Abstract
Recently, residual organotin compounds have generally been recognised as relevant sources of aquatic environmental pollutants. However, the effects of these contaminants on the glutathione (GSH)-antioxidant system of fishes have not been adequately studied. In the current study, the chronic effects of tributyltin (TBT) found within antifouling paints for ships, on the GSH antioxidant system and related gene expression in the liver of juvenile common carp (Cyprinus carpio) were investigated. Fishes were exposed to sub-lethal concentrations of TBT (75 ng/L, 0.75 and 7.5 μg/L) for 15, 30 and 60 days. GSH levels and GSH-related enzymes activities, including glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione S-transferase (GST), were quantified in the fish liver. The levels of malondialdehyde were also measured as a marker of oxidative damage. In addition, the expression levels of gstp1, gr and gpx1 in common carp chronically exposed to TBT were determined. The results of the current study indicate that chronic exposure of TBT results in reactive oxygen species stress in the liver of common carp, and mRNA expression levels are more sensitive than related enzyme levels. In short, the measured GSH-related indices could potentially be used as molecular indicators for monitoring organotin compounds in the aquatic environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidative stress has been defined as an imbalance of oxidants and antioxidants in favour of oxidants, potentially leading to cell damage (Azzi et al. 2004). To cope with the oxidative damage, organisms have evolved multiple systems of antioxidant defenses. Endogenous enzymatic and non-enzymatic antioxidants are essential for the conversion of reactive oxygen species (ROS) to harmless substances and for maintenance of cellular metabolism and function (Mates 2000; Zhang et al. 2008).
The aquatic environment is contaminated by a number of foreign chemical pollutants that have the potential to exert cytotoxic effects in fishes by production of ROS (Oliveira et al. 2008; Li et al. 2010). The glutathione (GSH)-related antioxidant system plays a central role in the intercellular defense mechanism counteracting oxidative stress, which is comprised of GSH and its associated enzymes, such as glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione S-transferase (GST) (Hidalgo et al. 2002). Under normal physiological conditions, the total amount of cellular GSH maintains a balance between its synthesis and its utilisation. Furthermore, molecular biomarkers are considered as sensitive indicators of the early effects of xenobiotics in organisms and have been useful tools for monitoring some effects of environmental pollutants, as the utilisation of molecular endpoints in ecotoxicology can provide rapid and valuable information on immediate organismal responses to chemical stressors (Beggel et al. 2012).
Among them, the adverse effects of organotin compounds, particularly tributyltin (TBT), have generated concern due to their wide presence, persistence and potential toxicity (Antizar-Ladislao 2008). Unfortunately, although there are documented cases of antioxidant responses of fishes exposed to environmental pollutants, there are limited data on the effects of residual organotin compounds on the GSH-antioxidant system of fishes. In China, cases of TBT in the environment were recorded at concentrations of 0.5 ng/L (detection limited) to hundreds of nano grams per litre as tin (Sn) in surface water (Gao et al. 2006).
In the present study, juvenile common carp (Cyprinus carpio), a fish species widely used in aquatic toxicology, was chosen as the test organism to determine the responses of the GSH-antioxidant system in the liver exposed to TBT over chronic time scales. This was done by analysing the GSH-antioxidant system (GSH contents and GR, GPx and GST activities) and oxidative stress indices (MDA) in fish liver, as well as the expression levels of the glutathione-related genes gstp1, gr and gpx1.
Materials and methods
Chemicals
TBT (90 %) was purchased from Sinopharm Chemical Reagent Co., Ltd (Beijing, China). A suitable amount of this compound was directly weighed into a brown vessel and dissolved in 50 ml acetone (ACT)-water (1:1) to form a stable concentration level. This stock solution was sealed and kept at 4 °C in a refrigerator until used. A working standard solution (100 μg/ml) was freshly prepared by diluting the stock solution with deionized water before use.
Fish
The juvenile common carp (9.65 ± 0.13 cm, 22 ± 1.8 g) obtained from a local hatchery (Jingzhou, China), were raised in a flow-through system with dechlorinated tap water (pH 7.4 ± 0.2; hardness 42.5 ± 1.3 CaCO3/L) at a constant temperature (20 ± 1 °C) with a photoperiod of 12:12 h (light:dark). Fish were acclimatised for 14 days before the beginning of the experiment and were fed commercial fish food (Tongwei, China). Waste and residue were removed daily while the test equipment and chambers were cleaned once a week. The fish were not fed for 24 h prior to experimentation to avoid prandial effects during the assay.
Exposure to TBT
A 100 l semi-static system was used in which 20 juvenile common carp were randomly distributed to each of ten aquaria. The nominal concentrations of TBT used were 75 ng/L (E1 group, according to environmental concentration), 0.75 μg/L (E2 group, 1 % 96 h-LC50) and 7.5 μg/L (E3 group, 10 % 96 h-LC50). Prior to the present study, the common carp 96 h (acute) LC50 for TBT had been determined (75.0 μg/L, unpublished). TBT was dissolved in ACT with a final concentration <0.01 %. Two other groups were used as control groups: a group exposed to clean freshwater and an ACT group exposed to the volume of ACT (v/v, 0.01 %) used for the highest TBT concentration. Each experimental condition was duplicated. The fish were fed daily with commercial fish pellets at 1 % total body weight at a fixed time and the extra food was removed. A total of 80 % of the exposed solution was renewed each day after 2 h of feeding to maintain the appropriate concentration of TBT and ACT and to maintain water quality. The test equipment was cleaned every 7 days. The test fish were exposed to TBT for 15, 30 and 60 days. At the end of each exposure period, three randomly selected fish from each aquarium were killed. The liver tissue was quickly removed, immediately frozen and stored at −80 °C until analysis.
To ensure agreement between nominal and actual compound concentrations in the aquaria, water samples were analysed during the experimental period by liquid chromatography–mass spectrometry/mass spectrometry. Water samples were collected from the test aquaria after 1 and 24 h of renewing the test solutions. The mean concentration of TBT in the water samples was always within 20 % of the intended concentration.
Biochemical variable measurement
Frozen samples for analysis of enzyme activities were defrosted and homogenised on ice with ten volumes of cold, 0.86 % physiological saline. The homogenate was centrifuged at 3,000 rpm at 4 °C for 10 min to obtain the supernatant for the enzyme activity assays of glutathione-S-transferase (GST; EC 2.5.1.18), glutathione reductase (GR; EC 1.6.4.2), glutathione peroxidase (GPx; EC 1.11.1.9), monoamine oxidase (MAO: EC 1.4.3.4) and the content of the malondialdehyde (MDA) and reduced glutathione (GSH). All biochemical variables were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Protein concentrations in the supernatants were determined using the Bradford assay with bovine serum albumin as the standard (Bradford 1976).
GST (EC 2.5.1.18) activity was measured according to Habig et al. (1974) using 1-chloro-2,4-dinitrobenzene (CDNB) as the substrate. GR (EC 1.6.4.2) activity was determined spectrophotometrically, measuring nicotinamide adenine dinucleotide phosphate (NADPH) oxidation at 340 nm (Carlberg and Mannervik 1975). GPx (EC 1.11.1.9) activity was measured by using H2O2 as a substrate and was determined spectrophotometrically at 340 nm and 37 °C, with the activity expressed as mU/mg protein (Lawrence and Burk 1976). MDA content was examined as an indicator of lipid peroxidation, which is based on the 2-thiobarbituric acid (2,6-dihydroxypyrimidine-2-thiol; TBA) reactivity, with the results expressed as nmol/mg protein (Jain et al. 1989). The GSH content was determined spectrophotometrically by monitoring the chromophoric product resulting from the reaction of the 5,5′-dithiobis-(2-nitrobenzoic acid) with GSH in the presence of NADPH and glutathione reductase at 412 nm (Wu et al. 2011). The GSH content was expressed as μg/mg protein.
Total RNA extraction and real-time PCR
Total RNA was extracted from fish tissues using a Trizol kit (TaKaRa, Dalian, China). After the total RNA was incubated with deoxyribonuclease I (TaKaRa, Dalian, China), the reverse transcription was performed on 1 μg of total RNA following a PrimeScript™ 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China) using the manufacturer’s recommended procedure. Levels of gstp1, gr and gpx1 in the fish tissues were determined using quantitative reverse transcription (RT)-PCR with SYBR Green chemistry on a Rotor-Gene 3000 (Applied Biosystems, USA) using the housekeeping gene, β-actin as an internal control according to the method of our laboratory (Li et al. 2014). The differences in expression levels were calculated using the 2−∆∆Ct method (Livak and Schmittgen 2001). The quantitative RT-PCR primers are shown in Table 1.
Statistical assays
All values were expressed as mean ± SD and analysed by SPSS for Win 13.0 software (SPSS Inc., Chicago, IL, USA). One-way ANOVA with Tukey’s test was used to determine whether results of treatments were significantly different from the control group (p < 0.05 or p < 0.01). In addition, principal component analysis (PCA) within Statistic 6.0 was used to define the most important parameters, which could be used as key factors for individual variations.
Results
Oxidative stress indices and glutathione-related antioxidant variables
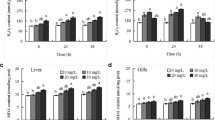
To verify the presence of oxidative imbalance induced by TBT, levels of MAD (as indicated by tissue ROS levels) and glutathione-related antioxidant variables were measured in all groups (Table 2). Compared with the control group, a significant increase (p < 0.05) in MAD levels was observed in the E2 group after 60 days and E3 group after 30 days exposure.
In the liver, a significant decrease (p < 0.05) in GSH levels in the E2 and E3 groups was observed after 30 days of exposure. After 60 days exposure, the significant decrease (p < 0.05) in GSH levels was maintained in the E2 group, whereas it resumed to control level in the E3 group. Hepatic GST activity was significantly induced in the E2 and E3 groups after 30 days of exposure, which was maintained in the E2 group after 60 days. Long-term exposure to TBT induced a significant increase (p < 0.05) in the activity of GR in the liver of fish in the E2 and E3 groups after 30 days exposure, which was maintained in that of the E2 group after 60 days exposure. In addition, there was a significant increase (p < 0.05) in the GPx activity in the liver of fish in the E2 and E3 groups after long-term exposure (30 and 60 days).
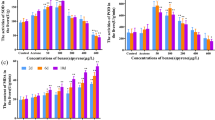
Expression of glutathione-related genes
The mRNA expression of glutathione-related genes (gstp1, gr and gpx1) in the liver of common carp was evaluated in our study (Fig. 1). The transcription levels of all genes increased and were significantly up-regulated (p < 0.05) in the E3 group after 15 days exposure, whereas the gpx1 gene especially was significantly induced in the E2 group. After 30 days exposure, the expression levels of the three genes were up-regulated in all TBT-treated groups in a dose-dependent manner. At the end of experiment, there was a decreasing trend in expression levels of the genes gstp1 and gr in the E3 group, although the expression levels were significantly higher (p < 0.05) than the control. In addition, the gpx1 gene began to decrease in the E2 group after 60 days exposure.
Chemometrics
Using PCA, all the variables measured in the current study were distinguished on the ordination plots corresponded to the first and second principle components (76.78 and 10.82 %, respectively) (Fig. 2). Furthermore, the observed relationships among the variables were confirmed (Table 3).
Discussion
Several studies have demonstrated that environmental pollution induces the production of ROS, which may be scavenged by the antioxidant defense system (Oruc et al. 2004). Oxidative stress, the final manifestation of a multi-step pathway, results in an imbalance between pro-oxidant and antioxidant defense mechanisms due to the depletion of antioxidants or excessive accumulation of ROS, or both, leading to damage. MDA, as a lipid peroxidation indicator, has been used to evaluate the oxidative stress of aquatic animals (Rajeshkumar et al. 2013). In this study, the significantly increasing MDA levels indicated the generation of serious oxidative stress in fish exposed to TBT.
Fish exposed to pollutants can purge the foreign contaminants by conjugation with GSH directly or by means of GSH-related antioxidant enzymes, which leads to a depletion of GSH levels (Oruc et al. 2004). Therefore, the change in GSH levels is considered as a potential indicator of environmental stress. The current study demonstrates that long-term exposure of TBT to the carp induced a significant decrease in GSH contents, probably by the result of impaired GSH synthesis. On the basis of the obtained results, the increase in GPx and GR activities could be interpreted as an adaptive response to oxidative stress. Similar to GR and GPx, GST activity can be induced due to an adaptive mechanism to counteract marginal oxidative stress. However, severe oxidative stress may suppress GST activity due to the exhaustion of GSH and/or disruption of its synthesis. The results from the current study, together with those of previous studies, demonstrate that an induction of GST activity may lead to a fall in GSH levels. The correlation between GST and GSH could be summarised as the activity of GST was restricted by GSH to some extent (Zhang et al. 2004).
Furthermore, the expression levels of glutathione-related genes were induced in the TBT-treated groups after 15 days exposure, while the activities of enzymes did not change significantly, indicating that mRNA expression levels are more sensitive than related enzyme activities in fish liver under TBT stress. On the basis of the results, all gene expressions were up-regulated in the E1 group (environmental level) after 30 days. This demonstrates that the expression of glutathione-related genes would be altered by TBT at environmental levels; therefore, the expression of glutathione-related genes could be a potential molecular variable for monitoring TBT in aquatic environments.
Conclusion
In conclusion, results of the present investigation indicate that long-term exposure to sub-lethal concentrations of TBT causes ROS stress in the liver of common carp reflected by the significantly higher MDA levels as well as the different responses of GSH-related antioxidant systems. On the basis of the above mentioned results, the fish liver demonstrated a higher adaptive competence expressed as antioxidant defenses activation. Moreover, the glutathione-related genes displayed a greater sensitivity than enzyme activities under TBT-induced stress. In summary, this study indicates that the GSH-related antioxidant system of fishes could potentially provide useful information for monitoring organotin compounds in aquatic environments. On the other hand, the exact molecular mechanism of regulation of the GSH-related antioxidant system in fishes under oxidative stress is still unclear, which requires further study.
Abbreviations
- TBT:
-
Tributyltin
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GST:
-
Glutathione-S-transferase
- GSH:
-
Reduced glutathione
- ACT:
-
Acetone
- GSSG:
-
Oxidized glutathione
- TCA:
-
Trichloroacetic acid
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- PCA:
-
Principal component analysis
References
Antizar-Ladislao B (2008) Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. a review. Environ Int 34:292–308
Azzi A, Davies KJA, Kelly F (2004) Free radical biology–terminology and critical thinking. FEBS Lett 558:3–6
Beggel S, Werner I, Connon RE, Geist JP (2012) Impacts of the phenylpyrazole insecticide fipronil on larval fish: time-series gene transcription responses in fathead minnow (Pimephales promelas) following short-term exposure. Sci Total Environ 426:160–165
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein dye binding. Anal Biochem 72:248–254
Carlberg I, Mannervik B (1975) Purification and characterization of flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Gao JM, Hu JY, Zhen H, Yang M, Li BZ (2006) Organotin compounds in the three gorges reservoir region of the Yangtze River. Bull Environ Contam Toxicol 76:155–162
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases—first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hidalgo MC, Exposito A, Palma JM, de la Higuera M (2002) Oxidative stress generated by dietary Zn-deficiency: studies in rainbow trout (Oncorhynchus mykiss). Int J Biochem Cell Biol 34:183–193
Jain SK, McVie R, Duett J, Herbst JJ (1989) Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 38:1539–1543
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium deficient rat liver. Biochem Biophys Res Commun 71:952–958
Li ZH, Zlabek V, Grabic R, Li P, Randak T (2010) Modulation of glutathione-related antioxidant defense system of fish chronically treated by the fungicide propiconazole. Comp Biochem Physiol C 152:392–398
Li ZH, Chen L, Wu YH, Li P, Li YF, Ni ZH (2014) Effects of waterborne cadmium on thyroid hormone levels and related gene expression in Chinese rare minnow larvae. Comp Biochem Physiol C-Toxicol Pharmacol 161:53–57
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-delta delta C) method. Methods 25:402–408
Mates JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
Oliveira M, Pacheco M, Santos MA (2008) Organ specific antioxidant responses in golden grey mullet (Liza aurata) following a short-term exposure to phenanthrene. Sci Total Environ 396:70–78
Oruc EO, Sevgiler Y, Uner N (2004) Tissue-specific oxidative stress responses in fish exposed to 2,4-D and azinphosmethyl. Comp Biochem Physiol C 137:43–51
Rajeshkumar S, Mini J, Munuswamy N (2013) Effects of heavy metals on antioxidants and expression of HSP70 in different tissues of Milk fish (Chanos chanos) of Kaattuppalli Island, Chennai, India. Ecotox Environ Safe 98:8–18
Wu H, Zhang R, Liu J, Guo Y, Ma E (2011) Effects of malathion and chlorpyrifos on acetylcholinesterase and antioxidant defense system in Oxya chinensis (Thunberg) (Orthoptera: Acrididae). Chemosphere 83:599–604
Zhang JF, Shen H, Wang XR, Wu JC, Xue YQ (2004) Effects of chronic exposure of 2,4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere 55:167–174
Zhang X, Yang F, Zhang X, Xu Y, Liao T, Song S, Wang H (2008) Induction of hepatic enzymes and oxidative stress in Chinese rare minnow (Gobiocypris rarus) exposed to waterborne hexabromocyclododecane (HBCDD). Aquat Toxicol 86:4–11
Acknowledgments
This study was supported by Special Scientific Research Funds for Central Non-profit Institutes, Chinese Academy of Fishery Sciences (2014A02YQ01), Technology Foundation for Selected Overseas Chinese Scholar of MOHRSS, and the CENAKVA CZ.1.05/2.1.00/01.0024, the Project LO1205 with a financial support from the MEYS of the CR under the NPU I program, the Project P503/11/1130 of the Grant agency of Czech Republic.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, ZH., Li, P. & Shi, ZC. Responses of the hepatic glutathione antioxidant defense system and related gene expression in juvenile common carp after chronic treatment with tributyltin. Ecotoxicology 24, 700–705 (2015). https://doi.org/10.1007/s10646-014-1416-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1416-2