Abstract
Industrialization and the rise in population have led to the larger utilization of resources which has become the supreme risk for the environment. Different types of pollutants enter the aquatic environment from various sources which create a threat for the aquatic organisms and humans. Many separation techniques like precipitation, adsorption, reactive distillation, ion exchange, electro dialysis, solvent extraction, and ultrafiltration are available, but these techniques have various limitations like the use of excessive and expensive chemicals, high energy requirement, sludge formation, requirement of utilities in large amount and so. The green emulsion liquid membrane (GELM) is an emerging and promising method that incorporates the traits of ELM for the removal of various pollutants, metal ions, acids, and so on. In the present scenario, much focus has been diverted towards the use of green solvents derived from vegetable and plant origin. These solvents are environmentally friendly and economically viable making the ELM process more reliable. The traditionally used petroleum-based solvents for ELM formation are expensive, toxic, volatile in nature, and are detrimental to the environment. The present study tries to address the recent advancement in the field of GELM. The different factors like concentration of surfactant, carrier, types of diluents, effect of volume ratio of external feed phase to emulsion, agitation speed, effect of internal aqueous phase concentration, and emulsification time play a substantial role in the removal of several pollutants from the aqueous streams through GELM have been discussed in detail. The statistical analysis of the operating variables as executed by different investigators is also mentioned.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Industrial development and booming of population growth are accompanying environmental pollution, especially in developing nations. Rapid evolution of chemical and process industries has led to the greater consumption of resources which has become an utmost threat to the ecosystem. In the era of urbanization, water resources are at high and constant risk of getting polluted. Significant quantities of pollutants such as acids, alkalis, dyes, pesticides, oils, grease, heavy metals, and pharmaceutical compounds are dumped into the freshwater bodies through municipal sewage waste, agricultural waste, industrial waste, landfill leachate, and the waste generated from medical institutions. This creates an ecological imbalance in the environment (Yan et al., 2021; Yaseen et al., 2021). To improve health conditions and to increase the life span of humans and animals, various pharmaceutical active compounds like analgesics, beta-blockers, lipid regulators, anti-inflammatory drugs, and estrogens are been utilised throughout the world (Olasupo & Suah, 2021). The pharmaceuticals compounds and different preservatives like acetaminophen, ibuprofen, ketoprofen, chlorpheniramine, diclofenac, norfloxacin, ethylparaben, methylparaben, and so on are noticed in the water bodies and their adverse effect are reported by the several authors (Ahmad et al., 2019; Dâas & Hamdaoui, 2014; Razo-Lazcano et al., 2018; Kohli et al., 2019a; Muthusaravanan et al., 2019; Kohli et al., 2020a; Shirasangi et al., 2021).
Moreover, low-level discharge of dyes generates numerous adverse effects like prevention of the light and decrease in gas solubility and is also harmful to aquatic life (Fetimi et al., 2021). Fetimi et al. (2021) reported that during the dyeing process, 10–25% of textile dyes are wasted and 2–20% are directly released as the aqueous stream in various water resources. The US Environment Protection Agency (EPA) observed that heavy metal contamination is a major and a critical issue nowadays (Sujatha et al., 2021a). The occurrence of heavy metals is one of the main reasons for the existence of different diseases like osteoporosis, vomiting, and neurological disorders (Zereshki et al., 2021). With the upward thrust in the financial system and technological enhancement, the electric and digital device production is increasing day by day and is also turning out to be the main aspect of solid waste generation. Printed circuit board is an important component of every electrical and electronic equipment which contains different rare earth metals and valuable metals such as gold, silver, and platinum. Hence recovery of these metals is very important (Zhou et al., 2021).
Numerous techniques such as precipitation, adsorption, reactive distillation, ion exchange, electro dialysis, solvent extraction, and ultrafiltration are being used in the area of separation science and technology, but these methods have some limitations. The precipitation method requires lots of non-eco-friendly chemicals (Ooi et al., 2015). The adsorption process too becomes expensive when pure adsorbents are used (Elsagh et al., 2017). Compounds with low volatility lead to higher energy consumption and also produce high boiling side products in reactive distillation (Kumar et al., 2019a). In the ion exchange method, recovery of product is more difficult, as it highly depends on the resin structure and the reuse of ionic material requires a chemical reagent which is expensive and creates further pollution (Peng & Guo, 2020).
The membrane-based separation processes are considered one of the cleanest and energy-saving processes for the treatment of aqueous as well as gaseous stream contaminants. Also, nowadays the liquid membrane is widely used in different areas like chemistry, chemical engineering, environmental science, and hydrometallurgy (Abbassian & Kargari, 2016). Amid the various membrane-based methods, liquid membrane (LM) has grown into an established unit operation for a diverse range of separations. LM is used in wastewater treatment and industries pertaining to chemical, pharmaceutical, food processing, biotechnology, textile, environmental engineering, pulp, and paper (San Román et al., 2010). LM consists of two phases of the same nature, but dissimilar composition being divided by the third phase having discrete features. The third phase should be insoluble in the other two phases and is said to be LM (Kislik, 2012). LMs are efficient for the elimination and reclamation of compounds from aquatic streams as they offer merits like low solvent consumption, little operational cost, instant extraction, and stripping in a single unit (Kohli et al., 2021a).
Liquid membranes are mainly classified into three different types: Bulk liquid membrane (BLM), supported liquid membrane (SLM), and emulsion liquid membrane (ELM) (Rouhani et al., 2020). BLM contains the external feed phase and internal aqueous phase which are separated with the help of a third liquid phase (membrane). The BLM is simple and continuous, but has few limitations like having small interfacial area and low mass transfer rate and requiring large amount of solvent and economically not affordable on the industrial scale (Chang, 2016). SLM is prepared by stabilizing the membrane fluid on the solid base. In the SLM, hollow fibers (Kohli et al., 2021b), flat sheets (Zante et al., 2020), and spiral wound (Wang et al., 2020) are most commonly used. SLM offers high interfacial area, low energy consumption, ease of use, and high selectivity but also suffers from limitations like membrane replacement and use of toxic and volatile solvents that make SLM more unstable (Parhi, 2013). Also, recently Sarang et al. (2022) too used the concept of an artificial neural network approach with a pseudo-emulsion hollow fiber strip method for the separation of ethylparaben and diclofenac.
In the current study, the authors have attempted to explore the green emulsion liquid membrane (GELM) technique as another approach for the separation of different metal ions and compounds from the aqueous streams in place of the conventionally used methods. Moreover, the investigation also focuses on sustainable and reliable advancement in the field of ELM. The working principle, transport mechanism, effect of different parameters like concentration of surfactant, carrier, types of diluents, effect of volume ratio of external feed phase to emulsion, agitation speed, effect of internal aqueous phase concentration, influence of emulsification time on the removal of several targeted solutes from the aqueous have been discussed in detail. Moreover, the influence of statistical tools on extraction efficiency and real-time diagnosis of water quality are also elaborated in depth.
2 Emulsion Liquid Membrane
ELM was first used by Li (1968) for the separation of hydrocarbons. ELM is a three-phase dispersion system: innermost phase, intermediate phase, and outer phase. The innermost phase is an internal phase (stripping agent), an intermediate phase is a membrane phase that contains the carrier dissolved in suitable diluents along with surfactant to emulsify the emulsion droplets, and the outer phase is the external feed phase. The external feed phase contains the solute to be separated from the feed solution. The concentration gradient is the driving force for the solute transport through the membrane (Jusoh et al., 2016). Some of the merits of ELM as compared to the other processes are as follows: (1) combines the approach of extraction and stripping in a single step; (2) diffusivity is higher as a smaller emulsion globule provides the larger interfacial area (Teng et al., 2013); (3) ELM could be 40% cheaper than solvent extraction method (Thakur et al., 2014); (4) saving of contacting equipment volume as separate contactors for extraction and stripping process are not required (Ahmad et al., 2011). ELM technique has great potential for a wide variety of applications including the removal, separation, recovery, and purification of solute from dilute streams. Table 1 shows the exhaustive information regarding the surfactant, carrier, diluent, and internal aqueous phase used for the removal of several targeted solutes by the ELM method.
2.1 Green Emulsion Liquid Membrane
For the formulation of ELM, the diluent is a significant component as it decides the total viscosity of the membrane phase and is also responsible for the emulsion stability (Ahmad et al., 2015). Traditionally the solvents from the petroleum feed stocks like kerosene, hexane, heptane, dichloroethane, and toluene are generally used as diluents for the membrane phase preparation in ELM. The petroleum-based diluents are generally used due to their properties like low viscosity, ready availability, and non-polarity, but these diluents also create several problems in the environment as they are non-renewable, non-biodegradable, flammable, difficult to handle and toxic in nature (Ahmad et al., 2016). According to the World health organization, the permitted amount of hydrocarbons in water should be less than 0.05 mg/L but the petroleum-based diluents are water soluble in the range of 10 mg/ L (Zereshki et al., 2021). These diluents will have a detrimental effect on the environment if they are discharged into the environment. Due to the limited resources of petroleum-based diluent, the price is inconsistent and it could affect the total cost for the ELM formation. For economic and environmental considerations, there is a paramount need to find a better substitute for petroleum-based diluents.
Green solvents or vegetable oils like palm oil, corn oil, sunflower oil, coconut oil, mahua oil, and so on have properties like non-toxicity, non-volatility, non-flammability, degradability, and inexpensive and are reusable which makes them better than petroleum-based diluents (Kumar et al., 2018a). In complying with the principle of a greener approach, different vegetable oils have been incorporated in the ELM formulation and the detailed information regarding the removal of targeted solute using green solvent as a membrane phase is mentioned in Table 2. Table 2 also describes the comprehensive details on the surfactant, carrier, diluent, internal aqueous phase, and extraction efficiency reported by the various investigators for the removal of different targeted solute from the feed phase through the GELM system.
2.2 Green Emulsion Liquid Membrane Formulation
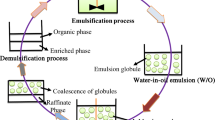
GELM process has four different stages: (1) emulsification, (2) dispersion and extraction, (3) settling, and (4) demulsification process. The primary emulsion is prepared by emulsifying the internal phase and membrane phase. This primary emulsion is dispersed into the external feed phase where the solute is extracted. Emulsion and feed solution are allowed to separate in the settling stage. The membrane phase is recovered through demulsification after the extraction of solute (Arabi Ardehali et al., 2020). Demulsification is carried out by two methods: (1) chemical demulsification and (2) physical demulsification. Chemical demulsification is not selected over physical demulsification as after chemical demulsification the oil phase cannot be reused. Electrical, ultrasonication, and thermal treatment are the methods used for physical demulsification (Lin et al., 2016). Figure 1 shows the schematic view of the GELM system.
Table 3 provides the manufacturer details of various components of GELM.
2.3 Transport Mechanism of GELM
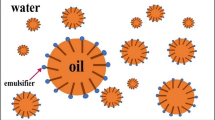
The membrane phase in GELM consists of the suitable organic phase of plant origin and an appropriate carrier (extractant) along with a surfactant is dissolved in it. The solute molecules of the feed phase will react with the carrier and the solute-carrier complex is formed at the feed-liquid membrane interface. At the liquid membrane-stripping side interface, the complex undergoes a reverse reaction removing the solute to the stripping phase as the complex is de-complexed due to its reaction with the stripping phase. The free carrier renews again and diffuses back across the liquid membrane and the cycle repeats (Kohli et al., 2021a). The feed phase will be depleted of the solute molecules and the stripping phase will be enriched with the solute molecules. Figure 2 represents the facilitated transport mechanism of the solute.
3 Effect of Different Parameters on the Removal of Targeted Solute
3.1 Effect of Surfactant Concentration
In ELM, surfactant plays a vital role in the transport rate of solute, break up of emulsion, stability, and swelling (Chakraborty et al., 2010). Surfactant is one of the important constituents of the membrane phase which decreases the interfacial tension between the water and oil by adsorbing at the liquid–liquid interface and helps to form the emulsion (Kumar et al., 2018c; Ting et al., 2022). The concentration of surfactant is an important parameter as studies have indicated that at low surfactant concentration, large emulsion globules are formed which provides a low interfacial area for mass transfer and hence reduces the separation efficiency. Also, low surfactant concentration in ELM is unable to reduce the interfacial tension between water and oil which leads to the separation of water and oil. The emulsion stability is also low at low surfactant concentration as the surfactant is not able to totally cover up the whole internal aqueous phase (Jusoh and Othman, 2017; Zereshki et al., 2018). The rise in surfactant concentration leads to the adsorption of more surfactant on the liquid–liquid interface which tends to enhance the emulsion stability and the strength of the adsorption layer. This reduces the surface tension of the membrane phase producing the smaller emulsion globules which provide a high mass transfer area between the donor and internal phase.
Jusoh and Othman (2017) witnessed less emulsion droplets formation with Span 80 concentration of 5% w/v and 7% w/v. At this high concentration of surfactant, the extraction efficiency was reduced due to the increase in the overall viscosity of the membrane phase. This hinders the mass transfer of solute molecules from the feed phase to the stripping phase due to the enhancement in the interfacial resistance and by the reaction with a carrier at the interface. Above a certain limit of concentration, most of the surfactant molecules forms aggregates in the bulk which is referred to as critical micelles concentration (CMC), and these aggregates act as a reservoir for water (Dâas & Hamdaoui, 2010). The reverse micelles promote the transportation of water from the external feed phase to the internal phase which leads to a larger osmotic difference and increases the swelling rate.
Othman et al. (2017) observed an increase in extraction of phenol from 46 to 99% as Span 80 concentration was increased from 1 to 5% w/v with palm oil as the diluent. Noah et al. (2018) observed a reduction in the size of the emulsion globule from 3.70 to 3.14 µm as the surfactant concentration increased from 1 to 3% w/v. At the optimum level of surfactant concentration, no breakage and swelling were observed which indicates the presence of the sufficient number of surfactant molecules. Perumal et al. (2019) too witnessed a rise in the percent extraction of chromium from 94 to 99.5% as the concentration of amphiphilic silica nanowires was raised from 5 to 25 mg per 7 mL in mahua oil (diluent) respectively. Sujatha et al. (2021a) too observed enhancement in extraction efficiency from 45 to 95% as the surfactant concentration raised from 1 to 2.66% v/v respectively during the removal of chromium (VI).
3.2 Effect of Carrier Concentration
Many investigators have explored the effect of carrier on the separation of different compounds. Carrier is an active compound that binds the compounds at the feed-membrane interface to form the compound-carrier complex. It is seen that a rise in carrier concentration to a certain limit increases the extraction, helps in proper complexation of the compound and a further rise in carrier concentration afterwards does not much affect the extraction efficiency as the membrane phase viscosity rises which increases the membrane phase resistance and hinders the extraction performance.
The viscosity of the membrane phase influences ELM’s stability. The rise in carrier concentration raises the viscosity of ELM (Lee, 2011). Kohli et al. (2018) too observed a decrease in the extraction of ethylparaben as the concentration of carrier TOA was raised beyond 3% w/v which happened due to the rise in the viscosity of the membrane phase. Sometimes, low carrier concentration does not enhance the ELM viscosity and results in instability and breakage of ELM (Othman et al., 2016). Moreover, the low concentration of carrier restricts the adequate carrier-complex formation. The osmotic pressure tends to be higher in the organic phase than in the external feed phase at higher carrier concentration (Choudhury et al., 2010; Kumbasar, 2010) and so swelling takes place. The mass transfer resistance increases, and extraction efficiency reduces at high carrier concentration (Jusoh et al., 2016; Othman et al., 2016). So, proper selection and optimum carrier concentration are required for the enhanced performance of an ELM system. Table 4 lists the separation of several targeted solutes using different surfactants, stripping phase, diluent, carrier, and their concentration. Figure 3 describes the impact of different carriers and their concentration on extraction efficiency as investigated by various investigators.
The critical micelles concentration (CMC) is believed to be the benchmark concentration for the surfactant to work efficiently in reducing the interfacial tension. Low CMC has two most important inferences for the field implementation. Firstly, the low CMC affects the amount of surfactant required to be handled in the field. Secondly, if only low surfactant concentration is required for effective working, then the total cost is also reduced (Abbas et al., 2022). CMC determination carried out by electric conductivity corresponded to the unexpected change in the specific conductance profile (de souza et al., 2021). Ahmad et al. (2012) reported that with non-ionic surfactants, droplet size is further reduced above the CMC during the removal of cadmium. Thus, CMC findings are remarkably important for the emulsion liquid membrane technique.
Solvent or diluent (vegetable oil / seed oil) is one of the key components of GELM. The diluent decides the overall viscosity of the liquid membrane. Jusoh et al. (2017) observed that a rise in carrier concentration does not contribute much towards the enhancement of liquid membrane viscosity, also palm oil viscosity (83 cP) was observed higher than Amberlite LA-2 (18 cP). Lower viscosity with high carrier concentration favours the formation of small emulsion globules during the dispersion process (Jusoh et al., 2020). The higher viscosity of membrane phase provides more stability, but it hinders the mass transfer and provides higher resistance for solute molecules (Zaulkiflee et al., 2021). In the membrane phase, the rise in carrier concentration reduces the interfacial tension which leads to the larger emulsion globule size whereas interfacial tension decreases with increases in the surfactant concentration up to a certain value (Sujatha & Rajasimman, 2021). So, the carrier concentration should be wisely selected to reduce the overall cost of the process and to achieve the finest extraction.
3.3 Effect of Types of Diluents
Diluents play an important role in the preparation of the organic membrane phase. The diluent viscosity should be low as it helps to facilitate the diffusion of the solute-carrier complex. The effect of diluent is significant as both physical and chemical interactions take place between diluent and carrier (Kohli et al., 2020b; Parhi, 2013). Moreover, in the GELM technique the part of the diluent is more substantial as the solvent used should be non-toxic and environmentally benign. The best and most suitable alternative to petroleum-based solvents is vegetable oils. Different vegetable oils like palm oil, corn oil, mahua oil, sunflower oil, rapeseed oil, coconut oil, neem oil, pungai oil, and waste cooking oil have been reported as biodegradable and environmentally benign diluent in the formulation of GELM. Seeds and fruits are good sources of vegetable oils. Vegetable oils contain nonpolar lipids (< 92%), polar lipids (< 4%), free fatty acids (< 2%), and unsaponifiable matter (phytosterols, tocopherols and hydrocarbons) (< 2%) (Othman et al., 2019).
As compared to petroleum-based diluents, vegetable oils have higher viscosity due to the intermolecular attractions of long chain fatty acids in vegetable oils and so it increases the mass transfer resistance (Mei et al., 2020; Othman et al., 2019). Many authors have reported that the waste vegetable oil doesn’t show good extraction efficiency as compared to the fresh vegetable oil as during frying at a higher temperature, waste vegetable oils generate a large amount of polar, surface active compounds and polymers due to the hydrolytic oxidation and polymerization reaction. Also, in comparison to vegetable oil-based diluents, petroleum-based diluents are non-renewable and expensive (Chang, 2017).
Noah et al. (2018) reported 100% extraction efficiency of chromium using green diluents like palm oil and corn oil and no reduction in extraction efficiency was observed due to the high viscosity of vegetable oil as compared to kerosene. Moreover, Perumal et al. (2019) also reported that high viscous oil like mahua oil provides high stability over low viscous oil like coconut oil and sunflower oil. Rosly et al. (2019) observed that the kerosene-based ELM had lower extraction (37%) efficiency as compared to palm oil-based ELM (77%). The results indicate that aliphatic hydrocarbons like kerosene have lower extraction efficiency due to their aliphatic nature as it contains a long carbon chain that provides nonpolar characteristics. However, palm oil contains oxygen atoms that enable phenol and triglycerides reaction by hydrogen bonding amid the molecules forming phenol triglycerides complex. Also, the phenolic compounds are dissolved due to the hydrophobic nature of palm oil (Rosly et al., 2019). Various vegetable oils such as waste cooking oil, canola oil, sunflower oil, corn oil, rice bran oil, and palm oil were used for the removal of various contaminants which shows the reliability and good capacity of the GELM (Harun et al., 2022; Rajasimman et al., 2021; Ting et al., 2022; Zaulkiflee et al., 2021).
In the last few decades, the worldwide demand for vegetable oil has drastically increased. Palm oil (Elaeis guineensis) is one of the most important oil crops in the world (Sakai et al., 2022). The palm oil boom has contributed to financial growth mainly in southeast Asia. Palm oil production is a driver of deforestation which creates air pollution due to the use of fire for land conversion (Ogahara et al., 2022). The conversion of forest into palm oil production has decreased the carbon stocks by over 50% and has enhanced the greenhouse gas emissions by four times as compared to land converted from old rubber plantations (Chiriacò et al., 2022). Due to deforestation, the problems associated with the loss of biodiversity and ecosystem function arise (Qaim et al., 2020). Also, the sheer scale at which palm oil is cultivated can harm the soil and leads to problems concerned to water quality and availability (Mukherjee & Sovacool, 2014).
Few authors have also reported problems associated with the land exploitation impact on native communities’ rights and land rights or on pesticides and their impact on the labourer’s health. Socioeconomic issues also include the rights of palm oil labourers in developing countries and support to small farmers to guarantee adequate remuneration and survival (Ruggeri & Samoggia, 2018; Dharmawan et al., 2020).
However, Chiriacò et al. (2022) reported that the cultivation of palm oil contributes positively to the socioeconomic growth of the local public, irrespective of the country of production or approach used, with a substantially positive impact on poverty reduction and economic growth. The production of palm oil is also considered one of the most environmentally sustainable products due to its high productivity, concerning the phenomenon of land grabbing in comparison to the production of other vegetable oils like soy, sunflower, or rapeseed. Also, palm oil is considered the best versatile oil to satisfy the uses of different sectors (Chiriacò et al., 2022).
3.4 Effect of Volume Ratio of External Feed Phase to Emulsion
The volume ratio of the external feed phase to emulsion also known as the treat ratio plays an important role in determining the extraction efficiency. The optimum ratio provides the best extraction efficiency. It is observed that the low treat ratio provides low extraction efficiency as emulsion cannot be dispersed properly and the formation of emulsion globules is reduced due to the osmotic pressure difference (Ahmad et al., 2017). The interfacial surface area decreases and the mass transfer area for extraction are reduced due to the breakage of the emulsion. Moreover, a high volume of emulsion creates problems in dispersion and increases the total viscosity of ELM (Jusoh and Othman, 2017).
An increase in the treat ratio increases the percent extraction as the osmotic pressure effect is reduced and the emulsion could properly disperse and ease into the feed phase. This leads to the formation of more emulsion globules. The globule size is small and so it can provide more interfacial area for complex formation on the feed-membrane interface (Othman et al., 2017, 2019). Further increment in treat ratio reduces the extraction capacity. The number of emulsion globules available is low and due to this, the interfacial surface area required for the extraction is reduced (Sujatha et al., 2021a; Zereshki et al., 2018). Thus, it also reduces the mass transfer of solute ions from the external feed phase to the emulsion. The breakage and rupture found in ELM are more at high ratio due to the large osmotic difference between the external feed phase and emulsion; as less number of emulsion, globules are available for extraction (Jusoh et al., 2020). Table 5 presents the effect of the treat ratio on the %extraction of targeted solute by GELM. Figure 4 shows the significance of the treat ratio on extraction efficiency as examined by many investigators.
3.5 Effect of Agitation Speed
The speed of agitation during the formation of emulsion is crucial. It influences the size of emulsion globules which eventually affects the interfacial mass transfer area, extraction efficacy, and stability of an ELM system. For a better and uniform dispersion of emulsion droplets in the external feed phase, a comparatively high agitation speed is required (Rosly et al., 2019). At lower agitation speed, the ELM formed is unstable due to the weak and slow dispersion of emulsion droplets into the external feed phase (Rosly et al., 2019; Kumar et al., 2019b). Less shear energy leads to slow or insufficient dispersion (Kumar et al., 2019b). It has been observed that larger emulsion globules tend to coalesce with one another which reduces the extraction efficiency and increases the membrane thickness leading to less mass transfer (Kumar et al., 2019b; Perumal et al., 2019). An increase in the speed of agitation increases the shear forces, which reduces the size of dispersed emulsion droplets and enhances the interfacial area for mass transfer between feed and ELM solution (Kumar et al., 2018a). Figure 5 describes the effect of agitation speed on the formation of emulsion globules.
At the optimum speed of agitation, shear forces hinder the coalescence process between the emulsion droplets and the membrane wall formed is thin. So, the extracted solute needs to travel very less distance to be stripped at the membrane-stripping interface which increases the extraction rate of solute (Ahmad et al., 2017; Othman et al., 2018). The speed of agitation beyond the optimum level provides high shear stress on the fine membrane droplets. The large energy introduced due to such a high speed of agitation leads to swelling where water transports from the external feed phase to the membrane phase and reduces the extraction efficiency (Kumar et al., 2018a; Othman et al., 2016). Also, several investigators have observed that at high speed of agitation, the membrane wall formed is much thinner and so the membrane breakage rate increases due to the rupture of emulsion globules. This allows the expulsion of extracted solute from the stripping phase to the external feed phase by back diffusion (Othman et al., 2017; Zereshki et al., 2018).
3.6 Effect of Internal Aqueous Phase Concentration
The concentration of the internal aqueous phase (stripping agent) is crucial for the development of emulsion. The internal aqueous phase affects the extraction efficiency, stripping efficiency, and emulsion breakage. The stripping agent has a vital role in the stripping process as the solute is stripped out from the solute-carrier complex which is present in the membrane phase. Othman et al. (2016) observed that the higher stripping rate can be achieved by increasing the internal aqueous phase concentration up to a certain limit only. Cloudiness of stripping solution is observed at low concentration of internal aqueous phase, and this leads to poor removal efficiency. This also indicates the inadequate amount of stripping agent availability in the internal phase which also reduces the stripping efficiency (Shokri et al., 2020). At optimum concentration of stripping agent, the extraction efficiency and stripping efficiency increase as enough number of stripping agent molecules are available for the stripping reaction. So, the carrier is again ready to form the new solute-carrier complex and more solute is being stripped into the internal aqueous phase within less time (Othman et al., 2016). Further rise in the internal aqueous phase concentration reduces the extraction and stripping efficiency and increases the emulsion breakage (Shokri et al., 2020).
The high concentration of the stripping agent has a strong pH gradient which causes a larger difference in osmotic pressure (Björkegren et al., 2015; Othman et al., 2016). The higher concentration of the stripping agent creates a larger density difference and increases the overall emulsion viscosity which hinders the mass transfer rate (Perumal et al., 2019). The excess amount of stripping agent molecules reacts (hydrolyzing) with the surfactant reducing the effective number of surfactant molecules and increasing the transport of the aqueous phase (Othman et al., 2017). This in turn leads to emulsion swelling and breakage. The swelling and emulsion breakage rate increases which ultimately destabilizes the emulsion (Zereshki et al., 2018). Table 6 shows the effect of internal aqueous phase concentration on the %extraction in GELM. Figure 6 describes the significance of various stripping agents and their concentration on extraction efficiency as studied by many researchers.
3.7 Effect of Emulsification Time
The internal phase droplet size and emulsion stability directly depend on the emulsification time. Extraction efficiency observed was poor for the shorter emulsification time. This is due to the formation of larger emulsion droplets which provides a smaller interfacial area for the transport of solute molecules (Ahmad et al., 2017). Many authors have observed poor extraction efficiency when the time is not sufficient to generate small emulsion globules. Furthermore, shorter emulsification time tends to the sudden breakage of emulsion. Sufficient time for emulsification is required for the surfactant to migrate and adsorb at the interface. This reduces the interfacial tension and results in small emulsion droplets which lead to the higher extraction of solute and better stability (Rosly et al., 2020).
Kumar et al. (2018c) observed that with the rise in the emulsification time from 10 to 20 min, the stability of GELM (diluent: rice bran oil) increased from 55 ± 2 to 121 ± 2 min respectively. Shokri et al. (2020) also observed the rise in emulsion stability from 20 to 118 min when emulsification time was increased from 3 to 10 min respectively. Exposure of emulsion constituents to the internal shear for a longer duration causes the creation of a large number of fine internal droplets which requires more time to coalesce. Therefore, the emulsion is more stable and the breakage phenomenon is hindered during the dispersion of the emulsion (Othman et al., 2018).
Also, a significant reduction is observed in extraction efficiency when emulsion formation is carried beyond the optimum emulsification time. This happens due to the coalescence of small internal phase droplets produced. Also, the emulsion breakage was observed to take place due to the membrane rupture. The longer emulsification time leads to a more viscous emulsion, which provides more resistance and results in poor extraction efficiency (Othman et al., 2017). Othman et al. (2018) studied the effect of emulsification time (3–9 min) during the removal of succinic acid by using palm oil as a diluent and observed 5 min as the most suitable emulsification time. Noah et al. (2018) observed a rise in emulsion droplets size from 2.67 to 3.60 µm as the emulsification time was raised from 3 to 10 min respectively with an increase in breakage from 10 to 30% while using palm oil (diluent).
3.8 Influence of Statistical Tools on Extraction Efficiency
Experimental design by response surface methodology (RSM) optimizes the process variables and improves the process’s effectiveness. RSM consists of mathematical and statistical techniques which are focussed on the fit of a polynomial equation to the experimental data. RSM can be soundly used where a single response or set of responses are affected by many variables. RSM is widely used as it generates large information and evaluates the interaction effect among the variables on the response (Bezerra et al., 2008).
Kumar et al. (2018b) investigated the extraction of lactic acid (LA) from an aqueous solution using green emulsion ionic liquid membrane (GEILM) with the help of a statistical approach comprising of 2-level fractional factorial design (FFD) and BBD (Box-Behnken design). The emulsion consisted of diluents like rice bran oil (70% v/v, natural green solvent) and hexane (30% v/v, organic solvent), surfactant Span 80, Aliquat336 as an ionic liquid, and internal phase reagent NaOH. The parameters namely, LA concentration, NaOH concentration, phase ratio, treat ratio, and stirring speed were screened out using FFD and then RSM was applied for optimization by BBD. BBD presented the design matrix of 42 experiments which were performed separately in triplicates and the average LA extraction efficiency was considered as the response. LA extraction achieved was 95 ± 3.5% under the optimum conditions as: LA concentration: 0.03 M, NaOH concentration: 0.25 M, phase ratio: 0.4 v/v, treat ratio: 2.4 v/v and stirring speed: 535 rpm.
Daraei et al. (2019) applied GELM consisting of Span 80 (surfactant), HCl (internal phase), and sunflower oil (diluent) for the removal of methyl violet 2B dye. Plackett–Burman screening design was first used to identify the important factors and then BBD was used for parametric optimization. BBD suggested 15 experimental runs based on different combinations of effective parameters. The model suggested the maximum dye removal as 97.54% with 4.9% wt Span 80, 0.84 M HCl, and equal amounts of GELM and contaminated water.
Kumar and Thakur (2019) studied the lactic acid extraction from an aqueous solution by the use of soybean oil (green solvent) and a synergistic mixture of the extractants: tri-n-octylamine (TOA) and Tri-n-octylmethylammonium chloride (TOMAC). The statistical optimization was carried out with the help of BBD and extraction efficiency achieved was 71.5% under the optimum conditions as: 0.02 M initial LA concentration, 0.5 (v/v) extractant ratio, 28.66% v/v mixed extractants concentration, 2 (v/v) phase ratio, 27 ºC temperature, 102 rpm stirring speed and 63 min contact time. BBD provided 62 experimental runs using different combinations of different process factors. It was observed that the synergistic effect of the mixed extractants leads to a higher value of distribution coefficient as compared to the single extractants.
Zereshki et al. (2021) investigated the removal of copper ions from aqueous solutions by GELM. BBD was used for statistical analysis and optimization. BBD provided 29 experiments for four variables namely surfactant (Span 80) concentration, internal phase (HCl) concentration, carrier (D2EHPA) concentration, and internal to organic phase (I/O) ratio. Sunflower oil was used as the solvent. It was observed that more than 94% of Cu(II) ions were extracted at the optimum conditions of 3.25% wt Span 80, 1.44 M HCl, 104.23 mM D2EHPA, and an I/O ratio of 1.
3.9 Real-Time Diagnosis of Water Quality
It has been observed that the existing situation of pollution intensity leads to the rise in a build-up of pollutants in marine waters. This also creates problems in assessing the impact on the ecological system. Varotsos and Krapivin (2018) proposed the use of geoecological information modelling system (GIMS) for dealing with this problem occurring in the Arctic Basin. GIMS consists of a series of precise models that describes ecological, hydrological, climatic, and hydrochemical processes in Arctic waters. The combination of GIMS with the Arctic basin ecosystem (ABE) model considers a variety of pollutants, like river runoffs, long-range atmospheric transport, and anthropogenic behaviour in the coastal zone. The main aspect of GIMS-ABE lies in the structural liberty of its blocks and the transfer of knowledge among them that occurs through inputs and outputs. Varotsos et al. (2019) also proposed the optical decision-making system for in-situ evaluation of water quality in natural water regions.
Monitoring the quality of water is important for controlling the pollutants which can cause harm to humans and the environment. With the advancement in technology and engineering materials, real-time analysis of water quality is possible. Silva et al. (2022) too discussed the development of low-cost technologies, which can speed up the compilation of data required for the monitoring of physical, chemical, and biological parameters of the water.
4 Concluding Remarks
Conventional techniques used for the separation of targeted solute from the aqueous streams have the disadvantage of high energy consumption, extreme use of chemicals, complicated operating conditions, and high capital and working cost. The liquid membrane on the other side is a more efficient technique as it provides simultaneous extraction and stripping in a solo unit, low consumption of chemicals, and low operational cost. Green emulsion liquid membrane is a type of LM technique that focuses on the removal of targeted solute through a greener approach. Green solvents being non-toxic, non-volatile, reusable, and degradable in nature makes them a suitable solvent to be used in the field of separation science. This saves the environment and also reduces the overall working cost of the system. Parametric studies indicate that several factors like concentration of surfactant, carrier, types of diluents, effect of volume ratio of external feed phase to emulsion, agitation speed, effect of internal aqueous phase concentration, and emulsification time play a substantial role in the removal of targeted solute from the aqueous streams. The statistical tools optimize the process variables and improve the process effectiveness. A broad scope exists towards the use of greener solvents and more studies can be done in order to explore more solvents of vegetable/plant origin. The GELM approach can further be used for the removal and recovery of several other metal ions and compounds from the aqueous streams.
Data Availability
All the data used in the paper are presented in the form of tables and/or figures.
References
Abbas, A. H., Abd Alsaheb, R. A., & Abdullah, J. K. (2022). Comparative study of natural chemical for enhanced oil recovery: Focus on extraction and adsorption at quartz sand surface. Petroleum. https://doi.org/10.1016/j.petlm.2022.01.007
Abbassian, K., & Kargari, A. (2016). Modification of membrane formulation for stabilization of emulsion liquid membrane for extraction of phenol from aqueous solutions. Journal of Environmental Chemical Engineering, 4(4), 3926–3933. https://doi.org/10.1016/j.jece.2016.08.030
Ahmad, A. L., Kusumastuti, A., Derek, C. J. C., & Ooi, B. S. (2011). Emulsion liquid membrane for heavy metal removal: An overview on emulsion stabilization and destabilization. Chemical Engineering Journal, 171(3), 870–882. https://doi.org/10.1016/j.cej.2011.05.102
Ahmad, A. L., Kusumastuti, A., Derek, C. J. C., & Ooi, B. S. (2012). Emulsion liquid membrane for cadmium removal: Studies on emulsion diameter and stability. Desalination, 287, 30–34. https://doi.org/10.1016/j.desal.2011.11.002
Ahmad, A. L., Kusumastuti, A., Shah Buddin, M. M. H., Derek, C. J. C., & Ooi, B. S. (2014). Emulsion liquid membrane based on a new flow pattern in a counter rotating Taylor-Couette column for cadmium extraction. Separation and Purification Technology, 127, 46–52. https://doi.org/10.1016/j.seppur.2014.02.029
Ahmad, A. L., Shah Buddin, M. M. H., Ooi, B. S., & K. adhi. (2015). Cadmium removal using vegetable oil based emulsion liqid membrane (ELM): Membrane breakage investigation. Jurnal Teknologi (sciences & Engineering), 75(1), 39–46.
Ahmad, A. L., Shah Buddin, M. M. H., Ooi, B. S., & Kusumastuti, A. (2016). Cadmium removal from aqueous solution by emulsion liquid membrane (ELM): Influence of emulsion formulation on cadmium removal and emulsion swelling. Desalination and Water Treatment, 57(58), 28274–28283. https://doi.org/10.1080/19443994.2016.1179674
Ahmad, A. L., Shah Buddin, M. M. H., Ooi, B. S., & Kusumastuti, A. (2017). Utilization of environmentally benign emulsion liquid membrane (ELM) for cadmium extraction from aqueous solution. Journal of Water Process Engineering, 15, 26–30. https://doi.org/10.1016/j.jwpe.2016.05.010
Ahmad, A. L., Zaulkiflee, N. D., Kusumastuti, A., & Buddin, M. M. H. S. (2019). Removal of Acetaminophen from Aqueous Solution by Emulsion Liquid Membrane: Emulsion Stability Study. Industrial and Engineering Chemistry Research, 58(2), 713–719. https://doi.org/10.1021/acs.iecr.8b03562
Anarakdim, K., Matos, M., Senhadji-Kebiche, O., & Benamor, M. (2017). Optimization of hexavalent chromium removal by emulsion liquid membrane (ELM) using sunflower oil as eco-friendly solvent. Desalination and Water Treatment, 72, 281–289. https://doi.org/10.5004/dwt.2017.20428
Anarakdim, K., Matos, M., Cambiella, A., Senhadji-Kebiche, O., & Gutiérrez, G. (2020). Effect of temperature on the heat treatment to recover green solvent from emulsion liquid membranes used in the extraction of Cr(VI). Chemical Engineering and Processing - Process Intensification, 158, 108178. https://doi.org/10.1016/j.cep.2020.108178
Anitha, M., Ambare, D. N., Singh, D. K., Singh, H., & Mohapatra, P. K. (2015). Extraction of neodymium from nitric acid feed solutions using an emulsion liquid membrane containing TOPO and DNPPA as the carrier extractants. Chemical Engineering Research and Design, 98, 89–95. https://doi.org/10.1016/j.cherd.2015.04.011
Arabi Ardehali, B., Zaheri, P., & Yousefi, T. (2020). The effect of operational conditions on the stability and efficiency of an emulsion liquid membrane system for removal of uranium. Progress in Nuclear Energy, 130, 103532. https://doi.org/10.1016/j.pnucene.2020.103532
Balasubramanian, A., & Venkatesan, S. (2012). Removal of phenolic compounds from aqueous solutions by emulsion liquid membrane containing Ionic Liquid [BMIM]+[PF6]− in Tributyl phosphate. Desalination, 289, 27–34. https://doi.org/10.1016/j.desal.2011.12.027
Begum, K. M. M. S., Venkatesan, S., & Anantharaman, N. (2012). Emulsion liquid membrane pertraction of metal ions from aqueous solutions and electroplating effluent using rotating disk contactor. Chemical Engineering Communications, 199(12), 1575–1595. https://doi.org/10.1080/00986445.2012.672497
Benyahia, N., Belkhouche, N., & Jönsson, J. Å. (2014). A comparative study of experimental optimization and response surface methodology of Bi(III) extraction by emulsion organophosphorus liquid membrane. Journal of Environmental Chemical Engineering, 2(3), 1756–1766. https://doi.org/10.1016/j.jece.2014.07.003
Bezerra, M. A., Santelli, R. E., Oliveira, E. P., Villar, L. S., & Escaleira, L. A. (2008). Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta, 76(5), 965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Björkegren, S., Karimi, R. F., Martinelli, A., Jayakumar, N. S., & Hashim, M. A. (2015). A new emulsion liquid membrane based on a palm oil for the extraction of heavy metals. Membranes, 5(2), 168–179. https://doi.org/10.3390/membranes5020168
Chakraborty, M., Bhattacharya, C., Datta, S. (2010). Emulsion liquid membranes: Definitions and classification, theories, module design, applications, new directions and perspectives (1st ed., pp. 141–199) Liquid Membranes. Elsevier B.V. https://doi.org/10.1016/B978-0-444-53218-3.00004-0
Chang, S. H. (2016). Types of bulk liquid membrane and its membrane resistance in heavy metal removal and recovery from wastewater. Desalination and Water Treatment, 57(42), 19785–19793. https://doi.org/10.1080/19443994.2015.1102772
Chang, S. H. (2017). Parametric studies on an innovative waste vegetable oil-based continuous liquid membrane (WVCLM) for Cu(II) ion separation from aqueous solutions. Journal of Industrial and Engineering Chemistry, 50, 102–110. https://doi.org/10.1016/j.jiec.2017.01.037
Chaouchi, S., & Hamdaoui, O. (2014). Acetaminophen extraction by emulsion liquid membrane using Aliquat 336 as extractant. Separation and Purification Technology, 129, 32–40. https://doi.org/10.1016/j.seppur.2014.03.021
Chaouchi, S., & Hamdaoui, O. (2015). Extraction of endocrine disrupting compound propylparaben from water by emulsion liquid membrane using trioctylphosphine oxide as carrier. Journal of Industrial and Engineering Chemistry, 22, 296–305. https://doi.org/10.1016/j.jiec.2014.07.023
Chaouchi, S., & Hamdaoui, O. (2016). Removal of 4-nitrophenol from water by emulsion liquid membrane. Desalination and Water Treatment, 57(12), 5253–5257. https://doi.org/10.1080/19443994.2015.1021104
Chiha, M., Hamdaoui, O., Ahmedchekkat, F., & Pétrier, C. (2010). Study on ultrasonically assisted emulsification and recovery of copper(II) from wastewater using an emulsion liquid membrane process. Ultrasonics Sonochemistry, 17(2), 318–325. https://doi.org/10.1016/j.ultsonch.2009.09.001
Chiriacò, M. V., Bellotta, M., Jusić, J., & Perugini, L. (2022). Palm oil’s contribution to the United Nations sustainable development goals: Outcomes of a review of socio-economic aspects. Environmental Research Letters, 17(6), 063007. https://doi.org/10.1088/1748-9326/ac6e77
Choudhury, A., Sengupta, S., Bhattacharjee, C., & Datta, S. (2010). Extraction of hexavalent chromium from aqueous stream by emulsion liquid membrane (ELM). Separation Science and Technology, 45(2), 178–185. https://doi.org/10.1080/01496390903409617
Dâas, A., & Hamdaoui, O. (2010). Extraction of bisphenol A from aqueous solutions by emulsion liquid membrane. Journal of Membrane Science, 348(1–2), 360–368. https://doi.org/10.1016/j.memsci.2009.11.026
Dâas, A., & Hamdaoui, O. (2014). Removal of non-steroidal anti-inflammatory drugs ibuprofen and ketoprofen from water by emulsion liquid membrane. Environmental Science and Pollution Research, 21(3), 2154–2164. https://doi.org/10.1007/s11356-013-2140-9
Daraei, P., Zereshki, S., & Shokri, A. (2019). Application of nontoxic green emulsion liquid membrane prepared by sunflower oil for water decolorization: Process optimization by response surface methodology. Journal of Industrial and Engineering Chemistry, 77, 215–222. https://doi.org/10.1016/j.jiec.2019.04.039
Daraei, P., Shokri, A., & Rostami, E. (2022). Extraction of vancomycin antibiotic from water using green emulsion liquid membrane based on sunflower oil. Journal of Membrane Science and Research, 8(2). https://doi.org/10.22079/jmsr.2021.526001.1454
Davoodi-Nasab, P., Rahbar-Kelishami, A., & Raji-Asadabadi, M. (2017). Fast and efficient chromium(VI) pertraction with aliquat 336 in emulsion liquid membrane using sunflower oil as a high potential solvent. Desalination and Water Treatment, 80, 234–246. https://doi.org/10.5004/dwt.2017.20990
Davoodi-Nasab, P., Rahbar-Kelishami, A., Safdari, J., & Abolghasemi, H. (2018). Evaluation of the emulsion liquid membrane performance on the removal of gadolinium from acidic solutions. Journal of Molecular Liquids, 262, 97–103. https://doi.org/10.1016/j.molliq.2018.04.062
Dharmawan, A. H., Mardiyaningsih, D. I., Komarudin, H., Ghazoul, J., Pacheco, P., & Rahmadian, F. (2020). Dynamics of rural economy: A socio-economic understanding of oil palm expansion and landscape changes in east Kalimantan, Indonesia. Land, 9(7). https://doi.org/10.3390/land9070213
de Souza, F. B., de Souza, A. A. U., Oliveira, J. V., & Ulson, S. M. D. A. G. (2021). Green extraction based on emulsion liquid membranes: Removal of Cr (III) from synthetic effluents. Environmental Nanotechnology, Monitoring & Management, 16, 100579. https://doi.org/10.1016/j.enmm.2021.100579
Elsagh, A., Moradi, O., Fakhri, A., Najafi, F., Alizadeh, R., & Haddadi, V. (2017). Evaluation of the potential cationic dye removal using adsorption by graphene and carbon nanotubes as adsorbents surfaces. Arabian Journal of Chemistry, 10, S2862–S2869. https://doi.org/10.1016/j.arabjc.2013.11.013
Fang, Z., Liu, X., Zhang, M., Sun, J., Mao, S., Lu, J., & Rohani, S. (2016). A neural network approach to simulating the dynamic extraction process of l-phenylalanine from sodium chloride aqueous solutions by emulsion liquid membrane. Chemical Engineering Research and Design, 105, 188–199. https://doi.org/10.1016/j.cherd.2015.11.012
Fetimi, A., Dâas, A., Benguerba, Y., Merouani, S., Hamachi, M., Kebiche-Senhadji, O., & Hamdaoui, O. (2021). Optimization and prediction of safranin-O cationic dye removal from aqueous solution by emulsion liquid membrane (ELM) using artificial neural network-particle swarm optimization (ANN-PSO) hybrid model and response surface methodology (RSM). Journal of Environmental Chemical Engineering, 9(5), 105837. https://doi.org/10.1016/j.jece.2021.105837
García, M. G., Acosta, A. O., & Marchese, J. (2013). Emulsion liquid membrane pertraction of Cr(III) from aqueous solutions using PC-88A as carrier. Desalination, 318, 88–96. https://doi.org/10.1016/j.desal.2013.03.025
Goyal, R. K., Jayakumar, N. S., & Hashim, M. A. (2011). Chromium removal by emulsion liquid membrane using [BMIM]+[NTf2]- as stabilizer and TOMAC as extractant. Desalination, 278(1–3), 50–56. https://doi.org/10.1016/j.desal.2011.05.001
Gupta, S., Chakraborty, M., & Murthy, Z. V. P. (2011). Response surface modelling and optimization of mercury extraction through emulsion liquid membrane. Separation Science and Technology, 46(15), 2332–2340. https://doi.org/10.1080/01496395.2011.595033
Hachemaoui, A., Belhamel, K., & Bart, H. J. (2010). Emulsion liquid membrane extraction of Ni(II) and Co(II) from acidic chloride solutions using bis-(2-ethylhexyl) phosphoric acid as extractant. Journal of Coordination Chemistry, 63(13), 2337–2348. https://doi.org/10.1080/00958972.2010.500375
Harun, M. H. Z. M., Ahmad, A. L., & Rajandram, L. (2022). Emulsion liquid membrane screening for ibuprofen removal from aqueous solution. Journal of Physical Science, 33(1), 109–122. https://doi.org/10.21315/jps2022.33.1.8
He, J., Li, Y., Xue, X., Ru, H., Huang, X., & Yang, H. (2015). Extraction of Ce(IV) from sulphuric acid solution by emulsion liquid membrane using D2EHPA as carrier. RSC Advances, 5(91), 74961–74972. https://doi.org/10.1039/c5ra11851d
Jiao, H., Peng, W., Zhao, J., & Xu, C. (2013). Extraction performance of bisphenol A from aqueous solutions by emulsion liquid membrane using response surface methodology. Desalination, 313, 36–43. https://doi.org/10.1016/j.desal.2012.12.002
Jusoh, N., Othman, N., & Nasruddin, N. A. (2016). Emulsion liquid membrane technology in organic acid purification. Malaysian Journal of Analytical Science, 20(2), 436–443. https://doi.org/10.17576/mjas-2016-2002-28
Jusoh, N., & Othman, N. (2017). Stability of palm oil-based emulsion liquid membrane for succinic acid extraction from aqueous solution. Journal of Applied Membrane Science & Technology, 19(1), 1–17. https://doi.org/10.11113/amst.v19i1.19
Jusoh, N., Noah, N. F. M., & Othman, N. (2019). Extraction and recovery optimization of succinic acid using green emulsion liquid membrane containing palm oil as the diluent. Environmental Progress and Sustainable Energy, 38(3), 1–9. https://doi.org/10.1002/ep.13065
Jusoh, N., Sulaiman, R. N. R., Othman, N., Noah, N. F. M., Rosly, M. B., & Rahman, H. A. (2020). Development of vegetable oil-based emulsion liquid membrane for downstream processing of bio-succinic acid. Food and Bioproducts Processing, 119, 161–169. https://doi.org/10.1016/j.fbp.2019.11.003
Kargari, A. (2013). Simultaneous extraction and stripping of 4-chlorophenol from aqueous solutions by emulsion liquid membrane. Desalination and Water Treatment, 51(10–12), 2275–2279. https://doi.org/10.1080/19443994.2012.734681
Kislik, V. S. (2012). Solvent Extraction: Classical and novel approaches (1st ed.). Elsevier.
Kohli, H. P., Gupta, S., & Chakraborty, M. (2018). Extraction of Ethylparaben by emulsion liquid membrane: Statistical analysis of operating parameters. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 539, 371–381. https://doi.org/10.1016/j.colsurfa.2017.12.002
Kohli, H. P., Gupta, S., & Chakraborty, M. (2019a). Separation of diclofenac using pseudo-emulsion hollow fiber membrane: Optimization by Box-Behnken response surface design. Journal of Water Process Engineering, 32, 100880. https://doi.org/10.1016/j.jwpe.2019.100880
Kohli, H. P., Gupta, S., & Chakraborty, M. (2019b). Stability and performance study of emulsion nanofluid membrane: A combined approach of adsorption and extraction of Ethylparaben. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 579, 123675. https://doi.org/10.1016/j.colsurfa.2019.123675
Kohli, H. P., Gupta, S., & Chakraborty, M. (2020a). Characterization and stability study of pseudo-emulsion hollow fiber membrane: Separation of Ethylparaben. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 587, 124308. https://doi.org/10.1016/j.colsurfa.2019.124308
Kohli, H. P., Gupta, S., & Chakraborty, M. (2020). Applicability of hollow fiber strip dispersion for the removal of metal ions from aqueous streams. Journal of The Institution of Engineers (India) Series E, 101(1), 91–97.
Kohli, H. P., Gupta, S., & Chakraborty, M. (2021a). Comparative studies on the separation of endocrine disrupting compounds from aquatic environment by emulsion liquid membrane and hollow fiber supported liquid membrane. International Journal of Chemical Reactor Engineering, 19(7), 689–698. https://doi.org/10.1515/ijcre-2020-0153
Kohli, H. P., Gupta, S., & Chakraborty, M. (2021b). Statistical analysis of operating variables for pseudo-emulsion hollow fiber strip dispersion technique: Ethylparaben separation from aqueous feed stream. Chemical Papers, 75(2), 629–640. https://doi.org/10.1007/s11696-020-01317-9
Kumar, A., Thakur, A., & Panesar, P. S. (2018a). Lactic acid extraction using environmentally benign Green emulsion ionic liquid membrane. Journal of Cleaner Production, 181, 574–583. https://doi.org/10.1016/j.jclepro.2018.01.263
Kumar, A., Thakur, A., & Panesar, P. S. (2018b). Statistical optimization of lactic acid extraction using green emulsion ionic liquid membrane (GEILM). Journal of Environmental Chemical Engineering, 6(2), 1855–1864. https://doi.org/10.1016/j.jece.2018.01.037
Kumar, A., Thakur, A., & Panesar, P. S. (2018c). Stability analysis of environmentally benign green emulsion liquid membrane. Journal of Dispersion Science and Technology, 39(10), 1510–1517. https://doi.org/10.1080/01932691.2017.1421079
Kumar, A., & Thakur, A. (2019). Statistical optimization of lactic acid extraction using green solvent and mixed extractants (TOA and TOMAC). Chemical Engineering Research Bulletin, 21(1), 20–35. https://doi.org/10.3329/cerb.v21i1.47369
Kumar, A., Thakur, A., & Panesar, P. S. (2019a). A review on emulsion liquid membrane (ELM) for the treatment of various industrial effluent streams. Reviews in Environmental Science and Biotechnology, 18(1), 153–182. https://doi.org/10.1007/s11157-019-09492-2
Kumar, A., Thakur, A., & Panesar, P. S. (2019b). Extraction of hexavalent chromium by environmentally benign green emulsion liquid membrane using tridodecyamine as an extractant. Journal of Industrial and Engineering Chemistry, 70, 394–401. https://doi.org/10.1016/j.jiec.2018.11.002
Kumbasar, R. A. (2010). Extraction and concentration of cobalt from acidic leach solutions containing Co-Ni by emulsion liquid membrane using TOA as extractant. Journal of Industrial and Engineering Chemistry, 16(3), 448–454. https://doi.org/10.1016/j.jiec.2010.01.045
Laguel, S., & Samar, M. H. (2019). Removal of Europium(III) from water by emulsion liquid membrane using Cyanex 302 as a carrier. Desalination and Water Treatment, 165, 269–280. https://doi.org/10.5004/dwt.2019.24551
Laki, S., Arabi Shamsabadi, A., Madaeni, S. S., & Niroomanesh, M. (2015). Separation of manganese from aqueous solution using an emulsion liquid membrane. RSC Advances, 5(102), 84195–84206. https://doi.org/10.1039/c5ra08547k
Laki, S., & Kargari, A. (2016). Extraction of silver ions from aqueous solutions by emulsion liquid membrane. Journal of Membrane Science and Research, 2(1), 33–40. https://doi.org/10.22079/jmsr.2016.15876
Lee, S. C. (2011). Extraction of succinic acid from simulated media by emulsion liquid membranes. Journal of Membrane Science, 381(1–2), 237–243. https://doi.org/10.1016/j.memsci.2011.07.039
Lende, A. B., & Kulkarni, P. S. (2015). Selective recovery of tungsten from printed circuit board recycling unit wastewater by using emulsion liquid membrane process. Journal of Water Process Engineering, 8, 75–81. https://doi.org/10.1016/j.jwpe.2015.09.003
Li, N. N. (1968). Separating hydrocarbons with liquid membranes. US Patent 3410794.
Lin, Z., Zhang, Z., Li, Y., & Deng, Y. (2016). Magnetic nano-Fe3O4 stabilized Pickering emulsion liquid membrane for selective extraction and separation. Chemical Engineering Journal, 288, 305–311. https://doi.org/10.1016/j.cej.2015.11.109
Liu, H., Zhang, Y., Huang, J., Liu, T., Xue, N., & Wang, K. (2017). Selective separation and recovery of vanadium from a multiple impurity acid leaching solution of stone coal by emulsion liquid membrane using di-(2-ethylhexyl)phosphoric acid. Chemical Engineering Research and Design, 122, 289–297. https://doi.org/10.1016/j.cherd.2017.04.026
Mei, X., Li, J., Jing, C., Fang, C., Liu, Y., Wang, Y., et al. (2020). Separation and recovery of phenols from an aqueous solution by a green membrane system. Journal of Cleaner Production, 251, 119675. https://doi.org/10.1016/j.jclepro.2019.119675
Mohammed, A. A., Atiya, M. A., & Hussein, M. A. (2020a). Simultaneous studies of emulsion stability and extraction capacity for the removal of tetracycline from aqueous solution by liquid surfactant membrane. Chemical Engineering Research and Design, 159, 225–235. https://doi.org/10.1016/j.cherd.2020.04.023
Mohammed, A. A., Atiya, M. A., & Hussein, M. A. (2020b). Studies on membrane stability and extraction of ciprofloxacin from aqueous solution using pickering emulsion liquid membrane stabilized by magnetic nano-Fe2O3. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 585, 124044. https://doi.org/10.1016/j.colsurfa.2019.124044
Mokhtari, B., & Pourabdollah, K. (2015). Emulsion liquid membrane for selective extraction of Bi(III). Chinese Journal of Chemical Engineering, 23(4), 641–645. https://doi.org/10.1016/j.cjche.2014.06.035
Mousavi, S. M., Kiani, S., Farmad, M. R., Hemati, A., & Abbasi, B. (2012). Extraction of arsenic(V) from water using emulsion liquid membrane. Journal of Dispersion Science and Technology, 33(1), 123–129. https://doi.org/10.1080/01932691.2010.548230
Mukherjee, I., & Sovacool, B. K. (2014). Palm oil-based biofuels and sustainability in southeast Asia: A review of Indonesia, Malaysia, and Thailand. Renewable and Sustainable Energy Reviews, 37, 1–12. https://doi.org/10.1016/j.rser.2014.05.001
Muthusaravanan, S., Vasudha Priyadharshini, S., Sivarajasekar, N., Subashini, R., Sivamani, S., Dharaskar, S., & Dhakal, N. (2019). Optimization and extraction of pharmaceutical micro-pollutant - Norfloxacin using green emulsion liquid membranes. Desalination and Water Treatment, 156, 238–244. https://doi.org/10.5004/dwt.2019.23833
Ng, Y. S., Jayakumar, N. S., & Hashim, M. A. (2010). Performance evaluation of organic emulsion liquid membrane on phenol removal. Journal of Hazardous Materials, 184(1–3), 255–260. https://doi.org/10.1016/j.jhazmat.2010.08.030
Noah, N. F. M., Othman, N., & Jusoh, N. (2016). Highly selective transport of palladium from electroplating wastewater using emulsion liquid membrane process. Journal of the Taiwan Institute of Chemical Engineers, 64, 134–141. https://doi.org/10.1016/j.jtice.2016.03.047
Noah, N. F. M., Jusoh, N., Othman, N., Sulaiman, R. N. R., & Parker, N. A. M. K. (2018). Development of stable green emulsion liquid membrane process via liquid–liquid extraction to treat real chromium from rinse electroplating wastewater. Journal of Industrial and Engineering Chemistry, 66, 231–241. https://doi.org/10.1016/j.jiec.2018.05.034
Nosrati, S., Jayakumar, N. S., & Hashim, M. A. (2011). Extraction performance of chromium (VI) with emulsion liquid membrane by Cyanex 923 as carrier using response surface methodology. Desalination, 266(1–3), 286–290. https://doi.org/10.1016/j.desal.2010.08.023
Ogahara, Z., Jespersen, K., Theilade, I., & Nielsen, M. R. (2022). Review of smallholder palm oil sustainability reveals limited positive impacts and identifies key implementation and knowledge gaps. Land Use Policy, 120, 106258. https://doi.org/10.1016/j.landusepol.2022.106258
Olasupo, A., & Suah, F. B. M. (2021). Recent advances in the removal of pharmaceuticals and endocrine-disrupting compounds in the aquatic system: A case of polymer inclusion membranes. Journal of Hazardous Materials, 406, 124317. https://doi.org/10.1016/j.jhazmat.2020.124317
Ooi, Z. Y., Harruddin, N., & Othman, N. (2015). Recovery of kraft lignin from pulping wastewater via emulsion liquid membrane process. Biotechnology Progress, 31(5), 1305–1314. https://doi.org/10.1002/btpr.2129
Ooi, Z. Y., Othman, N., & Choo, C. L. (2016). The role of internal droplet size on emulsion stability and the extraction performance of kraft lignin removal from pulping wastewater in emulsion liquid membrane process. Journal of Dispersion Science and Technology, 37(4), 544–554. https://doi.org/10.1080/01932691.2015.1050728
Othman, N., Zailani, S. N., & Mili, N. (2011a). Recovery of synthetic dye from simulated wastewater using emulsion liquid membrane process containing tri-dodecyl amine as a mobile carrier. Journal of Hazardous Materials, 198, 103–112. https://doi.org/10.1016/j.jhazmat.2011.10.014
Othman, N., Djamal, R., Mili, N., & Zailani, S. N. (2011b). Removal of red 3BS dye from wastewater using emulsion liquid membrane process. Journal of Applied Sciences, 11(8), 1406–1410. https://doi.org/10.3923/jas.2011.1406.1410
Othman, N., Yi, O. Z., Zailani, S. N., Zulkifli, E. Z., & Subramaniam, S. (2013). Extraction of rhodamine 6G dye from liquid waste solution: Study on emulsion liquid membrane stability performance and recovery. Separation Science and Technology, 48(8), 1177–1183. https://doi.org/10.1080/01496395.2012.731123
Othman, N., Noah, N. F. M., Harruddin, N., Abdullah, N. A., & Bachok, S. K. (2014). Selective extraction of palladium from simulated liquid waste solution by emulsion liquid membrane process using D2EHPA as a mobile carrier. Jurnal Teknologi (Sciences and Engineering), 69(9), 1–4. https://doi.org/10.11113/jt.v69.3386
Othman, N., Noah, N. F. M., Poh, K. W., & Yi, O. Z. (2016). High performance of chromium recovery from aqueous waste solution using mixture of palm-oil in emulsion liquid membrane. In Procedia Engineering, 148, 765–773. https://doi.org/10.1016/j.proeng.2016.06.611
Othman, N., Noah, N. F. M., Shu, L. Y., Ooi, Z. Y., Jusoh, N., Idroas, M., & Goto, M. (2017). Easy removing of phenol from wastewater using vegetable oil-based organic solvent in emulsion liquid membrane process. Chinese Journal of Chemical Engineering, 25(1), 45–52. https://doi.org/10.1016/j.cjche.2016.06.002
Othman, N., Jusoh, N., Mohar, M. S., Rosly, M. B., & Noah, N. F. M. (2018). Extraction of succinic acid from real fermentation broth by using emulsion liquid membrane process. Malaysian Journal of Analytical Sciences, 22(6), 1090–1101. https://doi.org/10.17576/mjas-2018-2206-20
Othman, N., Raja Sulaiman, R. N., Rahman, H. A., Noah, N. F. M., Jusoh, N., & Idroas, M. (2019). Simultaneous extraction and enrichment of reactive dye using green emulsion liquid membrane system. Environmental Technology (united Kingdom), 40(11), 1476–1484. https://doi.org/10.1080/09593330.2018.1424258
Parhi, P. K. (2013). Supported liquid membrane principle and its practices: A short review. Journal of Chemistry, 2013https://doi.org/10.1155/2013/618236
Peng, H., & Guo, J. (2020). Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environmental Chemistry Letters, 18(6), 2055–2068. https://doi.org/10.1007/s10311-020-01058-x
Peng, W., Jiao, H., Shi, H., & Xu, C. (2012). The application of emulsion liquid membrane process and heat-induced demulsification for removal of pyridine from aqueous solutions. Desalination, 286, 372–378. https://doi.org/10.1016/j.desal.2011.11.051
Perumal, M., Soundarajan, B., & Thazhathuveettil Vengara, N. (2019). Extraction of Cr (VI) by pickering emulsion liquid membrane using amphiphilic silica nanowires (ASNWs) as a surfactant. Journal of Dispersion Science and Technology, 40(7), 1046–1055. https://doi.org/10.1080/01932691.2018.1496829
Qaim, M., Sibhatu, K. T., Siregar, H., & Grass, I. (2020). Environmental, economic, and social consequences of the oil palm boom. Annual Review of Resource Economics, 12(1), 321–344. https://doi.org/10.1146/annurev-resource-110119-024922
Rajasimman, M., & Karthic, P. (2010). Application of response surface methodology for the extraction of chromium (VI) by emulsion liquid membrane. Journal of the Taiwan Institute of Chemical Engineers, 41(1), 105–110. https://doi.org/10.1016/j.jtice.2009.04.010
Rajasimman, M., Rajamohan, N., & Sujatha, S. (2021). Recovery of zinc from electroplating wastewater using green emulsion liquid membrane. Water Supply, 21(5), 2008–2018. https://doi.org/10.2166/ws.2020.294
Raji, M., Abolghasemi, H., Safdari, J., & Kargari, A. (2018). Selective extraction of dysprosium from acidic solutions containing dysprosium and neodymium through emulsion liquid membrane by Cyanex 572 as carrier. Journal of Molecular Liquids, 254, 108–119. https://doi.org/10.1016/j.molliq.2017.11.058
Raji, M., Abolghasemi, H., Safdari, J., & Davoodi-Nasab, P. (2020). Neodymium pertraction through sunflower oil-based emulsion liquimembrane: Stability and mass transfer investigation. Desalination and Water Treatment, 187, 333–344. https://doi.org/10.5004/dwt.2020.25450
Razo-Lazcano, T. A., del Pilar González-Muñoz, M., Stambouli, M., Pareau, D., Hernández-Perales, L., & Avila-Rodriguez, M. (2018). Chlorpheniramine recovery from aqueous solutions by emulsion liquid membranes using soy lecithin as carrier. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 536, 68–73. https://doi.org/10.1016/j.colsurfa.2017.07.050
Rosly, M. B., Jusoh, N., Othman, N., Rahman, H. A., Noah, N. F. M., & Sulaiman, R. N. R. (2019). Effect and optimization parameters of phenol removal in emulsion liquid membrane process via fractional-factorial design. Chemical Engineering Research and Design, 145, 268–278. https://doi.org/10.1016/j.cherd.2019.03.007
Rosly, M. B., Jusoh, N., Othman, N., Rahman, H. A., Sulaiman, R. N. R., & Noah, N. F. M. (2020). Stability of emulsion liquid membrane using bifunctional diluent and blended nonionic surfactant for phenol removal. Chemical Engineering and Processing - Process Intensification, 148, 107790. https://doi.org/10.1016/j.cep.2019.107790
Rouhani, S. H. R., Davarkhah, R., Zaheri, P., & Mousavian, S. M. A. (2020). Separation of molybdenum from spent HDS catalysts using emulsion liquid membrane system. Chemical Engineering and Processing - Process Intensification, 153, 107958. https://doi.org/10.1016/j.cep.2020.107958
Ruggeri, A., & Samoggia, A. (2018). Twitter communication of agri-food chain actors on palm oil environmental, socio-economic, and health sustainability. Journal of Consumer Behaviour, 17(1), 75–93. https://doi.org/10.1002/cb.1699
Sakai, K., Hassan, M. A., Vairappan, C. S., & Shirai, Y. (2022). Promotion of a green economy with the palm oil industry for biodiversity conservation: A touchstone toward a sustainable bioindustry. Journal of Bioscience and Bioengineering, 133(5), 414–424. https://doi.org/10.1016/j.jbiosc.2022.01.001
San Román, M. F., Bringas, E., Ibañez, R., & Ortiz, I. (2010). Liquid membrane technology: Fundamentals and review of its applications. Journal of Chemical Technology and Biotechnology, 85(1), 2–10. https://doi.org/10.1002/jctb.2252
Sarang, P., Kohli, H. P., Mungray, A. K., & Chakraborty, M. (2022). Artificial neural network approach towards the separation of ethylparaben and diclofenac using pseudo-emulsion hollow fiber strip dispersion technique. Chemical Data Collections, 40, 100890. https://doi.org/10.1016/j.cdc.2022.100890
Seifollahi, Z., & Rahbar-Kelishami, A. (2019). Amoxicillin extraction from aqueous solution by emulsion liquid membranes using response surface methodology. Chemical Engineering and Technology, 42(1), 156–166. https://doi.org/10.1002/ceat.201800089
Silva, G. M. E., Campos, D. F., Brasil, J. A. T., Tremblay, M., Mendiondo, E. M., & Ghiglieno, F. (2022). Advances in technological research for online and in situ water quality monitoring—A review. Sustainability, 14(9), 5059. https://doi.org/10.3390/su14095059
Shirasangi, R., Kohli, H. P., Gupta, S., & Chakraborty, M. (2020). Separation of Methylparaben by emulsion liquid membrane: Optimization, characterization, stability and multiple cycles studies. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 597, 124761. https://doi.org/10.1016/j.colsurfa.2020.124761
Shirasangi, R., Kohli, H. P., Gupta, S., & Chakraborty, M. (2021). Separation of methylparaben from aqueous source stream by pseudo-emulsion hollow fiber membrane strip dispersion technique: Optimization of process parameters using Grey-Taguchi method. Chemical Engineering and Processing - Process Intensification, 161, 108302. https://doi.org/10.1016/j.cep.2021.108302
Shokri, A., Daraei, P., & Zereshki, S. (2020). Water decolorization using waste cooking oil: An optimized green emulsion liquid membrane by RSM. Journal of Water Process Engineering, 33, 101021. https://doi.org/10.1016/j.jwpe.2019.101021
Srivastava, A., Bhagat, A., Sharma, U., Dohare, R. K., Singh, K., & Upadhyaya, S. (2017). Comparative study of arsenic(V) removal from aqueous solution using Aliquat-336 and 2-ethyl hexanol through emulsion liquid membrane. Journal of Water Process Engineering, 16, 64–68. https://doi.org/10.1016/j.jwpe.2016.12.007
Sujatha, S., & Rajasimman, M. (2021). Development of a green emulsion liquid membrane using waste cooking oil as diluent for the extraction of arsenic from aqueous solution – Screening, optimization, kinetics and thermodynamics studies. Journal of Water Process Engineering, 41, 102055. https://doi.org/10.1016/j.jwpe.2021.102055
Sujatha, S., Rajamohan, N., Anbazhagan, S., Vanithasri, M., & Rajasimman, M. (2021a). Extraction of nickel using a green emulsion liquid membrane – Process intensification, parameter optimization and artificial neural network modeling. Chemical Engineering and Processing - Process Intensification, 165, 108444. https://doi.org/10.1016/j.cep.2021.108444
Sujatha, S., Rajamohan, N., Vasseghian, Y., & Rajasimman, M. (2021b). Conversion of waste cooking oil into value-added emulsion liquid membrane for enhanced extraction of lead: Performance evaluation and optimization. Chemosphere, 284, 131385. https://doi.org/10.1016/j.chemosphere.2021.131385
Sujatha, S., Rajamohan, N., Anbazhagan, S., Vanithasri, M., & Rajasimman, M. (2022). Parameter screening, optimization and artificial neural network modeling of cadmium extraction from aqueous solution using green emulsion liquid membrane. Environmental Technology and Innovation, 25, 102138. https://doi.org/10.1016/j.eti.2021.102138
Thakur, A., Panesar, P. S., & Saini, M. S. (2014). Response surface modeling of lactic acid extraction by emulsion liquid membrane: Box-Behnken experimental design. International Journal of Chemical and Molecular Engineering, 8, 880–889. https://doi.org/10.5281/zenodo.1094449
Tang, B., Yu, G., Fang, J., & Shi, T. (2010). Recovery of high-purity silver directly from dilute effluents by an emulsion liquid membrane-crystallization process. Journal of Hazardous Materials, 177(1–3), 377–383. https://doi.org/10.1016/j.jhazmat.2009.12.042
Teng, T. T., Muthuraman, G., Mubeena, K., & Sathya, M. (2013). Emulsion liquid membrane: Removal and recovery of organic and inorganic ions. Journal of Membrane Science & Technology, 03(02), 3–4. https://doi.org/10.4172/2155-9589.1000e117
Ting, H. C., Khan, H. W., Reddy, A. V. B., Goto, M., & Moniruzzaman, M. (2022). Extraction of salicylic acid from wastewater using ionic liquid-based green emulsion liquid membrane: COSMO-RS prediction and experimental verification. Journal of Molecular Liquids, 347, 118280. https://doi.org/10.1016/j.molliq.2021.118280
Varotsos, C. A., & Krapivin, V. F. (2018). Pollution of arctic waters has reached a critical point: an innovative approach to this problem. Water, Air, and Soil Pollution, 229(11). https://doi.org/10.1007/s11270-018-4004-x
Varotsos, C. A., Krapivin, V. F., & Mkrtchyan, F. A. (2019). New optical tools for water quality diagnostics. Water, Air, and Soil Pollution, 230(8). https://doi.org/10.1007/s11270-019-4228-4
Wang, T., Xie, T., & Xu, C. (2020). Numerical investigations of micro-SLM extraction/stripping in a spiral channel. Chemical Engineering Science, 212, 115344. https://doi.org/10.1016/j.ces.2019.115344
Xue, J. Q., Liu, N. N., Li, G. P., & Dang, L. T. (2016). Optimization of cyanide extraction from wastewater using emulsion liquid membrane system by response surface methodology. Water Science and Technology, 74(4), 779–786. https://doi.org/10.2166/wst.2016.220
Yan, B., Huang, X., Chen, K., Liu, H., Wei, S., Wu, Y., & Wang, L. (2021). A study of synergetic carrier emulsion liquid membrane for the extraction of amoxicillin from aqueous phase using response surface methodology. Journal of Industrial and Engineering Chemistry, 100, 63–74. https://doi.org/10.1016/j.jiec.2021.05.041
Yang, L., Xiao, J., Shen, Y., Liu, X., Li, W., Wang, W., & Yang, Y. (2017). The efficient removal of thallium from sintering flue gas desulfurization wastewater in ferrous metallurgy using emulsion liquid membrane. Environmental Science and Pollution Research, 24(31), 24214–24222. https://doi.org/10.1007/s11356-017-0040-0
Yaseen, M., Farooq, M. M. U., Ahmad, W., & Subhan, F. (2021). Fabrication of rGO-CuO and/or Ag2O nanoparticles incorporated polyvinyl acetate based mixed matrix membranes for the removal of Cr6+ from anti-corrosive paint industrial wastewater. Journal of Environmental Chemical Engineering, 9(2), 105151. https://doi.org/10.1016/j.jece.2021.105151
Zaheri, P., & Davarkhah, R. (2017). Rapid removal of uranium from aqueous solution by emulsion liquid membrane containing thenoyltrifluoroacetone. Journal of Environmental Chemical Engineering, 5(4), 4064–4068. https://doi.org/10.1016/j.jece.2017.07.076
Zante, G., Boltoeva, M., Masmoudi, A., Barillon, R., & Trébouet, D. (2020). Selective separation of cobalt and nickel using a stable supported ionic liquid membrane. Separation and Purification Technology, 252, 117477. https://doi.org/10.1016/j.seppur.2020.117477
Zaulkiflee, N. D., Ahmad, A. L., & Yaacob, M. (2021). Acetaminophen extraction study using vegetable oil-based emulsion liquid membrane: The juxtaposition of carrier and internal phase. Journal of Membrane Science and Research, 7(4), 282–287. https://doi.org/10.22079/JMSR.2021.120282.1338
Zereshki, S., Daraei, P., & Shokri, A. (2018). Application of edible paraffin oil for cationic dye removal from water using emulsion liquid membrane. Journal of Hazardous Materials, 356, 1–8. https://doi.org/10.1016/j.jhazmat.2018.05.037
Zereshki, S., Shokri, A., & Karimi, A. (2021). Application of a green emulsion liquid membrane for removing copper from contaminated aqueous solution: Extraction, stability, and breakage study using response surface methodology. Journal of Molecular Liquids, 325, 115251. https://doi.org/10.1016/j.molliq.2020.115251
Zhao, L., Fei, D., Dang, Y., Zhou, X., & Xiao, J. (2010). Studies on the extraction of chromium(III) by emulsion liquid membrane. Journal of Hazardous Materials, 178(1–3), 130–135. https://doi.org/10.1016/j.jhazmat.2010.01.052
Zhou, W., Liang, H., & Xu, H. (2021). Recovery of gold from waste mobile phone circuit boards and synthesis of nanomaterials using emulsion liquid membrane. Journal of Hazardous Materials, 411https://doi.org/10.1016/j.jhazmat.2020.125011
Zing-Yi, O., Othman, N., Mohamad, M., & Rashid, R. (2014). Removal performance of lignin compound from simulated pulping wastewater using emulsion liquid membrane process. International Journal of Global Warming, 6(2–3), 270–283. https://doi.org/10.1504/IJGW.2014.061021
Author information
Authors and Affiliations
Contributions
Akash R. Raval: Conceptualization, methodology, investigation, writing, original draft preparation, and data curation; Himanshu P. Kohli: Visualization, data curation, writing, editing and reviewing; Omprakash K. Mahadwad: Supervision and reviewing.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• GELM: A sustainable and reliable advancement in the field of ELM.
• Oils based on vegetable and plant origin are used as green solvents.
• GELM formulation and its transport mechanism are discussed.
• Parametric and statistical studies of GELM.
• Real-time diagnosis of water quality.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raval, A.R., Kohli, H.P. & Mahadwad, O.K. A Comprehensive Review on Green Emulsion Liquid Membrane and Its Applicability Towards the Removal of Contaminants from the Aquatic Streams. Water Air Soil Pollut 233, 379 (2022). https://doi.org/10.1007/s11270-022-05849-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05849-6