 48 h of recovery in eprinomectin-free water. Data are expressed as means and standard deviations (┬) in six animals for each group. Different letters (a, b, c) under the bar indicate statistically significant differences between treatments using analysis of variance (ANOVA) and Tukey’s test (p < 0.05; n = 6 per group); at each evaluation time point
Brain NOx levels were significantly higher (p = 0.021) in fish exposed to 1.80 and 3.97 μg/L of eprinomectin compared to the control (0.0 μg/L) after 48 h of exposure and remained significantly higher after 48 h of recovery in eprinomectin-free water (p = 0.003) (Fig. 1).
In liver at 48 h, NOx levels were significantly higher (p = 0.018) in fish exposed to 1.12 and 3.97 μg/L of eprinomectin in water compared to the control (0.0 μg/L). After 48 h of recovery in eprinomectin-free water, all groups exposed to the contaminant showed a significant increase in NOx levels in the liver compared to the control group (p = 0.031) (Fig. 2).
In gills at 48 h, NOx levels were significantly higher (p < 0.001) in fish exposed to 1.80 and 3.97 μg/L eprinomectin compared to the control (0.0 μg/L). After 48 h of recovery in eprinomectin-free water, levels in fish exposed to 1.80 μg/L eprinomectin did not differ significantly from those of the control group, while NOx levels remained significantly increased in the group exposed to 3.97 μg/L eprinomectin (p < 0.001) (Fig. 3).
There was no significant difference (p > 0.05) with respect to time within the same exposure group for all tissues analyzed (Figs. 1, 2, and 3). There was also no mortality in fish at any of the concentrations or times of exposure to eprinomectin or even during the recovery period.
Discussion
Our results indicate, for the first time, that eprinomectin promotes increased NOx levels in the brain, liver, and gills of silver catfish. The brain and liver were the most affected organs during exposure to eprinomectin due to the significant increase in NOx levels and the lack of recovery in eprinomectin-free water, revealing its persistence and the possibility of causing damage for long periods of time, as well as the possible capacity to accumulate in the tissues. This may have a direct relationship with the mode of action of avermectins, which can act on the central nervous system of fish (Serafini et al. 2019c), and by the metabolism of detoxification of fish, whose main organ responsible is the liver (Serafini et al. 2019b).
Baldissera et al. (2018a) showed a significant increase in splenic and serum NOx levels after exposure to 22 mg/L of ectoparasitic trichlorfon for 48 h, which remained elevated after 48 h of recovery in trichlorfon-free water. According to these authors, the exacerbated increases and the absence of reduction in NOx levels to physiological levels suggests that NO is an important mediator of the inflammatory process and tissue damage during exposure to environmental concentrations of trichlorfon. Exposure to the pesticide pyriproxyfen caused toxicity in larvae of zebrafish (Danio rerio) via an increase in NOx levels, which contributes to its toxic effects (Maharajan et al. 2018). In vitro exposure to pesticides carbaryl (105 μM), kepone (144 μM), and malathion (170 μM) elicited increased brain NOx levels due to interactions with ionic calcium, exerting toxic effects in the brain (Rao et al. 1999) that can be considered a possible mechanism of action of eprinomectin. Increased brain NOx levels in common carp (Cyprinus carpio) exposed to pesticide imidacloprid was associated with upregulation of iNOs and eNOS activity, which can be another pathway involved on eprinomectin-induced NOx formation (Ozdemir et al. 2018). In this sense, increased NOx levels over long periods can indicate oxidative and inflammatory damage in silver catfish. When evaluating our results and those of studies by Serafini et al. (2019b), (2019c), it is possible to emphasize that there are pro-oxidant and pro-inflammatory profiles in the brain, liver, and gills of fish exposed to eprinomectin that can contribute to the compromise of their physiological functions and survival.
Conclusions
Elevation of NO levels occurred in brain, liver, and gill tissues following eprinomectin exposure. This can be interpreted as negative effects on fish health, because increased NO metabolism causes nitrosative stress and inflammatory responses, changes that increase tissue and cellular damage. Despite removing the fish for 48 h from the water contaminated by eprinomectin, the alterations in the levels of NOx did not return to baseline levels.
References
Alak G, Yeltekin AÇ, Tas IH, Ucar A, Parlak V, Topal A, Kocaman EM, Atamanalp M (2017) Investigation of 8-OHdG, CYP1A, HSP70 and transcriptional analyses of antioxidant defense system in liver tissues of rainbow trout exposed to eprinomectin. Fish Shellfish Immunol 65:136–144. https://doi.org/10.1016/j.fsi.2017.04.004
Bahadoran Z, Carlstrom M, Mirmiran P, Ghasemi A (2020) Nitric oxide: to be or not to be an endocrine hormone? Acta Physiol 229:e13443. https://doi.org/10.1111/apha.13443
Baldissera MD, Souza CF, Doleski PH, Moreira KLS, Da Rocha MIUM, Da Veiga ML, Santos RCV, Baldisserotto B (2017) Xanthine oxidase activity exerts a pro-oxidant and pro-inflammatory profile in gills of experimentally infected silver catfish with Streptococcus agalactiae. Aquaculture 477:71–75. https://doi.org/10.1016/j.aquaculture.2017.04.025
Baldissera MD, Souza CF, Descovi SN, Zanella R, Stefani LM, Da Silva AS, Baldisserotto B (2018a) Purinergic signalling as a potential pathway for trichlorfon induced-inflammation and impairment of the immune response using freshwater silver catfish. Aquaculture 497:91–96. https://doi.org/10.1016/j.aquaculture.2018.07.037
Baldissera MD, Souza CF, Santos RCV, Baldisserotto B (2018b) Purinergic system displays an anti-inflammatory profile in serum of silver catfish experimentally infected with Streptococcus agalactiae: an attempt to ameliorate the inflammatory response. Microb Pathog 114:193–196. https://doi.org/10.1016/j.micpath.2017.11.060
Da Cunha MA, Zeppenfeld CC, Garcia LO, Loro VL, Da Fonseca MB, Emanuelli T, Veeck APL, Copatti CE, Baldisserotto B (2010) Anesthesia of silver catfish with eugenol: time of induction, cortisol response and sensory analysis of fillet. Ciência Rural 40:2107–2114. https://doi.org/10.1590/S0103-84782010005000154
Flora Filho R, Zilberstein B (2000) Óxido nítrico: o simples mensageiro percorrendo a complexidade. Metabolismo, síntese e funções. Rev Assoc Méd Bras 46:265–271. https://doi.org/10.1590/S0104-42302000000300012
Kozan E, Sevimli FK, Birdane FM, Adanir R (2008) Efficacy of eprinomectin against Toxocara canis in dogs. Parasitol Res 102(397–400):2008–2400. https://doi.org/10.1007/s00436-007-0776-4
Kvaternick V, Kellermann M, Knaus M, Rehbein S, Rosentel J (2014) Pharmacokinets and metabolism of eprinomectin in cats when administered in a novel topical combination of fipronil, (S)-methoprene, eprinomection and praziquantel. Vet Parasitol 202:2–9. https://doi.org/10.1016/j.vetpar.2014.02.031
Maharajan K, Muthulakshmi S, Nataraj B, Ramesh M, Kadirvelu K (2018) Toxicity assessment of pyriproxyfen in vertebrate model zebrafish embryos (Danio rerio): a multi biomarker study. Aquat Toxicol 196:132–145. https://doi.org/10.1016/j.aquatox.2018.01.010
Merck (1996) Ivomec Eprinex (eprinomectin) pour-on for beef and dairy cattle: environmental assessment. Report NADA 141-079EA. Merck and Company, Rahway
Ozdemir S, Altun S, Arslan H (2018) Imidacloprid exposure cause the histopathological changes, activation of TNF-α, iNOS, 8-OHdG biomarkers, and alteration of caspase 3, iNOS, CYP1A, MT1 gene expression levels in common carp (Cyprinus carpio L.). Toxicol Rep 5:125–138. https://doi.org/10.1016/j.toxrep.2017.12.019
Rao MR, Kanji VK, Sekhar V (1999) Pesticide induced changes of nitric oxide synthase in rat brain in vitro. Drug Chem Toxicol 22:411–420. https://doi.org/10.3109/01480549909017844
Serafini S, Soares JG, Perosa CF, Picoli F, Segat JC, Da Silva AS, Baretta D (2019a) Eprinomectin antiparasitic affects survival, reproduction and behavior of Folsomia candida biomarker, and its toxicity depends on the type of soil. Environ Toxicol Pharmacol 72:103262. https://doi.org/10.1016/j.etap.2019.103262
Serafini S, Souza CF, Baldissera MD, Baldisserotto B, Picoli F, Segat JC, Baretta D, Da Silva AS (2019b) Fish exposed to eprinomectin show hepatic oxidative stress and impairment in enzymes of the phosphotransfer network. Aquaculture 508:199–205. https://doi.org/10.1016/j.aquaculture.2019.04.081
Serafini S, Souza CF, Baldissera MD, Baldisserotto B, Segat JC, Baretta D, Zanella R, Da Silva AS (2019c) Fish exposed to water contaminated with eprinomectin show inhibition of the activities of AChE and Na+/K+-ATPase in the brain, and changes in natural behavior. Chemosphere 223:124–130. https://doi.org/10.1016/j.chemosphere.2019.02.026
Souza CF, Baldissera MD, Moreira KLS, Da Rocha MIUM, Da Veiga ML, Santos RCV, Baldisserotto B (2017) Involvement of xanthine oxidase activity with oxidative and inflammatory renal damage in silver catfish experimentally infected with Streptococcus agalactiae: interplay with reactive oxygen species and nitric oxide. Microb Pathog 111:1–5. https://doi.org/10.1016/j.micpath.2017.08.010
Tatsch E, Bochi GV, Pereira RS, Kober H, Agertt VA, De Campos MMA, Gomes P, Duarte MMMF, Moresco RN (2011) A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem 44:348–350. https://doi.org/10.1016/j.clinbiochem.2010.12.011
Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzellie S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton C, Harris C, Roberts DD, Wink DA (2008) The chemical biology of nitric oxide. Implications in cellular signaling. Free Radic Biol Med 45:18–31. https://doi.org/10.1016/j.freeradbiomed.2008.03.020
Acknowledgments
The authors would like to thank the CAPES (Brazil) and CNPq (Brazil) for their technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics committee
This study was approved by the Ethical and Animal Welfare Committee of the Universidade do Estado de Santa Catarina (protocol number 4679260518).
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Serafini, S., de Freitas Souza, C., Baldissera, M.D. et al. Nitric oxide levels in brain, liver, and gills of silver catfish (Rhamdia quelen) exposed to the antiparasitic eprinomectin. Fish Physiol Biochem 46, 1867–1872 (2020). https://doi.org/10.1007/s10695-020-00836-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00836-2