Abstract
The present paper focuses on the working and application of hollow fiber strip dispersion system for the removal of metal ions from aqueous streams. Conventional separation methods like chemical precipitation, adsorption, ion exchange and solvent extraction are in use to eliminate and recover heavy metals from aqueous solutions, but suffer from their inherent limitations like less efficiency, high operating costs, secondary sludge production and disposal. Liquid membrane in comparison with these methods offers little investment, high flux, less solvent and energy consumption. Supported liquid membrane is preferred for the removal and reclamation of metal ions as this technique offers simultaneous extraction and stripping. Hollow fiber supported liquid membrane is being used successfully for the separation of various metal ions, but instability and extended functioning are the concerned issues for industrial usage. Pseudo-emulsion hollow fiber strip dispersion system solves the instability issue by maintaining uninterrupted supply of organic membrane phase in pores and successfully removes the metal ions. This paper mainly focuses on the effect of various parameters like concentration of metal, carrier and type of diluent on the removal of metal ions from aqueous streams. Permeation model development and evaluation of mass transfer coefficients are also discussed here.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liquid membrane system consists of two phases of the same nature, but diverse composition being divided by the third phase having dissimilar nature. The third phase should be insoluble in the other two phases and is referred as liquid membrane. Supported liquid membrane (SLM) is one of the configuration of liquid membrane. Liquid imbedded in the pores of thin microporous solid support due to the capillary action is said to be SLM [1, 2]. The simplicity of SLM has made them applicable in various fields like hydrometallurgy, biotechnology, wastewater treatment, pharmaceutical industry, analytical and environmental chemistry [3].
SLM is currently in practice for the elimination of treasured metal ions from various means. Here, extraction and stripping is combined in one sole step leading to high selectivity and savings of chemicals used for precipitation and stripping process. Process like chemical precipitation, adsorption, ion exchange and solvent extraction used in industries for the removal of metals from aqueous streams suffers from limitations like reduction in efficiency, sensitive operating conditions, cost of operation, formation of sludge and its disposal. SLM can be the feasible option as it offers advantages like low energy requirement, high flux, low capital and low operating cost [4].
Classification of SLM
SLM can be classified as flat sheet supported liquid membrane (FSSLM) and hollow fiber supported liquid membrane (HFSLM) depending on size, shape, surface area and use. The main driving force consists of concentration gradient and diffusion of molecules [4].
Flat Sheet Supported Liquid Membrane
Microporous solid support is used for the liquid membrane which is immobilized with the extractant and is fixed amid two half cells by gaskets forming two sections comprising source and receiving phase, respectively. Stirring of both phases is done by mechanical stirrers. FSSLM is generally used for laboratory scale [4, 5].
Hollow Fiber Supported Liquid Membrane
The removal of metal ions by HFSLM is carried out through the use of a hollow fiber module. The shell from external side consists of a solo nonporous material and consists of many thin fibers packed in rows from inner side. Pump allows the feed phase to be passed from fibers and stripping phase through shell side. Instability and long time performance are some of the concerned industrial issues. Hollow fiber modules offer large surface area per unit of module volume up to 500 m−1 but are expensive. Commercially accessible modules can have the membrane area up to 220 m2 [4, 6].
Metal Transport Mechanism, Stability and Working Principle of SLM
Metal Ion Transport Mechanism Through SLM
The extraction mechanism for the removal of n-valent metal ion from aqueous feed phase is described as follows:
where Mn+ is n-valent cation, RH is a monobasic acid, and bar shows membrane phase species.
Coupled counter current transport of metal takes place via SLM from feed phase to stripping phase. Mn+ and H+ travel in opposite path by diffusion through the membrane by extractant RH. MRn complex is formed due to the reaction of carrier RH with Mn+ liberating H+. Complex then diffuses through membrane phase and reacts with H+ at membrane–stripping phase interface discharging Mn+ while H+ binds with the carrier molecule and subsequently regenerates the carrier which again diffuses back to feed–membrane phase interface [4, 7].
Stability of Pseudo-emulsion Hollow Fiber Strip Dispersion (PEHFSD) system
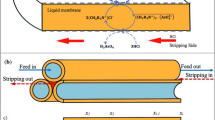
HFSLM suffers from instability as the membrane phase loses from the pores gradually due to interfacial shear force and osmotic pressure difference [8]. Figure 1a describes the HFSLM system in which the vacant pores are observed from which the membrane phase has left out, leading to instability which affects both the selectivity and flux. Aqueous phase will fill these pores progressively which reduces the separation efficacy of membrane module.
HFSLM with strip dispersion can be used to increase the stability. Here, aqueous stripping solution is dispersed in an organic membrane solution with an extractant in a mixer. This results in the formation of water-in-oil dispersion which is pumped to one side of microporous support. As shown in Fig. 1b, the continuous supply of organic membrane solution in the pores maintains the stability, continuity and effective functioning of the system. This process combines SLM and emulsion liquid membrane (ELM) and hence can be referred as pseudo-emulsion hollow fiber strip dispersion system [3, 9]. PEHFSD incorporates the benefits of ELM by offering high surface area and avoids the problem of stability [10, 11].
Working Principle of PEHFSD System
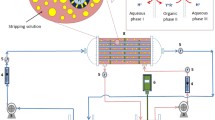
Figure 2 shows the experimental setup for PEHFSD system. A single hollow fiber module is used for extraction and stripping. A temporary emulsion formed between organic and stripping solutions in PEHFSD is referred as pseudo-emulsion [12]. The inner shell comprises numerous thin fibers arranged in rows. Stirred tanks are used for both feed and pseudo-emulsion phase. Pseudo-emulsion phase is prepared by the addition of stripping phase in the tank having solvent and extractant. Both the phases are passed counter currently by gear pumps. Feed phase and pseudo-emulsion phase pass via fibers and shell side, respectively, while maintaining the flow rates. Solvent having the extractant immobilizes the pores of fibers due to its hydrophobic characteristic. The pressure is kept higher on fiber side than on shell side to maintain the boundary layer at pores opening. Metal ion from the feed phase is extracted into the organic phase of pseudo-emulsion and is consecutively stripped by the stripping phase of pseudo-emulsion. Organic and stripping phase of pseudo-emulsion separates immediately as the stirring is stopped and the metal ion can be recovered easily. Samples are collected at time intervals to determine the concentration [8, 13].
Development of Model
The estimation of overall permeability coefficients for PEHFSD system operating in a recycling manner depends on the first-order mass transfer model with immediate reaction on the stripping side [10]. Recycling of both feed and stripping solutions takes place. Several investigators have already developed similar models while working on PEHFSD system for the elimination of different metal ions like Y(III), U(VI), Au(I), Cr(VI), Hg(II) and Zn(II) [8, 10, 14,15,16,17].
The model for transport of metal ion through PEHFSD functioning in the recycling manner comprises equations showing: (i) change of metal ion concentration in feed and stripping streams while flowing in the membrane module and (ii) change of metal ion concentration in feed and pseudo-emulsion tanks where aqueous solutions are constantly recycled through proper mixing.
Equations describing the above material balance can be fixed for the following first-order kinetic expression [8, 13, 18, 19]:
where [C] shows metal ion concentration, Vf exhibits feed volume, and S is a parameter which depends on the fibers/module geometry, linear velocity of fluids and total permeability for the system. Overall permeability coefficient (PC) for a system running in the recycling manner can be attained by the experimental value of slope S, as:
where L represents fiber length, uf describes the mean velocity of the feed solution, ri shows inner radius of hollow fiber, and Qf denotes feed flow rate.
Three mass transfer resistances based on overall permeability coefficient contribute to design of HFSLM module for the separation and concentration of metal ion which are given as:
- (1)
Liquid moving inside the fiber.
- (2)
Metal carrier complex diffusion across the organic phase immobilized on microporous structure of the fiber wall.
- (3)
Aqueous boundary layer formed on the outer side of fiber.
The reciprocal of overall permeability coefficient is shown as [10, 16]:
where rlm shows hollow fiber log mean radius, ro describes outer radius of hollow fiber, and ki and ko represent interfacial coefficient to inside and outside aqueous boundary layer, respectively.
Permeability of membrane (PM) is given as follows [15, 16]:
where km represents membrane phase mass transfer coefficient and DC shows distribution coefficient of metal ion.
Substituting Eq. 5 into Eq. 4 gives
The influence of outer aqueous phase is ignored when the reaction is rapid on reception side, and then Eq. 6 is given as:
Estimation of Coefficients for Mass Transfer
Inner aqueous boundary layer mass transfer (ki) can be given as [16, 20]:
where Df shows coefficient of diffusion for metal ion in feed phase given by Stokes–Einstein equation as [16]:
where kB shows Boltzmann constant, T exhibits absolute temperature, µ represents viscosity of solvent, and dm is the diameter of metal ion.
Mass transfer coefficient for membrane (km) can be assessed by [16]:
where ε and τ represent porosity and tortuosity for the hollow fiber, respectively, Dm shows coefficient of diffusion for metal ion in membrane, and dorg denotes membrane thickness.
Application of Hollow Fiber Strip Dispersion (HFSD) for Metal Ion Removal
Solvent extraction is extensively used in industries for macro-concentrations of metals, but reclamation of metal ions at lower concentration is challenging. Moreover, HFSD on an industrial scale has been found to be appropriate with prolonged endurance [21]. Table 1 lists out the literature review on %removal of several metal ions by HFSD/PEHFSD using various carrier, diluent and stripping solution.
Effect of Various Parameters of HFSD/PEHFSD
Impact of Metal Concentration
Different researchers have investigated the effect of feed phase metal concentration on its removal. It is seen that with increasing concentration of metal ion, concentration gradient rises which eventually increases the initial mass transfer coefficient, but overall mass transfer coefficient reduces. With limited amount of carrier present in membrane phase, overall mass transfer resistance increases at higher initial metal content.
Sonawane et al. [15] studied the transport of different initial Cr(VI) concentrations (20–120 mg/L) in feed phase using Cyanex 923 by PEHFSD. Decrease in %metal transport was observed at higher metal concentration as chemical reactions might take place instantaneously between carrier and Cr(VI), forming a complex which creates high diffusional resistance to the membrane phase.
Alguacil et al. [23] studied the removal of Co(II) by extractant DP-8R in Exxsol D100 through PEHFSD. Overall mass transfer coefficient was found to decrease with increase in initial Co(II) concentration from 0.17 to 1.7 mM in the feed phase as distribution coefficient decreased with rise in initial metal concentration.
Agarwal et al. [25] investigated the effect of Cu(II) concentration (0.1–1 g/L) in the feed phase and its permeation by Acorga M5640 through PEHFSD. Overall mass transfer coefficient was also found to decrease with rise in Cu(II) content due to the rise in overall mass transfer resistance.
Pirom et al. [8] studied the transport of yttrium(III) at different concentration (1–4 mM) by PEHFSD using D2EHPA. The %extraction decreased with rise in initial Y(III) concentration. They suggested that the slow diffusion of Y(III) complex in the organic solution caused decline in mass transfer of Y(III) ion.
Effect of Carrier Concentration
Several investigators have studied the influence of carrier concentration on the removal of metal ion. It is observed that permeability increases with rise in carrier concentration to certain extent and thereafter the permeability decreases due to the rise in organic phase viscosity at higher carrier concentration which increases the membrane phase resistance.
Ramakul et al. [22] studied the synergistic effect amid Cyanex 272 and TBP during the extraction of yttrium ions in lanthanide series from rare earths mixture through HFSLM. Around 75% selective extraction of yttrium ions was achieved using mixture of TBP in Cyanex 272 in the ratio of 0.2 M: 0.4 M. Yttrium was removed selectively because of its smallest ionic radius.
Sonawane et al. [15] studied the effect of carrier Cyanex 923 concentration (2–20% v/v) on chromium permeation through PEHFSD. More than 95% Cr(VI) transport was observed at 10% v/v, and then further increase in the carrier concentration did not affect the Cr(VI) transport.
Vijayalakshmi et al. [29] observed increase in %transport of Y(III) through HFSLM from 62 to 92% with rise in the concentration of carrier DNPPA from 0.15 to 0.4 M, respectively. Increasing the DNPPA concentration increased the distribution coefficient of Y(III) amid feed and organic phases which helped in the higher transfer of Y(III) ions across the membrane.
Reis et al. [19] observed decrease in overall mass transfer coefficient from 3 × 10−6 to 4.8 × 10−7 m/s on reducing the concentration of carrier N-decoxy-1-(pyridin-3-yl)ethaneimine from 0.1 to 0.05 M, respectively, during the removal of Zn(II) through PEHFSD.
Table 2 lists out the effect of carrier concentration on the separation of metal ions by PEHFSD system along with the overall permeability coefficient values.
Effect of Diluents
Diluents are used for preparing different concentrations of carrier which is used for the removal of metal ions. The viscosity of the diluent should be low to facilitate diffusion of solute-complex in the membrane. Both physical and chemical interactions occur amid diluent and carrier, and so the influence of diluent is substantial [4].
Alguacil et al. [31] investigated the effect of diluents like n-decane, n-heptane, toluene and cumene on Cr(III) transport through PEHFSD. Overall permeability coefficient for Cr(III) transport was highest with n-decane. The intrinsic properties of diluent play a crucial part in the performance of liquid membrane.
Alguacil et al. [23] evaluated the performance of Exxsol D100 and Solvesso 100 during the removal of Co(II) by PEHFSD. Solvesso 100 offered greater overall mass transfer coefficient than Exxsol D100, and they correlated it with the inherent characteristics of Solvesso 100 like surface tension, volatility, viscosity and water solubility.
Pirom et al. [8] studied the influence of kerosene and toluene on the removal of yttrium(III) through PEHFSD. They found that diluent polarity index affected the efficacy of an extractant–carrier system in a membrane. The lower polarity index of kerosene helped in the formation and reformation of Y(III) complex. The lower viscosity of kerosene improved the overall removal capacity by reducing the mass transfer resistance.
Concluding Remarks
HFSLM offers effective removal and recovery of metal ions from aqueous streams but suffers from instability concern. PEHFSD resolves this issue as it maintains stability of membrane by continuous supply of membrane phase in the pores and so is used by many investigators for effective removal of the metal ions from aqueous streams. So wide scope for SLM exists in the field of separation and its application should be extended toward the removal of other organic compounds from wastewater.
References
V.S. Kislik, in Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater Treatment, ed. by V.S. Kislik (Elsevier, Great Britain, 2010), p. 5
V.S. Kislik, Solvent Extraction: Classical and Novel Approaches, 1st edn. (Elsevier, Great Britain, 2012), pp. 501–502
P. Dżygiel, P.P. Wieczorek, in Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater Treatment, ed. by V.S. Kislik (Elsevier, Great Britain, 2010), p. 76
P.K. Parhi, J. Chem. (2013). https://doi.org/10.1155/2013/618236
K. Nath, Membrane Separation Processes, 1st edn. (Prentice-Hall of India Private Limited, New Delhi, 2008), p. 244
A. Gabelman, S.T. Hwang, J. Membr. Sci. (1999). https://doi.org/10.1016/S0376-7388(99)00040-X
Z. Ren, W. Zhang, Y. Liu, Y. Dai, C. Cui, Chem. Eng. Sci. (2007). https://doi.org/10.1016/j.ces.2007.06.005
T. Pirom, A. Arponwichanop, U. Pancharoen, T. Yonezawa, S. Kheawhom, Sci. Rep. (2018). https://doi.org/10.1038/s41598-018-25771-4
H.P. Kohli, S. Gupta, M. Chakraborty, Colloids Surf. A (2020). https://doi.org/10.1016/j.colsurfa.2019.124308
S.C. Roy, J.V. Sonawane, N.S. Rathore, A.K. Pabby, P. Janardan, R.D. Changrani, P.K. Dey, S.R. Bharadwaj, Sep. Sci. Technol. (2008). https://doi.org/10.1080/01496390802064141
H.P. Kohli, S. Gupta, M. Chakraborty, J. Wat. Process Eng. (2019). https://doi.org/10.1016/j.jwpe.2019.100880
J.V. Sonawane, A.K. Pabby, A.M. Sastre, AIChE J. (2008). https://doi.org/10.1002/aic.11371
K. Wieszczycka, M. Regel-Rosocka, K. Staszak, A. Wojciechowska, M.T.A. Reis, M.R.C. Ismael, M.L.F. Gameiro, J.M.R. Carvalho, Sep. Purf. Technol. (2015). https://doi.org/10.1016/j.seppur.2015.09.017
J.V. Sonawane, A.K. Pabby, A.M. Sastre, J. Membr. Sci. (2007). https://doi.org/10.1016/j.memsci.2007.05.016
J.V. Sonawane, A.K. Pabby, A.M. Sastre, J. Hazard. Mater. (2010). https://doi.org/10.1016/j.jhazmat.2009.09.085
S. Gupta, M. Chakraborty, Z.V.P. Murthy, Sep. Purf. Technol. (2013). https://doi.org/10.1016/j.seppur.2013.04.020
S. Agarwal, M.T.A. Reis, M.R.C. Ismael, J.M.R. Carvalho, Sep. Purif. Technol. (2014). https://doi.org/10.1016/j.seppur.2014.02.039
S. Agarwal, M.T.A. Reis, M.R.C. Ismael, J.M.R. Carvalho, Sep. Purif. Technol. (2016). https://doi.org/10.1016/j.seppur.2016.03.031
M.T.A. Reis, M.R.C. Ismael, A. Wojciechowska, I. Wojciechowska, P. Aksamitowski, K. Wieszczycka, J.M.R. Carvalho, Sep. Purf. Technol. (2019). https://doi.org/10.1016/j.seppur.2019.04.076
F.J. Alguacil, M. Alonso, F.A. Lopez, A. Lopez-Delgado, I. Padilla, H. Tayibi, Chem. Eng. J. (2010). https://doi.org/10.1016/j.cej.2009.11.016
N.S. Rathore, A. Leopold, A.K. Pabby, A. Fortuny, M.T. Coll, A.M. Sastre, Hydrometallurgy (2009). https://doi.org/10.1016/j.hydromet.2008.08.009
P. Ramakul, T. Supajaroon, T. Prapasawat, U. Pancharoen, A.W. Lothongkum, J. Ind. Eng. Chem. (2009). https://doi.org/10.1016/j.jiec.2008.09.011
F.J. Alguacil, I. Garcia-Diaz, F. Lopez, A.M. Sastre, Sep. Purif. Technol. (2011). https://doi.org/10.1016/j.seppur.2011.05.029
L. Pei, L. Wang, W. Guo, N. Zhao, J. Membr. Sci. (2011). https://doi.org/10.1016/j.memsci.2011.05.037
S. Agarwal, M.T.A. Reis, M.R.C. Ismael, M.J.N. Correia, J.M.R. Carvalho, Sep. Purif. Technol. (2013). https://doi.org/10.1016/j.seppur.2012.09.026
F.J. Alguacil, I. Garcia-Diaz, F.A. Lopez, J. Ind. Eng. Chem. (2013). https://doi.org/10.1016/j.jiec.2012.12.003
A. Mondal, S. Ghosh, A. Bhowal, S. Datta, Sep. Sci. Technol. (2013). https://doi.org/10.1080/01496395.2012.723103
S. Chaturabul, W. Srirachat, T. Wannachod, P. Ramakul, U. Pancharoen, S. Kheawhom, Chem. Eng. J. (2015). https://doi.org/10.1016/j.cej.2014.12.034
R. Vijayalakshmi, S. Chaudhury, M. Anitha, D.K. Singh, S.K. Aggarwal, H. Singh, Int. J. Miner. Process. (2015). https://doi.org/10.1016/j.minpro.2015.02.003
S.A. Allahyari, S.J. Ahmadi, A. Minuchehr, A. Charkhi, RSC Adv. (2017). https://doi.org/10.1039/c6ra26463h
F.J. Alguacil, M. Alonso, F.A. Lopez, A. Lopez-Delgado, Sep. Purif. Technol. (2009). https://doi.org/10.1016/j.seppur.2009.01.012
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kohli, H.P., Gupta, S. & Chakraborty, M. Applicability of Hollow Fiber Strip Dispersion for the Removal of Metal Ions from Aqueous Streams. J. Inst. Eng. India Ser. E 101, 91–97 (2020). https://doi.org/10.1007/s40034-020-00163-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40034-020-00163-4