Abstract
Over the past few decades, ELM based separation processes have become an attractive and efficient way to remove toxic metal ions from the various aqueous waste effluent streams. This paper provides a comprehensive review of the generation, characterization, and applications of emulsion liquid membrane (ELM) from the more than 280 papers and books published so far. First, the ELM formation and the transport mechanism are introduced. Then the ELM stability and emulsion splitting methods are discussed in detail, followed by the latest research and findings of the extraction of copper and nickel using the ELM technique during the past few decades, including a thorough discussion of ELM operating parameters that impact ELM stability and extraction. In addition, the kinetics study and industrial application of this technique for the removal of coppers/nickel solutes are also presented and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Heavy Metal Ions from Wastewater and Methods of Treatments
As defined, heavy metal ions usually refer to elements having atomic weights between 63.5 and 200.6 with a specific gravity greater than 5.0 [1, 2]. They exist in all forms of waste effluents, including mining tailings, manufacturing discharges, smelting, and metal plating processes [3]. They are usually not considered biodegradable. Many heavy metal ions are toxic, or the accumulation of heavy metal ions can be harmful to living organisms [4,5,6]. For example, in excess amounts, copper can cause many problems in human beings such as vomiting, convulsions, and cramps [6]; nickel is a human carcinogen, and it may cause serious lung and kidney problems such as pulmonary fibrosis and gastrointestinal distress [4]; mercury may cause nausea, nephrotic syndrome, and neurotic disorder in human beings [7]; lead, one of the most common heavy metal in wastewater, may cause brain dysfunction and severe kidney disease [8]; cadmium and chromium may cause lung problems such as lung inflammation, scarring, or even cancer [5, 9]. In order to minimize the negative effects brought by these heavy metal ions to the environment, governments around the world have set strict regulations regarding the heavy metal ion concentrations allowed in wastewater discharges. The types of heavy metals and their permissible limits are given in Table 1 [10].
One main source of wastewater is acid mine drainage (AMD). Although the formation of AMD is a complicated process with a combination of chemical and microbiological process, it generally includes the oxidation of pyrite and pyrrhotite [11,12,13,14], as such it often contains iron as well as many different heavy metal ions. These heavy metal ion concentrations may vary and were sometimes distributed unevenly among the tailings, causing difficulty in treatment. Examples of AMD obtained globally are given in Table 2.
As concluded by Fu and Wang (2011) [5], various methods have been implemented toward removing heavy metals ions from wastewater, including solvent extraction [21,22,23,24,25,26]; chemical precipitation [27,28,29], ion exchange [30, 31], adsorption [32], solid membrane filtration [33, 34], liquid membrane technique [35,36,37,38], coagulation and flocculation [39, 40], floatation [41], and electrochemical treatment [42,43,44]. Their advantages and disadvantages are listed in Table 3.
Although the techniques listed above can be used to treat industrial effluents containing a high level of metal ions, when facing wastewater that contains a much lower metal ion concentration (less than 500 mg/L), most techniques would incur a lower removal or separation efficiency and high operating cost. The emulsion liquid membrane technique can be used to solve these problems because it has a high removal efficiency and very large contacting surface area. Thus, this paper will focus on the emulsion liquid membrane technique and the details of which will be described in the following sections.
2 Emulsion Liquid Membrane Generation
ELM based various extraction systems are essentially double emulsions that exist as W/O/W (water-in-oil-in-water) and O/W/O (oil-in-water-in-oil), where metal ions from wastewater were usually recovered by utilizing the W/O/W type emulsions, here the organic phase separates the encapsulated internal phase droplets in the emulsion from the aqueous feed [102,103,104,105,106,107,108]. Two steps are usually involved in the formation of ELM, which are the formation of primary emulsion and the formation of emulsion liquid membrane. In order to prepare the primary emulsion, water or oil droplets need to be dispersed into many smaller ones to suspend in the surrounding liquid matrix. It should be noted the deformation of a droplet is opposed by Laplace pressure – a pressure difference between the convex and concave side of a curved interface [109].
where γ represents the surface tension and R1 and R2 represent the principal radii of curvature. Generally, a larger external stress needs to be applied to the mother droplet, which means that a very large pressure gradient occurs in order to deform the droplets. Although this could be achieved by high stresses in laminar flow, in most cases, when droplets are suspended in water or another low-viscosity liquid of turbulent flow, inertial effects (liquid chaotic motion such as pressure fluctuations or cavitation) play the main role, although high shear stresses are sometimes required [110, 111]. The following devices are generally used for emulsification: mechanical stirrers, high-pressure homogenizers (such as ultrasonicator), and rotor–stator systems (such as colloidal mills); the comparisons of these devices are made in Table 4.
The addition of an emulsifier (surfactant) is necessary to form a stable emulsion. The emulsifier usually has a polar head (hydrophilic) and a non-polar tail (lipophilic), and the non-polar tail will go into the oil phase while the polar head remains in the water phase [109]. Figure 1 shows a typical structure of an emulsifier molecule in O/W emulsion.
The emulsifier not only lowers the surface tension (γ) in order to facilitate the break-up of emulsion droplets but also to prevent the formed emulsion droplets from recoalescing [102, 103].
After the primary emulsion is formed (it could be W/O or O/W emulsion), an emulsion liquid membrane will be generated by stirring the primary emulsion in water or oil depending on the liquid matrix, the stirring is usually conducted using a mechanical stirrer under appropriate speed. During this process, an additional surfactant may be required depending on the system [120]. A typical structure of an ELM droplet can be seen in Fig. 2.
3 ELM Mechanisms
For the extraction of heavy metal ions using emulsion liquid membrane, a solution–diffusion model is the most widely accepted mechanism [38, 102]. It can be divided into two types, including simple permeation mechanism and facilitated transport mechanism. They are discussed in the following subsections separately.
3.1 Simple Permeation Mechanism
Here the solute in the external liquid phase is dissolved into the liquid membrane, and the dissolved solute has a certain diffusivity that can be transported into the internal receiving phase. The solute species dissolve in the liquid membrane and diffuse across the membrane due to an imposed concentration gradient. Permeation stops when concentration equilibrium is reached. The solute does not react chemically with the liquid membrane and is supposed to be in the same form in the feed, liquid membrane, and receiving phases [121]. However, this is rare and in most cases the second mechanism applies.
3.2 Facilitated Transport Mechanism
For this type, the efficiency and selectivity of transport across the liquid membrane can usually be enhanced by a carrier in the liquid membrane. A carrier reacts rapidly and reversibly with the desired solute to form a complex. This process is known as facilitated or carrier-mediated liquid membrane separation. In many cases, the facilitated transport is combined with coupling counter- or cotransport of different ions through the liquid membrane. The coupling effect supplies the energy for uphill transport of the solute. In order for the maximum amount of solute to diffuse into the membrane phase and transport the solute into the receiving phase, two mechanisms may be utilized:
-
The first one is the passive transport mechanism, which is done through the incorporation of a stripping liquor inside the receiving phase. Once the solute moving in the membrane phase reacts with the stripping agent, a membrane insoluble product is produced [122,123,124,125]. The examples include phenol removal when NaOH is used as the receiving stripping liquor and nickel ion removal when using oxalic acid solutions as the stripping liquor [126, 127].
-
The second one is more widely used, where a carrier (or extractant) exists in the membrane phase; the solute diffusion and chemical reactions occur in both the membrane phase and internal receiving phase [128,129,130,131]. Depending on the system requirement, the carrier can be acidic, basic, or chelating. Especially for metal ion transport, the carriers are usually acid such as –COOH or chelating products such as commercially available LIX products [35,36,37]. The whole process is as follows: First, when a solute enters into the interface between the membrane and the external phase, it will usually form a solute–carrier complex that is soluble in the membrane phase – this reaction is reversible [37]. This complex can diffuse freely inside the membrane phase, and once it reaches the interface between the membrane and internal phase, it will dissociate and the solute will dissolute into the internal phase [132, 133]. It can be seen that this process combines both solvent extraction and stripping in the same unit [37, 134]. Through this way, the carrier molecule can be regenerated and reused many times, meaning that a lower amount of carrier is required when using ELM than conventional solvent extraction. The percentage of metal removal can be calculated by comparing the difference of metal concentration in aqueous phase before and after the extraction process. Figure 3 shows a typical process for copper ion extraction by ELM using a CuSO4 solution as an example. In this example, HR represent a copper extractant (such as LIX 984 N) and CuR2 represents the nickel-extractant complex.
The extraction of copper ions by ELM technique: (a) The formation of ELM globules in CuSO4 solution; (b) The formation of copper-extractant complex by solvent extraction; (c) The copper-extractant complex moves freely inside the ELM globule; (d) The regeneration of copper ions inside the internal liquor by stripping
4 Emulsion Stability & Splitting Technique
4.1 Emulsion Stability
Emulsion stability is a perennial issue, with formed emulsion being generally thermodynamically unstable [102]. Take water in oil (W/O) emulsion for example, during the emulsification process, large water globules will be breaking into many smaller droplets and these droplets are uniformly dispersed in the oil matrix. This not only significantly increases the total surface area of water droplets but also increases the total configurational entropy [107]. This phenomenon can be shown in the second law of thermodynamics:
Here ΔG is the change in Gibbs energy during the emulsion formation process; ΔA is the change of total surface area of droplets; γ is the surface tension between the mother droplet and the surrounding immiscible phase; TΔS is equal to the entropy of dispersions during emulsification. Usually ΔAγ is much higher than TΔS, this means that the Gibbs energy formed cannot be compensated by the entropy dispersion in the droplets [109]. Thus, this formation is thermodynamically unstable.
Emulsion stability is usually a relative term, the stability measurement of emulsion droplets can be very complicated, and sometimes it is difficult to perform a quantitative measurement. As such, various methods have been proposed, including droplet counting and size distribution analysis using optical microscope or dynamic light scattering; zeta-potential measurements to predict the electrostatic repulsion force between droplets; other stability tests such as shaking, thermal cycling and freeze–thaw, etc. [112].
Before the emulsion droplets are deformed, they are balanced by interaction forces, including the van der Waals attraction, electrostatic repulsion from the double layers, or steric repulsion depending on the type of surfactant that is used [111]. Once the balance force can no longer support the emulsion droplets, the following emulsion breakdown process may occur: flocculation, sedimentation, creaming, Ostwald ripening (disproportionation), phase inversion, and coalescence [109]; the schematic representation of each term is shown in Fig. 4.
Various emulsion breakdown processes (adapted from Tadros (2009)) [109]
In most circumstances, for example, a water in oil (W/O) emulsion, the breakdown of the emulsion usually involves four steps. The first step is that some large groups are formed due to the flocculation of many small dispersed internal water droplets; the second step is that these small water droplets coalescence into large drops and the numbers of emulsion droplets are thus decreased; the third step involves the sinkage of formed large water droplets by moving into the water and oil interface; then the last step is that these drops coagulate, meaning the emulsion droplets are broken [120].
4.2 Emulsion Splitting Technique
Although emulsion droplets will deform inherently, some emulsion droplets are relatively stable and can last for several weeks or months. De-emulsification or ELM splitting can be complicated, depending on the characteristics and properties of the emulsion as well as the system requirement. Various methods have been proposed, including heating, such as conventional and microwave demulsification [135]; mechanical methods such as gravity separation and centrifugal force [136]; chemical methods such as the addition of de-emulsifier [137]; electrical methods such as high-voltage electrostatic splitter [138]. The comparison of the advantages and disadvantages of these techniques can be found in Table 5.
These methods may be used solely or combined. It should be noted that there are many ways to quantify the demulsification efficiency, including dewatering ratio, remaining water content, comparison of emulsion volume, and comparison of oil concentration in feed and permeate [145].
5 Extraction of Heavy Metal Ions using ELM
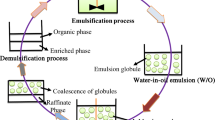
The extraction of heavy metal ions from waste stream using ELM has been a popular topic ever since ELM was invented in 1968. Studies generally focus on the extraction efficiency, process kinetics, process optimization, separation mechanism, and mass transfer modeling in the extraction of metals such as copper, nickel, zinc, cadmium, cobalt, mercury, and chromium. The ELM formed is always a water-in-oil-in-water (W/O/W) emulsion, where the internal water phase is usually a very strong acid (such as H2SO4) or base (such as NaOH); the external phase is the waste water stream, and the oil phase is an inexpensive diluent (such as kerosene) containing an oil-soluble metal extractant (such as LIX 64 N) and hydrophobic surfactant (such as SPAN 80) [35]. A process schematic of utilizing ELM to extract heavy metal ions is shown in Fig. 5.
A summary of the compositions of ELM for some commonly seen heavy metal ions (Cu, Ni, Zn, Co, Cr, Cd) removal is listed in Table 6.
It should be noted that ELM not only can be used to remove metal ions from single cation bearing solutions, it is also efficient in separating metal ions from others. This can be attributed to the metal selectivity of the extractant or the stripping liquor. Examples include, but are not limited to, the separation of cobalt and nickel; zinc and copper; and chromium and nickel.
Many reports have focused on the metal ions removal from single cation solutions using ELM, these metals include copper, nickel, cobalt, zinc, chromium, cadmium, mercury, lead, silver, gallium, molybdenum, uranium, ruthenium, platinum, and palladium. Each metal has different physical and chemical properties during this process and needs to be discussed separately. Only copper and nickel are discussed in this review because they are the most studied and representative metals from wastewater. Copper is one of the most widely used metals and chalcopyrite (CuFeS2) is the primary copper mineral for the production of copper, whereas nickel minerals rarely form separate aggregates, but are mostly dispersed in other sulfide minerals, mainly pyrrhotite (Fe(1−x)S) and chalcopyrite (CuFeS2). Thus, they may be present at relatively high concentrations in aqueous effluents from copper and nickel mines, such as Copper Cliff mine from Sudbury, Ontario, Canada.
5.1 Copper
As one of the most common metals in the world, copper has been extensively utilized in recorded history. The removal of copper from wastewater has also been an area of research interest. In the past few decades, much research has been reported on copper removal by ELM. These can be generally classified into three categories: process operating parameters, kinetic studies during the copper permeation process, and industrial applications. These are discussed in the following sections.
5.1.1 Copper Process Operating Parameters
Many different operating parameters are associated with the ELM extraction process, including the ones during the formation of primary emulsion: extractant concentration, surfactant concentration, the internal phase concentration, the volume ratio of internal phase/organic phase, the primary emulsion formation speed, the primary emulsion formation time; and other parameters associated with the formation of ELM and copper extraction, including the volume ratio of primary emulsion/external phase, the pH of the external phase, the copper concentration of the feed phase, the double emulsion stirring time, and double emulsion stirring speed [146, 147, 152, 194]. These parameters have been investigated by many researchers. It should be noted that each work was performed under different conditions, thus the conclusions usually differ. However, many common characteristics have been found as follows:
Extractant Concentration
Extractant concentration is vital since metal ions loading in the membrane phase usually governs the rate of extraction. The extractant usually act as a “shuttle” during the transportation of metal ions through the liquid membrane [204]. Thus, the extractant must be able to form a complex with the solute and move freely in the membrane phase, in the meanwhile, the extractant should not be soluble in the internal or external water phase; besides that, the extractant should be able to increase the emulsion stability and selectivity. Most research used oxime as the copper extractant because the oxime carrier contained some p-nonylphenol that could prevent crud formation and allow clear phase separation. Both type and concentration of extractant have impacts on emulsion stability and extraction efficiency, where an increment in both has been observed with the increase in extractant concentration [205]. Manzak and Tutkun elucidated that with an increase in extractant concentration, the complex forming tendency also increases with the solute [206]. Zheng et al. noted that a higher extractant concentration is preferred during the process since it will favor complex formation at the membrane-feed stream interface, thus the copper ions transfer flux will be increased [207]. However, as stated by Valenzuela et al. and Sengupta et al., an excessive increase in extractant concentration will make the oxime aggregate, increasing the viscosity of the membrane [35, 148]. The increased membrane phase viscosity effects the dispersion behavior of an emulsion, which is further responsible for the reduction in the interfacial mass transfer area [208], thus impeding the diffusion of copper into the ELM [35, 148]. It is also reported by other researchers that a high extractant concentration may cause the hydrolysis of surfactant molecules, further leading to a high degree of osmotic swelling, and finally, poor ELM stability [209, 210]. Manzak and Melek observed a dilution of the internal phase by increasing the extractant concentration, causing an increase in emulsion swelling and a reduction in extraction efficiency [211]. Bhatti et al. also recently reported that the emulsion droplet size tends to increase with the increase in extractant due to enhanced ionic charge on the emulsion droplet surface [212].
Surfactant Concentration
Surfactants in general have a vital role in emulsion stability and removal of low concentration metal ions [213]. They usually have hydrophilic heads and hydrophobic tails in their chemical structure, making them able to absorb on the oil and water interface, thus decreasing the interfacial surface tension, reducing the emulsion droplet size, and developing resistance to droplet coalescence. It is well known that thermodynamic instability of emulsion droplets arises from coalescence of droplets, and stable ELM leads to the formation of smaller internal phase droplets to provide large interfacial area [214,215,216]. In general, surfactants with low hydration capacity and diffusivity are highly preferable for obtaining stable ELM, but too much stable ELM is also not desirable due to the difficulty in the de-emulsification process [217]. It should be noted that the surfactant concentration should only be within an appropriate range (2–5%) for ELM applications [210], where a very high surfactant concentration will increase the diffusional resistance due to the increased interfacial viscosity [147], thus hindering the movement of internal phase droplets within the large emulsion globule, which will further decrease the mass transfer coefficient [218,219,220]. It was reported by Mikucki and Osseo-Asare that with an increase in surfactant concentration, the amount of copper extracted initially increased [221, 222]. However, once the surfactant concentration increases beyond a certain point, even though the emulsion droplets were more stable, Mikucki and Osseo-Asare found that both the interfacial viscosity and the chances of association of hydrophobic tails of the surfactant with the internal droplets were increased, and the membrane thickening also brought an increased diffusion distance for metal ions [221]. Manzak and Tutkun also studied the effect of surfactant concentration on the lactic acid extraction efficiency and reported 5% surfactant concentration as an optimum value [206]. In addition to the effects mentioned above, the surfactant also has an impact on the osmotic swelling of the internal water phase, where the transfer of water molecules into the internal phase may be due to the hydration of surfactant molecules. Wan and Zhang found that the emulsion swelling rate increases with the increase of surfactant concentration up to a certain value of surfactant concentration; thereafter, it reduces on further enhancement in its concentration [217].
Internal Phase Concentration
The internal phase concentration plays a vital role in the emulsion stability [223] and extraction efficiency [224]. From the ELM transport mechanism point of view, the main driving force is the difference in H+ concentration between external phase and internal phase. The extraction efficiency increases with the increase of internal phase concentration due to the difference in chemical potential, but after reaching the optimum value, it starts to reduce due to the rise in osmotic pressure between the external phase and internal stripping phase; this causes membrane swelling and led to poor ELM stability [205, 209, 212]. Zheng et al. pointed out that although mass transfer of the stripping phase was not the rate controlling step in the extraction process, a higher concentration of H+ in the internal phase would lead to a very large concentration gradient across the membrane, which could increase the speed of copper complex dissociation, favoring copper transport across the membrane [207]. Also, it was reported that when using NaOH as the internal phase, when increased beyond a certain limit, NaOH hydrolyzed the Span 80 and the emulsion stability decreased. Abbassian and Kargari reported that an increased amount of internal phase reagent reduces the thickness of membrane phase which further leads to membrane breakage [225]. Also, a much higher internal concentration will degrade the extractant as well as lower its acid-activity coefficient.
The Volume Ratio of Internal Phase/organic Phase
The volume ratio of internal phase/organic phase is usually defined as phase ratio, and in general, the phase ratio affects emulsion stability and extraction efficiency [147], and phase inversion. As reported by Tang et al., a low phase ratio may slow down the diffusion process and also increase the consumption of membrane phase [226]. In general, with the increase of the phase ratio to a certain value, the extraction efficiency increases, but thereafter, it causes instability in the emulsion [206]. Sengupta et al. noted that an increase in phase ratio not only increased the overall extracting capacity of the ELM but also increased the extraction rate [147, 227] because the denser packing of stripping droplets helped form a thinner membrane; thus, the diffusion path length for the copper complex within the emulsion globule was shortened. As a contrast, Kumbasar observed a much higher phase ratio will bring insufficient availability of the membrane phase volume for enclosing all the internal reagent molecules [184]. In his previous research, he also noticed that with the increase in volume of the internal phase, the emulsion became unstable due to the release of the internal reagent into the external phase, which may be due to the increase in internal droplets diameter [220]. This may also cause a reduction in the interfacial reaction area and thus lead to a decrease in extraction efficiency [181, 228].
The Primary Emulsion Formation Speed & Time
The emulsification speed is considered as the hydrodynamic pressure that is developed by the homogenizer to push the liquid through the fine channels of the homogenizer shaft causing a reduction in the mean droplet size [119, 229]. Bhatti et al. reported the effect of emulsification speed on the size (2.91 µm at 10,000 rpm and 2.56 µm at 20,000 rpm) of mean emulsion droplets [212]. Venkatesan and Begum varied the emulsification speed and reported the minimum breakage for the emulsification speed of 10,000 rpm for 6 min of emulsification time [119]. Most research revealed that the size of internal droplets decreased with the increase of the emulsification speed and time up to a certain level. Higher emulsification speed will usually produce smaller diameters and much more emulsion droplets than that of lower emulsification speed. It should be noted that ultrasound processing is also a very efficient emulsification technique. With ultrasound, the drop size is much smaller than that given by mechanical agitation under the same conditions. However, only a few studies focused on using this technique for preparing the W/O emulsions because the production of W/O emulsions with high viscosity of continuous phase needs higher threshold ultrasonic intensity compared to O/W emulsions [112]. A study also showed that under certain experimental conditions, the percentage of emulsion breakage decreases with the increase of the ultrasound power until a certain level. Ahmed found that lower emulsion breakage was obtained at an ultrasonic power of 20 W, an emulsification time of 3 min and a distance of 20 mm of the probe from the bottom of the emulsification cell [112].
Emulsification time has a very strong effect on the size of emulsion droplets, which has a direct impact on the emulsion stability as well as on the extraction efficiency. A short emulsification time gives large size emulsion droplets, which enhance the droplet coalescence. A long emulsification time increases the membrane-breakage, owing to the larger exposure of inner phase droplets to high shear rate, resulting in an increase in the water transfer rate into the inner stripping phase, which causes the membrane breakage [173, 230]. Djenouhat et al. observed that the emulsification time of 3 min was sufficient to make a stable emulsion using ultra-sonication [231].
The Volume Ratio of Primary Emulsion/external Phase
The volume ratio of external phase/primary emulsion usually refers to the treat ratio, and an increase in treat ratio usually makes emulsion droplets more widely spread, increasing the distance between emulsion droplets, thus reducing the unit interfacial reaction area, leading to a decrease in the extraction efficiency [205]. The ratio was selected to be over 1 most of the time, since at a treat ratio of 1, extraction efficiency was found to be reduced. This may be due to the difficulties in dispersing the emulsion in the external phase because of the large volume of the emulsion increases the emulsion viscosity, resulting in an inverse in the interfacial area. Goyal et al. found a treat ratio of 2 (v/v) as an optimum value during the chromium (Cr(VI)) removal process [177, 224]. Djenouhat et al. selected the treat ratio of 3.33 to provide good distribution of W/O emulsion in the external feed phase [231]. It should be noted that although the extraction efficiency is impacted by varying the treat ratio, the influence on the emulsion stability was not found to be significant [144, 209].
The pH of the External Phase
As discussed earlier, the difference in H+ concentration between the external feed phase and internal phase has been considered as the main driving force of ELM extraction. The extraction efficiency will be affected by the pH of the external feed phase since a change in pH may bring osmotic pressure difference, which increases the transportation of water molecules into the internal phase leading to membrane swelling and hence further affecting emulsion instability [205, 209]. The solute transfer rate decreases for low values of pH due to the competition of hydrogen ions with the solute molecules [205]. In the case of phosphorous based extractant (trinoctyl phosphoric acid) and high molecular weight tertiary amines (tri-n-octyl amine (TOA), alamine336), the extraction rate has been reported to decrease with the increase in feed phase pH [232]. Kondo et al. reported that in the low pH range, the initial extraction rate varied inversely as the 0.5 power of hydrogen-ion concentration, but in the high pH range it became independent of pH [233]. In addition, rupture of emulsion globules may also occur by changing the pH of the feed phase.
Double Emulsion Stirring Speed & Time
With the increase of double emulsion stirring speed, the applied shear force by the impeller on the emulsion globules increases, which further helps in producing the small size internal phase droplets, bringing good ELM stability and high extraction efficiency; meanwhile, the distribution of small emulsion globule also increases, which further enhances the interfacial area [173, 225]. Low stirring speed is not desirable due to the formation of large size emulsion globules, which further reduces the available interfacial area of mass transfer [104]. However, too high a value can lead to internal phase swelling, droplet coalescence, and globule rupture. Frankenfeld et al., Valenzuela et al., and Sengupta et al. stated the emulsion globules were more likely to rupture and swell under very high stirring speed and time because very intense drop–drop interactions existed; therefore, an appropriate stirring speed needs to be selected for optimal performance of the emulsion liquid membrane [35, 147, 234].
Emulsion stability is also closely related to the double emulsion stirring time on the extraction process. In general, a longer contact time generally results in higher water transport into the internal phase causing the membrane to swell and emulsion breakage increases [181, 204]. Kulkarni and Mahajani reported the effect of contact time on the extraction of molybdenum and found that the mean diameter of emulsion droplets was 2.15 µm initially, while the diameter become 3.40 µm after 8 min of contact with the external phase [235]. Kulkarni et al. found the optimum contact time of 3 min during molybdenum extraction process along with 90% extraction efficiency and 10% emulsion swelling [214].
To summarize, it has been observed that the copper extraction reaction usually occurs in a short time; in order to ensure a higher copper extraction rate, an adequate membrane viscosity and higher interfacial area between the membrane phase and continuous phase should be maintained. These can be achieved by adjusting the operating parameters during the process. According to Chiha et al., a D2EHPA concentration of 20% (w/w), a H2SO4 concentration of 0.3 mol/L, and a double emulsion stirring speed of 200 rpm are optimum for high copper removal [146]. However, most experiments were performed using the one factor at a time (OFAT) method, which means that all other factors were fixed while an optimization value was achieved for one factor by changing its values at different levels. This method may not guarantee the conditions obtained are indeed optimal. In addition, interactions between factors such as chemical and physical interactions do exist and they have not been explored thoroughly. Recently, researchers have started to use the design of experiment (DOE) method to explore the interaction between the main factors and their effects on Cu(II) ion removal during copper extraction using the ELM process [154,155,156, 166, 203, 236,237,238]. The authors have been using fractional factorial design (FFD) to screen the important variables [236], and then using the response surface methodology (RSM) method to optimize the copper extraction process, and thus determine the main effects and interactions between experimental factors on copper recovery [236,237,238]. From FFD results, based on the normal probability plot and their contributions to the response, four main important factors were selected: extractant concentration; W/O/W emulsion stirring time; W/O/W emulsion stirring speed; and CuSO4 solution/emulsion volume ratio. From RSM results, they found that H2SO4 concentration was found to chemically interfere or interact with other factors such as extraction concentration and CuSO4 solution/emulsion volume ratio, and these chemical interactions could have significant effects on the response. Those interactions can be explained as: with an increase in internal H2SO4 concentration, a higher driving force for extraction is provided and the extractant regeneration is also increased; also, an increase in CuSO4 solution/emulsion volume ratio will lead to a higher internal water swelling rate, which could negatively affect the internal H2SO4 concentration [237, 238].
5.1.2 Kinetics During the Copper Permeation Process
Much research has also been focused on the kinetics study of the copper permeation process. A typical copper complexion with LIX extractant (RH) can be described as [37, 126, 239, 240]:
All kinetics studies fall within the following categories:
Thermodynamic Equilibrium Constant
The thermodynamic equilibrium constant K can be defined as:
It was reported by Raghuraman et al. that the K value is 328.0 and 75.48 when using tetradecane as the organic solvent with LIX 860 and LIX 984 as the copper extractant, separately [239]. They also noted that this K value could be used over a large range of pH and ionic strength. Frankenfeld et al. reported that K was not only a function of the specific liquid ion exchange agent but was also affected by the organic solvent properties [234].
Copper Mass Transfer Coefficient
Physical mass transfer parameters for copper within the membrane were investigated by Völkel et al., and they found that the copper mass transfer was affected by many factors such as the membrane viscosity, extractant concentration, copper concertation, as well as stripping solution concentration, and its dropsize distribution [241]. The mass transfer value ranged from 0.1 × 10−3 to 1.18 × 10−3 cm/s, where it increased with higher stirring speed since it caused an increased degree of dispersion [241]. Valenzuela et al. calculated the mass transfer coefficients of copper and LIX 860 in the membrane are 3.56 × 10−3 and 1.1 × 10−4 cm/s, separately [37]. Hu and Wiencek found that the copper reaction mass transfer rate was 2.23 × 10−6 cm/s [242]. In addition, they showed that ELM can extract copper even from a very low feed concentration (580 ppb) [242].
Gameiro et al. reported the effects of various parameters on copper mass transfer rate under an assumption of a copper-LIX 54 effective diffusivity of 2.53 × 10−10 cm2/s and a stripping rate constant of 1.2 × 10−7 m/s. They found that an increase in stripping liquor concentration, extractant concentration, and stirring speed coupled with a decrease in copper concentration and the volume ratio of external phase to internal phase would enhance the extraction rate [116]. In further research, they reported that the extraction mechanism was significantly affected by the LIX 54 concentration, such that at a value of 0.2 kmol/m3, the process was controlled by copper diffusion in the aqueous boundary layer, while in the range of 0.015–0.10 kmol/m3, it was controlled by chemical reaction and copper diffusion in the aqueous boundary layer [150].
In contrast, Teramoto et al. found that the copper permeation rate was negatively affected by the feed solution flow rate while it was positively affected by the copper concentration using SME529 as copper extractant [240]. In subsequent research investigating the influence of other process parameters, they found that higher H+ concentration in feed phase brought down the mass transfer rate, while stripping liquor concentration and stirring speed had a positive effect on the mass transfer rate. However, the extractant concentration did not show significant effects during this process, whereas the addition of a small amount of anionic surfactant such as sodium dodecyl sulfate to the feed phase was found to increase the copper mass transfer rate significantly [240].
Copper-extractant Complex Diffusion Coefficient
Chakraborty et al. extracted copper using D2EHPA as the extractant, and the diffusivity of copper-D2EHPA complex inside the membrane was 3.29 × 10−10 cm2/s [157]. Valenzuela et al. found that the diffusion coefficient of extractant and the forward reaction constant to form the copper-LIX 860 complex were 1.23 × 10−5 cm2/s and 5.2 × 1011 cm3/mol·s, respectively, and a model was thus proposed [243]:
where Cu0 and HX0 represent initial copper and extractant concentrations, while Q is a constant. Lazarova and Boyadzhiev calculated the copper diffusion coefficient between the organic and aqueous phase to be approximately 76.5 [244].
5.1.3 Industrial Applications
Although much research has been performed on copper removal in research labs, few reports have been focused on the industrial application of this technique. In general, the pilot-scale plant contained an emulsion-membrane generation circuit, extractant tanks, settlers, electrical coalesce, and electronwinning (EW) cell. The process is usually counter-current with copper solution and emulsion flowing in opposite directions. Wright et al. reported their results processing 5600 gallons of copper solution at a copper mine in Arizona utilizing ELM, with normal plant pregnant leach liquor (PLS) containing 1.43 g/L Cu; PLS diluted with mine water to 0.5 g/L Cu; and PLS diluted with mine water to 0.32 g/L Cu were treated, and copper extractions averaged 98.0%, 95.7%, and 91.6% for these three solutions, respectively (flow sheet as shown in Fig. 6) [151]. Also, 165 g/L H2SO4 was used as the stripping liquor while the membrane swelling and leakage were promising, averaging less than 8% and 0.1%, respectively.
Draxler et al. showed that by using a counter-current extraction column, two copper solutions were reduced from an initial concentration of 8 g/L, 0.8 g/L to a final concentration of 0.027 g/L and 0.003 g/L, respectively, while the throughput was 20 L/h and 40 L/h [142]. In other work, it was found that 30 g/L copper can be obtained in the receiving phase using 250 g/L H2SO4 while the initial copper concentration has been reduced from 8 g/L to 0.04 g/L at a throughout of 200 L/h (flow sheet as shown in Fig. 7) [136]. Li and Cahn utilized a continuous feed 4-stage cascade mixer treating aqueous flow that had a copper concentration of 2.5 g/L and a throughput of 17.4 L/h. They found over 95% copper recovery in four mixers in cascade to give a total residence time of 12.5 min while the copper concentration in the internal phase reached 30 g/L using 150 g/L H2SO4 as stripping liquor [153]. Their economic assessment showed that the liquid membrane process had a significantly lower operating cost than solvent extraction and a considerably reduced capital cost (35–40%).
5.2 Nickel
Nickel is often found to co-exist with cobalt ions in wastewater stream, with these two metals having similar chemical properties due to their adjacent position in the periodic table. Thus, a lot of work has been focused on the removal and separation of nickel from cobalt using the ELM technique. The membrane or the stripping liquid is used as a tool to selectively extract or strip metals. Various kinds of extractants have been used for this process, whereas for copper extraction, LIX products dominate. In this section, only nickel extraction by ELM will be discussed; a review of the separation of cobalt and nickel by ELM will be discussed in our future work. Table 7 details a number of nickel extractants and their physical properties [245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261].
A number of research publications focused on the discussion of operating process parameters and process kinetics as well as industrial applications. They are discussed in more detail below.
5.2.1 Nickel Process Operating Parameters
Since the ELM removal technique for copper and nickel ions follows a similar process, the effects of operating parameters on ELM stability and extraction for nickel removal are overall very similar with those for copper removal. However, since nickel co-exists with cobalt in most circumstances, for most applications, the extraction and separation of nickel and cobalt was a focus, and the discussion of nickel extraction process operating parameters can be categorized into the following sections.
Emulsion stability
Chakraborty et al. studied the emulsion stability during the nickel extraction process with an initial nickel concentration of 100–300 ppm, 100–200 ppm, and 75–100 ppm, respectively [157, 262, 263]. They investigated the impact of parameters including the feed solution pH, emulsion stirring speed, surfactant concentration, and internal aqueous phase concentration. They found that the emulsion was less stable at pH 1.5 than 3.5 because the rate of SPAN 80 hydrolysis was much faster at pH 1.5 [157]. In addition, with an increase of stirring speed from 400 to 750 rpm, the emulsion breakage also increased from around 10% to more than 40% [157]. Chakraborty et al. also noted that an intermediate value (4 wt%) of SPAN 80 was needed in order to stabilize the emulsion to solve the hydrolysis problem; while NaOH should not be used as the internal stripping phase for nickel extraction since over 40% emulsion breakage would occur. Additionally, it was noted that H2SO4 was better than HCl with a lower emulsion breakage. All this suggests that the emulsion stability was affected not only by the hydrogen ion concentration but also the associated anions [157].
Kulkarni et al. also worked on determining the effect of the type of stripping liquor on membrane stability, and they obtained a different result with an initial nickel concentration of 400 ppm [161]. They reported that sulfuric acid yielded the maximum membrane swelling due to the highest affinity toward water. With an increase in acid concentration, the difference of osmotic pressure between the internal and external phase also increased, resulting in a linearly increased emulsion swelling rate. They also found that dodecane had higher extraction and lower swelling rate than toluene, xylene, and heptane [161]. The difference came from its high viscosity as well as hydrophobicity, which could maintain the membrane stability by lowering water transport into the membrane. Although it was found that a higher emulsion/feed phase volume ratio would increase the degree of extraction, a trade-off was needed to maximize the enrichment ratio of nickel in the internal stripping phase, where they found a value between 1:5 to 1:7 was ideal [161].
ELM Extraction and Separation
Kumbasar et al. performed many different tests to extract nickel or separate nickel from cobalt in acid leach solutions, ammoniacal solutions, or electroplating bath solutions with an initial nickel concentration of 100–500 ppm, 100–500 ppm, 300–500 ppm, and 100–1000 ppm, respectively [158, 264,265,266]. They found that the extractant concentration was the most important parameter during the process, and that a synergistic extractant can significantly improve the extraction efficiency, such as a mixture of LIX 63 and 2BDA or LIX 63 and D2EHPA [158, 264]. Unlike in copper extraction, the feed solution pH is a key parameter to selectively extract the targeted nickel, and high nickel removal only occurred within a narrow pH range (4.0–5.75) [158]. They also performed process optimization using the OFAT method, and found that in one case a more than 40% increase in nickel extraction could be achieved when operating parameters were optimized [158]. Table 8 lists the optimum parameters when extracting nickel from simulated spent Cr/Ni electroplating bath solutions.
Under these optimum conditions, a nickel removal efficiency of more than 99% could be achieved. Although a high HCl concentration is needed to strip nickel from the complex, it should also be noted that when extracting nickel from ammoniacal solutions, a low concentration of EDTA acid (0.025 mol/L) was sufficient [265].
Li et al. also used ELM to separate nickel from cobalt while using EDTA as a stripping reagent since the Ni-EDTA complex is more stable than the Co-EDTA complex with an initial nickel concentration of 10–300 ppm [267]. They found that an addition of NaAc-HAc buffer solution could increase the solution ionic strength, thus making the metal separation easier. In Eyupoglu and Kumbasar’s work where the initial nickel concentration was both 100–500 ppm [264, 265], the separation factor of nickel and cobalt was calculated using Eq. 6:
where CNi and CCo are the concentrations of Ni and Co in the stripping and initial feed solutions. They found that a value of 580 was achieved under optimum conditions.
Kulkarni et al. reported that maximum nickel extraction could be achieved with an emulsion/feed solution volume ratio fixed at 1:3 while having a HNO3 concentration of 1.0 mol/L as the internal stripping liquor (initial nickel concentration from 400–600 ppm) [159]. This value was low when compared with Kumbasar et al. [266], the reason being the increased swelling behavior of internal droplets with a drastic increase of HNO3 concentration, where the mass transfer area was reduced and thus the nickel extraction was impacted [158]. This finding was confirmed by Hachemaoui et al., where they used 0.5 mol/L HCl as the internal stripping liquor treating 250 ppm nickel under the optimum condition, 8% D2EHPA was used while the SPAN 80 concentration was determined to be 2% [268]. The nickel and cobalt extraction efficiency can be up to 97.2% and 98.2% when the treatment ratio of emulsion to feed phase and the phase ratio of internal stripping phase and oil phase was 0.2 and 0.5, respectively [268]. However, due to the low concentration of HCl, there was not a good separation between nickel and cobalt (separation factor around 1.0).
5.2.2 Kinetics During Nickel Permeation Process
The reaction between Ni2+ and D2EHPA is as follows [239]:
Thermodynamic Equilibrium Constant
Raghuraman et al. performed research into the equilibrium constant during nickel extraction by D2EHPA, and they found that if the percentage of D2EHPA consumed during the reaction was less than 10%, a constant equilibrium constant K of 6.95 × 10−5 was obtained. With an increase in the percentage of D2EHPA reacted, the value of K increased [239]. This was attributed to the polymerization of the Ni-D2EHPA complex in the membrane phase. Kakoi et al. measured the equilibrium constant of nickel extraction under three different extractants; LIX 860 was found to have the highest equilibrium constant (9.0 × 10−6), followed by LIX 84 (1.2 × 10−6) and LIX 65 N (8.1 × 10−7) [269]. This was most likely due to the reaction rate of LIX 860 being higher than the other two extractants. They also found that the nickel extraction rate improved significantly by using an amphoteric surfactant rather than a nonionic surfactant [269]. Juang and Jiang reported that the K value was 1.40 × 10−4 while using n-heptane as the organic solvent and 6.4% (v/v) D2EHPA as the nickel extractant. They found that the volume ratio of emulsion phase over external phase ϕ1 and the volume ratio of internal stripping phase and oil phase ϕ2 were the most important factors impacting the nickel recovery [160]. This conclusion was contrary to those of others, where extractant concentration and pH are considered to be vital [157, 267].
Nickel Mass Transfer Coefficient
Yamashita et al. reported that the nickel permeation rate in ELM depended on the hydrogen activity in the external phase, the first order of extractant concentration (LIX 63 or DOLPA), and the second order of surfactant concentration. The overall rate constant was calculated to be 8.3 × 1010 (m3/mol·s) [270]. Mickler et al. found that a higher nickel diffusion rate was obtained using 4-acyl-5-pyrazolones than β-diketones. This could be due to the lower acidity of β-diketones [271]. They also found that the permeation of nickel was hindered by the increased ammonia concentration of the ammoniacal solutions.
Nickel-extractant Complex Diffusion Coefficient
In the work of Chakraborty et al., a mass transfer analysis of nickel extraction by ELM using D2EHPA as nickel extractant and HCl as stripping agent was performed [262]. They calculated the effective diffusivity of the Ni-D2EHPA complex in the emulsion to be 0.7832 × 10−10 m2/s, and they obtained the following equation [157, 267].
where CD represents the distribution coefficient of nickel between membrane and external phase while CS is the equilibrium solute concentration in the aqueous phase. Based on these findings, in their later work they found that injection method of emulsion, stirring speed, oil phase viscosity, composition of inner water phase, and solute permeation rate all affected the emulsion droplet size [263].
Properties Between Different Phases of ELM
Kulkarni and his co-workers performed a series of tests to obtain the nickel distribution ratio of the membrane phase over the aqueous phase (KD) [159]. Those tests were performed under different pH since the extraction of nickel is pH dependent. Table 9 shows their experimental results.
It can be seen that the KD increased with pH while the value for dodecane was always higher than xylene under the same pH. This could be understood through the fact that the acidity difference between the internal phase and external phase affected the nickel permeation rate.
Serga et al. investigated the influence of direct current on the extraction of nickel cations from acidic solutions. They added 5–20 vol% of tributyl phosphate (TBP) or 1–2 vol% n-trioctylamine (Oct3N) to 20 vol% D2EHPA to significantly improve the electrical conductivity of the ELM [272]. When the applied current density was ≤ 2.1 mA/cm2 and ≥ 4.9 mA/cm2 for the mixture of D2EHPA with TBP and Oct3N, respectively, they found nickel removal was higher than 99% for both cases. Additionally, complete extraction of nickel from a more acidic solution was achieved when direct current was applied. The flux of nickel cations increased with an increase in current density, nickel concentration, and surfactant concentration [272]. The flux of nickel into the membrane phase and internal phase were calculated and listed in Table 10.
5.2.3 Industrial Applications
Very few reports have focused on the industrial application of removing nickel using ELM since the application of nickel permeation to the hydrometallurgical industry is restricted because the selectivity of the nickel extractants to other metals is generally poor. However, in solutions where no disturbing impurities are present, such as in the electroplating industry, the recovery of nickel by ELM has gained importance. Draxler and his co-workers reported the separation of metals, including zinc, copper, nickel, cadmium, lead, and chromium, in a pilot plant using a counter-current extraction column (height 2.7 m, diameter 50 mm) [136, 142, 273]. The system parameters (throughput, phase ratio, stirring speed and residence time) were optimized, and in their trial run, they found that nickel concentration was reduced from 2200 mg/L to 360 mg/L at a throughput of 20 L/h, while zinc was reduced from 4500 mg/L to 4 mg/L [142]. They found the extraction equilibria of zinc and nickel are about the same, but the kinetics, especially the kinetics of the stripping reaction, is much lower for the nickel transport. Comparative studies in a counter-current column showed that under given conditions the residence time is not sufficient for a complete separation of nickel. In addition, the stripping reaction of nickel is extremely slow (4 h) in a shaker, so that no conventional extraction equipment is suitable for nickel extraction. Therefore, multistage mixer-settlers with sufficient residence times are necessary for a complete separation of nickel. Then they performed tests under the same conditions to extract nickel in a two-stage counter-current mixer-settler [136], finding that the nickel concentration was reduced to approximately 200 mg/L in the first stage and to 3 mg/L in the second stage. They found that the maximum concentration obtained in the receiving phase was 52 g/l. This value is less than their expected value and because the surfactant ECA 4360 they used represents a considerable mass transfer resistance and cannot be used alone, and with the addition of SPAN 80, although the mass transfer resistance decreases at a constant emulsion stability, this addition causes swelling of the emulsion by water transport from the outer to the inner aqueous phase and thus the concentrated inner phase is diluted. In order to solve the problem, a combination of extraction and permeation was used, as shown in Fig. 8. The final cleaning procedure takes place in the extraction stage, where no osmosis effects occur. In this way, a separation up to 3 mg/l is possible together with product-concentrations up to 80 g/l. This process was realized in a plant in a bicycle factory in Austria with a throughput of 150 L/h.
6 Conclusions
Emulsion liquid membrane is a very promising method for heavy metals recovery from industrial waste water. The most widely used surfactant, stripping agent, and diluent for the removal of copper and nickel are span 80, sulfuric acid, and kerosene. The emulsion developed in the ELM system is normally formulated to resist emulsion instability to ensure peak performance for extraction where swelling and leakage were found to be the main problem in practical use, which is to say, the composition and properties of each component in the emulsion can significantly affect the emulsion performance, in term of emulsion breakdown resistance and extraction process. Also, many studies have focused on the effect of surfactant & carrier type and concentration, phase ratio & stirring time and speed, as well as stripping solution composition on the emulsion stability. A successful ELM application not only depends on the selection of suitable emulsification but also on emulsion formulation, which is related to the emulsion stability that still remains a great challenge in the application of the ELM at industrial scale.
The emerging future research challenges of this technique such as scale-up and process intensification remain the greatest obstacles for its commercial/industrial scale applications. The current upcoming technology is applying ultrasound rather than mechanical agitation to produce nano-emulsion since a small droplet diameter of emulsion is a key criterion that will provide a stable emulsion and a larger mass transfer area. In the meanwhile, the incoming few decades should see the applications of green solvents as diluent in various separation processes to solve the major environment related problems. Also, improving the economic potential of ELM has always been the interest; thus, the need for continuous optimization study is important to make the overall emulsification process commercially viable.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
References
Hawkes SJ (1997) What is a “heavy metal”? J Chem Educ 74(11):1374
Srivastava NK, Majumder CB (2008) Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J Hazard Mater 151(1):1–8
Bradl HB (2005) Heavy Metals in the Environment: [Origin, Interaction and Remediation]. Elsevier Academic Press, Amsterdam, Netherland
Borba CE, Guirardello R, Silva EA, Veit MT, Tavares CRG (2006) Removal of nickel(II) ions from aqueous solution by biosorption in a fixed bed column: Experimental and theoretical breakthrough curves. Biochem Eng J 30(2):184–191
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92(3):407–418
Paulino AT, Minasse FAS, Guilherme MR, Reis AV, Muniz EC, Nozaki J (2006) Novel adsorbent based on silkworm chrysalides for removal of heavy metals from wastewaters. J Colloid Interface Sci 301(2):479–487
Namasivayam C, Kadirvelu K (1999) Uptake of mercury (II) from wastewater by activated carbon from unwanted agricultural solid by-product: coirpith. Carbon 37(1):79–84
Naseem R, Tahir SS (2001) Removal of Pb(II) from aqueous solution by using bentonite as an adsorbent. Water Res 35(16):3982–3986
Khezami L, Capart R (2005) Removal of chromium(VI) from aqueous solution by activated carbons: kinetic and equilibrium studies. J Hazard Mater 123(1–3):223–231
Simate GS, Ndlovu S (2014) Acid mine drainage: Challenges and opportunities. J Environ Chem Eng 2(3):1785–1803
Demopoulos GP (1998) Aqueous processing and its role in the production of inorganic materials and environmental protection. Can Metall Q 37(1):1–18
Kuyucak N (2002) Acid mine drainage prevention and control options. CIM Bull 95(1060):96–102
Tarkan HM, Finch JA (2005) Air-assisted solvent extraction: towards a novel extraction process. Miner Eng 18(1):83–88
Patterson JW (1985) Industrial Wastewater Treatment Technology, 2nd edn. Butterworth, Stoneham, MA, USA
Harris DL, Lottermoser BG, Duchesne J (2003) Ephemeral acid mine drainage at the Montalbion silver mine, north Queensland. Aust J Earth Sci 50(5):797–809
Matlock MM, Howerton BS, Atwood DA (2002) Chemical precipitation of heavy metals from acid mine drainage. Water Res 36(19):4757–4764
Gray NF (1998) Acid mine drainage composition and the implications for its impact on lotic systems. Water Res 32(7):2122–2134
Lin C, Wu Y, Lu W, Chen A, Liu Y (2007) Water chemistry and ecotoxicity of an acid mine drainage-affected stream in subtropical China during a major flood event. J Hazard Mater 142(1–2):199–207
Feng D, Aldrich C, Tan H (2000) Treatment of acid mine water by use of heavy metal precipitation and ion exchange. Miner Eng 13(6):623–642
Murray J, Nordstrom DK, Dold B, Kirschbaum A (2021) Seasonal fluctuations and geochemical modeling of acid mine drainage in the semi-arid Puna region: The Pan de Azúcar Pb–Ag–Zn mine, Argentina. J S Am Earth Sci 109:103197
Acheampong MA, Meulepas RJW, Lens PNL (2010) Removal of heavy metals and cyanide from gold mine wastewater. J Chem Technol Biotechnol 85(5):590–613
Agrawal A, Manoj MK, Kumari S, Bagchi D, Kumar V, Pandey BD (2008) Extractive separation of copper and nickel from copper bleed stream by solvent extraction route. Miner Eng 21(15):1126–1130
Bari MF, Begum MN, Jamaludin SB, Hussin K (2009) Solvent extraction separation and recovery of copper, nickel and zinc from printed circuit board by Cyanex 272. Miner Process Extr Metall 54(4):227–234
Cheng CY, Barnard KR, Zhang W, Zhu Z, Pranolo Y (2016) Recovery of nickel, cobalt, copper and zinc in sulphate and chloride solutions using synergistic solvent extraction. Chin J Chem Eng 24(2):237–248
Dupont D, Raiguel S, Binnemans K (2015) Sulfonic acid functionalized ionic liquids for dissolution of metal oxides and solvent extraction of metal ions. Chem Commun 51(43):9006–9009
Khopkar SM (2009) Solvent Extraction: Separation of Elements with Liquid Ion Exchangers. New Age Science Limited, Tunbridge Wells, UK
Alvarez MT, Crespo C, Mattiasson B (2007) Precipitation of Zn(II), Cu(II) and Pb(II) at bench-scale using biogenic hydrogen sulfide from the utilization of volatile fatty acids. Chemosphere 66(9):1677–1683
Chen Q, Luo Z, Hills C, Xue G, Tyrer M (2009) Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res 43(10):2605–2614
Fu F, Zeng H, Cai Q, Qiu R, Yu J, Xiong Y (2007) Effective removal of coordinated copper from wastewater using a new dithiocarbamate-type supramolecular heavy metal precipitant. Chemosphere 69(11):1783–1789
Ye C, Yang H, Lin J, Zeng H, Yu F (2011) Study on ion exchange property of removing Mn2+ and Fe2+ in groundwater by modified zeolite. Desalin Water Treat 30(1–3):114–121
Argun ME (2008) Use of clinoptilolite for the removal of nickel ions from water: Kinetics and thermodynamics. J Hazard Mater 150(3):587–595
Inglezakis VJ, Stylianou MA, Gkantzou D, Loizidou MD (2007) Removal of Pb(II) from aqueous solutions by using clinoptilolite and bentonite as adsorbents. Desalination 210(1–3):248–256
Chang IS, Lee CH (1998) Membrane filtration characteristics in membrane-coupled activated sludge system—the effect of physiological states of activated sludge on membrane fouling. Desalination 120(3):221–233
Juang RS, Shiau RC (2000) Metal removal from aqueous solutions using chitosan-enhanced membrane filtration. J Membr Sci 165(2):159–167
Valenzuela F, Fonseca C, Basualto C, Correa O, Tapia C, Sapag J (2005) Removal of copper ions from a waste mine water by a liquid emulsion membrane method. Miner Eng 18(1):33–40
Valenzuela F, Cabrera J, Basualto C, Sapag J, Romero J, Sánchez J, Rios G (2007) Separation of zinc ions from an acidic mine drainage using a stirred transfer cell–type emulsion liquid membrane contactor. Sep Sci Technol 42(2):363–377
Valenzuela F, Araneda C, Vargas F, Basualto C, Sapag J (2009) Liquid membrane emulsion process for recovering the copper content of a mine drainage. Chem Eng Res Des 87(1):102–108
San Roman MF, Bringas E, Ibanez R, Ortiz I (2010) Liquid membrane technology: fundamentals and review of its applications. J Chem Technol Biotechnol 85(1):2–10
Amuda O, Amoo I, Ipinmoroti K, Ajayi O (2006) Coagulation/flocculation process in the removal of trace metals present in industrial wastewater. J Appl Sci Environ Manag 10(3):159–162
Johnson PD, Girinathannair P, Ohlinger KN, Ritchie S, Teuber L, Kirby J (2008) Enhanced removal of heavy metals in primary treatment using coagulation and flocculation. Water Environ Res 80(5):472–479
Matis KA, Zouboulis AI, Lazaridis NK (2003) Heavy metals removal by biosorption and flotation. Water Air Soil Pollut Focus 3(3):143–151
Khelifa A, Moulay S, Naceur AW (2005) Treatment of metal finishing effluents by the electroflotation technique. Desalination 181(1–3):27–33
Merzouk B, Gourich B, Sekki A, Madani K, Chibane M (2009) Removal turbidity and separation of heavy metals using electrocoagulation–electroflotation technique: A case study. J Hazard Mater 164(1):215–222
Shafaei A, Rezayee M, Arami M, Nikazar M (2010) Removal of Mn2+ ions from synthetic wastewater by electrocoagulation process. Desalination 260(1–3):23–28
Huisman JL, Schouten G, Schultz C (2006) Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 83(1–4):106–113
Baltpurvins KA, Burns RC, Lawrance GA, Stuart AD (1997) Effect of electrolyte composition on zinc hydroxide precipitation by lime. Water Res 31(5):973–980
Kongsricharoern N, Polprasert C (1995) Electrochemical precipitation of chromium (Cr6+) from an electroplating wastewater. Water Sci Technol 31(9):109–117
Özverdi A, Erdem M (2006) Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J Hazard Mater 137(1):626–632
Gonzalez-Munoz MJ, Rodriguez MA, Luque S, Alvarez JR (2006) Recovery of heavy metals from metal industry waste waters by chemical precipitation and nanofiltration. Desalination 200(1–3):742–744
Papadopoulos A, Fatta D, Parperis K, Mentzis A, Haralambous KJ, Loizidou M (2004) Nickel uptake from a wastewater stream produced in a metal finishing industry by combination of ion-exchange and precipitation methods. Sep Purif Technol 39(3):181–188
Matlock MM, Henke KR, Atwood DA (2002) Effectiveness of commercial reagents for heavy metal removal from water with new insights for future chelate designs. J Hazard Mater 92(2):129–142
Chang YK, Chang JE, Lin TT, Hsu YM (2002) Integrated copper-containing wastewater treatment using xanthate process. J Hazard Mater 94(1):89–99
Ying X, Fang Z (2006) Experimental research on heavy metal wastewater treatment with dipropyl dithiophosphate. J Hazard Mater 137(3):1636–1642
Kang SY, Lee JU, Moon SH, Kim KW (2004) Competitive adsorption characteristics of Co2+, Ni2+, and Cr3+ by IRN-77 cation exchange resin in synthesized wastewater. Chemosphere 56(2):141–147
Motsi T, Rowson NA, Simmons MJH (2009) Adsorption of heavy metals from acid mine drainage by natural zeolite. Int J Miner Process 92(1–2):42–48
Abo-Farha SA, Abdel-Aal AY, Ashour IA, Garamon SE (2009) Removal of some heavy metal cations by synthetic resin purolite C100. J Hazard Mater 169(1–3):190–194
Doula MK, Dimirkou A (2008) Use of an iron-overexchanged clinoptilolite for the removal of Cu2+ ions from heavily contaminated drinking water samples. J Hazard Mater 151(2–3):738–745
Yanagisawa H, Matsumoto Y, Machida M (2010) Adsorption of Zn (II) and Cd (II) ions onto magnesium and activated carbon composite in aqueous solution. Appl Surf Sci 256(6):1619–1623
Jusoh A, Shiung LS, Noor MJMM (2007) A simulation study of the removal efficiency of granular activated carbon on cadmium and lead. Desalination 206(1–3):9–16
Guo M, Qiu G, Song W (2010) Poultry litter-based activated carbon for removing heavy metal ions in water. Waste Manage 30(2):308–315
Wang H, Zhou A, Peng F, Yu H, Yang J (2007) Mechanism study on adsorption of acidified multiwalled carbon nanotubes to Pb (II). J Colloid Interface Sci 316(2):277–283
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354(6348):56
Odom TW, Huang JL, Kim P, Lieber CM (1998) Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 391(6662):62
Pillay K, Cukrowska EM, Coville NJ (2009) Multi-walled carbon nanotubes as adsorbents for the removal of parts per billion levels of hexavalent chromium from aqueous solution. J Hazard Mater 166(2–3):1067–1075
Rao GP, Lu C, Su F (2007) Sorption of divalent metal ions from aqueous solution by carbon nanotubes: a review. Sep Purif Technol 58(1):224–231
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Coll Interface Sci 140(2):114–131
Agouborde L, Navia R (2009) Heavy metals retention capacity of a non-conventional sorbent developed from a mixture of industrial and agricultural wastes. J Hazard Mater 167(1–3):536–544
Jiang MQ, Jin XY, Lu XQ, Chen ZL (2010) Adsorption of Pb (II), Cd (II), Ni (II) and Cu (II) onto natural kaolinite clay. Desalination 252(1–3):33–39
Gu X, Evans LJ (2008) Surface complexation modelling of Cd (II), Cu (II), Ni (II), Pb (II) and Zn (II) adsorption onto kaolinite. Geochim Cosmochim Acta 72(2):267–276
Mohan D, Chander S (2006) Removal and recovery of metal ions from acid mine drainage using lignite—a low cost sorbent. J Hazard Mater 137(3):1545–1553
Al-Jlil SA, Alsewailem FD (2009) Saudi Arabian clays for lead removal in wastewater. Appl Clay Sci 42(3–4):671–674
Apiratikul R, Pavasant P (2008) Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Biores Technol 99(8):2766–2777
Aman T, Kazi AA, Sabri MU, Bano Q (2008) Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surf, B 63(1):116–121
El-Sikaily A, El Nemr A, Khaled A, Abdelwehab O (2007) Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated carbon. J Hazard Mater 148(1–2):216–228
Landaburu-Aguirre J, García V, Pongrácz E, Keiski RL (2009) The removal of zinc from synthetic wastewaters by micellar-enhanced ultrafiltration: statistical design of experiments. Desalination 240(1–3):262–269
Samper E, Rodríguez M, De la Rubia MA, Prats D (2009) Removal of metal ions at low concentration by micellar-enhanced ultrafiltration (MEUF) using sodium dodecyl sulfate (SDS) and linear alkylbenzene sulfonate (LAS). Sep Purif Technol 65(3):337–342
Shahalam AM, Al-Harthy A, Al-Zawhry A (2002) Feed water pretreatment in RO systems: unit processes in the Middle East. Desalination 150(3):235–245
Mohsen-Nia M, Montazeri P, Modarress H (2007) Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 217(1–3):276–281
Cséfalvay E, Pauer V, Mizsey P (2009) Recovery of copper from process waters by nanofiltration and reverse osmosis. Desalination 240(1–3):132–142
Nguyen CM, Bang S, Cho J, Kim KW (2009) Performance and mechanism of arsenic removal from water by a nanofiltration membrane. Desalination 245(1–3):82–94
Figoli A, Cassano A, Criscuoli A, Mozumder MSI, Uddin MT, Islam MA, Drioli E (2010) Influence of operating parameters on the arsenic removal by nanofiltration. Water Res 44(1):97–104
Murthy ZVP, Chaudhari LB (2009) Separation of binary heavy metals from aqueous solutions by nanofiltration and characterization of the membrane using Spiegler-Kedem model. Chem Eng J 150(1):181–187
Sadrzadeh M, Mohammadi T, Ivakpour J, Kasiri N (2009) Neural network modeling of Pb2+ removal from wastewater using electrodialysis. Chem Eng Process 48(8):1371–1381
Cifuentes L, García I, Arriagada P, Casas JM (2009) The use of electrodialysis for metal separation and water recovery from CuSO4–H2SO4–Fe solutions. Sep Purif Technol 68(1):105–108
Saf AÖ, Alpaydin S, Sirit A (2006) Transport kinetics of chromium (VI) ions through a bulk liquid membrane containing p-tert-butyl calix [4] arene 3-morpholino propyl diamide derivative. J Membr Sci 283(1–2):448–455
Behr JP, Kirch M, Lehn JM (1985) Carrier-mediated transport through bulk liquid membranes: dependence of transport rates and selectivity on carrier properties in a diffusion-limited process. J Am Chem Soc 107(1):241–246
Kocherginsky NM, Yang Q, Seelam L (2007) Recent advances in supported liquid membrane technology. Sep Purif Technol 53(2):171–177
Balchen M, Gjelstad A, Rasmussen KE, Pedersen-Bjergaard S (2007) Electrokinetic migration of acidic drugs across a supported liquid membrane. J Chromatogr A 1152(1–2):220–225
Canet L, Ilpide M, Seta P (2002) Efficient facilitated transport of lead, cadmium, zinc, and silver across a flat-sheet-supported liquid membrane mediated by lasalocid A. Sep Sci Technol 37(8):1851–1860
Parthasarathy N, Pelletier M, Buffle J (1997) Hollow fiber based supported liquid membrane: a novel analytical system for trace metal analysis. Anal Chim Acta 350(1–2):183–195
Chaudry MA, Ahmad S, Malik MT (1998) Supported liquid membrane technique applicability for removal of chromium from tannery wastes. Waste Manage 17(4):211–218
El Samrani AG, Lartiges BS, Villiéras F (2008) Chemical coagulation of combined sewer overflow: Heavy metal removal and treatment optimization. Water Res 42(4–5):951–960
Chang Q, Wang G (2007) Study on the macromolecular coagulant PEX which traps heavy metals. Chem Eng Sci 62(17):4636–4643
Hankins NP, Lu N, Hilal N (2006) Enhanced removal of heavy metal ions bound to humic acid by polyelectrolyte flocculation. Sep Purif Technol 51(1):48–56
Bojic AL, Bojic D, Andjelkovic T (2009) Removal of Cu2+ and Zn2+ from model wastewaters by spontaneous reduction–coagulation process in flow conditions. J Hazard Mater 168(2–3):813–819
Waters A (1990) Dissolved air flotation used as primary separation for heavy metal removal. Filtr Sep 27(2):70–73
Polat H, Erdogan D (2007) Heavy metal removal from waste waters by ion flotation. J Hazard Mater 148(1–2):267–273
Capponi F, Sartori M, Souza ML, Rubio J (2006) Modified column flotation of adsorbing iron hydroxide colloidal precipitates. Int J Miner Process 79(3):167–173
Heidmann I, Calmano W (2008) Removal of Zn (II), Cu (II), Ni (II), Ag (I) and Cr (VI) present in aqueous solutions by aluminium electrocoagulation. J Hazard Mater 152(3):934–941
Nataraj SK, Hosamani KM, Aminabhavi TM (2007) Potential application of an electrodialysis pilot plant containing ion-exchange membranes in chromium removal. Desalination 217(1–3):181–190
Ölmez T (2009) The optimization of Cr (VI) reduction and removal by electrocoagulation using response surface methodology. J Hazard Mater 162(2–3):1371–1378
Kislik VS (ed) (2010) Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater Treatment. Elsevier, Amsterdam, Netherlands
van der Graaf S, Schroën CGPH, Boom RM (2005) Preparation of double emulsions by membrane emulsification-a review. J Membr Sci 251(1):7–15
Kumbasar RA (2008) Transport of cadmium ions from zinc plant leach solutions through emulsion liquid membrane-containing Aliquat 336 as carrier. Sep Purif Technol 63(3):592–599
Kumbasar RA (2009) Selective extraction of nickel from ammoniacal solutions containing nickel and cobalt by emulsion liquid membrane using 5, 7-dibromo-8-hydroxyquinoline (DBHQ) as extractant. Miner Eng 22(6):530–536
Kumbasar RA (2010) Extraction and concentration of cobalt from acidic leach solutions containing Co–Ni by emulsion liquid membrane using TOA as extractant. J Ind Eng Chem 16(3):448–454
Wiencek J (1994) Emulsion liquid membranes for wastewater treatment: Equilibrium models for some typical metal-extractant systems. Environ Sci Technol 28(6):1090–1098
Zhang YL, Liu PH, Zhang QY, Cheng W (2010) Separation of cadmium(II) from spent nickel/cadmium battery by emulsion liquid membrane. Can J Chem Eng 88(1):95–100
Tadros TF (2009) Emulsion Science and Technology: A General Introduction. Wiley-VCH, Weinheim
Schuster D. (Ed.). (1996) Encyclopedia of Emulsion Technology (Vol. 4). CRC Press, Boca Raton, USA
Sjoblom J. (Ed.). (2005). Emulsions and Emulsion Stability: Surfactant Science Series/61 (Vol. 132). CRC Press, Boca Raton, USA
Ahmad AL, Kusumastuti A, Derek CJC, Ooi BS (2011) Emulsion liquid membrane for heavy metal removal: An overview on emulsion stabilization and destabilization. Chem Eng J 171(3):870–882
Kargari A, Kaghazchi T, Soleimani M (2004) Role of emulsifier in the extraction of gold (III) ions from aqueous solutions using the emulsion liquid membrane technique. Desalination 162:237–247
Canselier JP, Delmas H, Wilhelm AM, Abismail B (2002) Ultrasound emulsification—an overview. J Dispersion Sci Technol 23(1–3):333–349
Abismaıl B, Canselier JP, Wilhelm AM, Delmas H, Gourdon C (1999) Emulsification by ultrasound: drop size distribution and stability. Ultrason Sonochem 6(1–2):75–83
Gameiro MLF, Bento P, Ismael MRC, Reis MTA, Carvalho JM (2007) Extraction of copper from ammoniacal medium by emulsion liquid membranes using LIX 54. J Membr Sci 293(1–2):151–160
Reis MTA, Carvalho JM (2004) Modelling of zinc extraction from sulphate solutions with bis (2-ethylhexyl) thiophosphoric acid by emulsion liquid membranes. J Membr Sci 237(1–2):97–107
Venkatesan S, Begum KMS (2009) Emulsion liquid membrane pertraction of benzimidazole using a room temperature ionic liquid (RTIL) carrier. Chem Eng J 148(2–3):254–262
Venkatesan S, Begum KMS (2009) Emulsion liquid membrane pertraction of imidazole from dilute aqueous solutions by Aliquat-336 mobile carrier. Desalination 236(1–3):65–77
Garti N (1997) Double emulsions—scope, limitations and new achievements. Colloids Surf, A 123(124):233–246
Way JD, Noble RD, Flynn TM, Sloan ED (1982) Liquid membrane transport: a survey. J Membr Sci 12(2):239–259
Cahn RP, Li NN (1974) Separation of phenol from waste water by the liquid membrane technique. Sep Sci 9(6):505–519
Terry RE, Li NN, Ho WS (1982) Extraction of phenolic compounds and organic acids by liquid membranes. J Membr Sci 10(2–3):305–323
Bunge AL, Noble RD (1984) A diffusion model for reversible comsumption in emulsion liquid membranes. J Membr Sci 21(1):55–71
Boyadzhiev L, Bezenshek E, Lazarova Z (1984) Removal of phenol from waste water by double emulsion membranes and creeping film pertraction. J Membr Sci 21(2):137–144
Teramoto M, Takihana H, Shibutani M, Yuasa T, Hara N (1983) Extraction of phenol and cresol by liquid surfactant membrane. Sep Sci Technol 18(5):397–419
Chan CC, Lee CJ (1987) A mass transfer model for the extraction of weak acids/based in emulsion liquid-membrane systems. Chem Eng Sci 42(1):83–95
Fang J, Li M, Xu Z (2003) Separation of cobalt from a nickel-hydrometallurgical effluent using an emulsion liquid membrane. Sep Sci Technol 38(14):3553–3574
Hachemaoui A, Belhamel K (2017) Simultaneous extraction and separation of cobalt and nickel from chloride solution through emulsion liquid membrane using Cyanex 301 as extractant. Int J Miner Process 161(34):7–12
Hasan MA, Atwa ST, El-Taweel AA (2015) Liquid emulsion membrane stability studies for removal of nickel from liquid aqueous waste. Asian J Chem 27(7):2712–2718
Kulkarni PS, Tiwari KK, Mahajani VV (2002) Membrane-liquid emulsion membrane process using a liquid emulsion membrane process using methane sulfonic acid as a strippant. Can J Chem Eng 80(3):402–409
Wan YH, De Wang X, Zhang XJ (1997) Treatment of high concentration phenolic waste water by liquid membrane with N503 as mobile carrier. J Membr Sci 135(2):263–270
Sastre A, Madi A, Cortina JL, Miralles N (1998) Modelling of mass transfer in facilitated supported liquid membrane transport of gold (III) using phospholene derivatives as carriers. J Membr Sci 139(1):57–65
Mokhtari B, Pourabdollah K (2015) Emulsion liquid membrane for selective extraction of Bi(III). Chin J Chem Eng 23(4):641–645
Kovaleva LA, Minnigalimov RZ, Zinnatullin RR (2011) Destruction of water-in-oil emulsions in radio-frequency and microwave electromagnetic fields. Energy Fuels 25(8):3731–3738
Draxler J, Marr R (1986) Emulsion liquid membranes part I: Phenomenon and industrial application. Chem Eng Process 20(6):319–329
Berkhof R, Kwekkeboom H, Balzer D, Ripke N (1992) U.S. Patent No. 5,164,116. Washington, DC: U.S. Patent and Trademark Office
Abou-Nemeh I, Moors M, Van Peteghem AP (1992) Electrostatic splitting of the emulsion used in liquid surfactant membranes process for metals separation. Sep Sci Technol 27(10):1319–1335
Devulapalli R, Jones F (1999) Separation of aniline from aqueous solutions using emulsion liquid membranes. J Hazard Mater 70(3):157–170
Chan CC, Chen YC (2002) Demulsification of W/O emulsions by microwave radiation. Sep Sci Technol 37(15):3407–3420
Sun D, Duan X, Li W, Zhou D (1998) Demulsification of water-in-oil emulsion by using porous glass membrane. J Membr Sci 146(1):65–72
Draxler J, Fürst W, Marr R (1988) Separation of metal species by emulsion liquid membranes. J Membr Sci 38(3):281–293
Guzmán-Lucero D, Flores P, Rojo T, Martínez-Palou R (2010) Ionic liquids as demulsifiers of water-in-crude oil emulsions: study of the microwave effect. Energy Fuels 24(6):3610–3615
Mortaheb HR, Kosuge H, Mokhtarani B, Amini MH, Banihashemi HR (2009) Study on removal of cadmium from wastewater by emulsion liquid membrane. J Hazard Mater 165(1–3):630–636
Shah Buddin MMH, Ahmad AL, Abd Khalil AT, Puasa SW (2020) A review of demulsification technique and mechanism for emulsion liquid membrane applications. J Dispers Sci Technol 1–18
Chiha M, Hamdaoui O, Ahmedchekkat F, Pétrier C (2010) Study on ultrasonically assisted emulsification and recovery of copper (II) from wastewater using an emulsion liquid membrane process. Ultrason Sonochem 17(2):318–325
Sengupta B, Sengupta R, Subrahmanyam N (2006) Copper extraction into emulsion liquid membranes using LIX 984N-C®. Hydrometallurgy 81(1):67–73
Sengupta B, Bhakhar MS, Sengupta R (2007) Extraction of copper from ammoniacal solutions into emulsion liquid membranes using LIX 84 I®. Hydrometallurgy 89(3–4):311–318
Lu D, Chang Y, Wang W, Xie F, Asselin E, Dreisinger D (2015) Copper and cyanide extraction with emulsion liquid membrane with LIX 7950 as the mobile carrier: part 1, Emulsion stability. Metals 5(4):2034–2047
Gameiro MLF, Ismael MRC, Reis MTA, Santos SM, Carvalho JM (2010) Extraction of copper from aqueous solutions by liquid membrane processes. Solvent Extr Ion Exch 28(1):85–108
Wright JB, Nilsen DN, Hundley G, Galvan GJ (1995) Field test of liquid emulsion membrane technique for copper recovery from mine solutions. Miner Eng 8(4–5):549–556
Strzelbicki J, Charewicz W (1978) Separation of copper by liquid surfactant membranes. J Inorg Nucl Chem 40(7):1415–1421
Li NN, Cahn RP, Naden D, Lai RWM (1983) Liquid membrane processes for copper extraction. Hydrometallurgy 9(3):277–305
Zereshki S, Shokri A, Karimi A (2021) Application of a green emulsion liquid membrane for removing copper from contaminated aqueous solution: Extraction, stability, and breakage study using response surface methodology. J Mol Liq 325:115251
Shamkhi HA, Albdiri AD, Jabir FA, Petruzzelli D (2021) Removal of Pb2+, Cu2+, and Cd2+ ions from a saline wastewater using emulsion liquid membrane: applying response surface methodology for optimization and data analysis. Arab J Sci Eng 1–15
Murugan P, Bhuvaneshwari S, Vidhyeswari D (2021) The extraction and process optimization of Cu (II) and Cd (II) using pickering emulsion liquid membrane. Water Sci Technol 83(8):1863–1877
Chakraborty M, Bhattacharya C, Datta S (2003) Mathematical modeling of simultaneous copper (II) and nickel (II) extraction from wastewater by emulsion liquid membranes. Sep Sci Technol 38(9):2081–2106
Eyupoglu V, Kumbasar RA (2015) Extraction of Ni (II) from spent Cr–Ni electroplating bath solutions using LIX 63 and 2BDA as carriers by emulsion liquid membrane technique. J Ind Eng Chem 21:303–310
Kulkarni PS, Tiwari KK, Mahajani VV (1999) Study in extraction of nickel by emulsion liquid membrane process. Indian J Chem Technol 6:329–335
Juang RS, Jiang JD (1995) Recovery of nickel from a simulated electroplating rinse solution by solvent extraction and liquid surfactant membrane. J Membr Sci 100(2):163–170
Kulkarni PS, Tiwari KK, Mahajani VV (2001) Recovery of nickel via liquid emulsion membrane process using methane sulfonic acid as a strippant. Sep Sci Technol 36(4):639–656
Yuliusman, Silvia, Nurqomariah A, Fajaryanto R (2019, March). Extraction of Co and Ni metals using emulsion liquid membrane and liquid-liquid extraction with Cyanex 272 as extractant. In AIP Conference Proceedings (Vol. 2085, No. 1, p. 020071). AIP Publishing LLC
Sujatha S, Rajamohan N, Anbazhagan S, Vanithasri M, Rajasimman M (2021) Extraction of nickel using a green emulsion liquid membrane–Process intensification, parameter optimization and artificial neural network modeling. Chem Eng Process Process Intensif 165:108444
Nafish AR, Salsabila CS, Ayu MP, Isa M (2020) Ni metal extraction with surfactant variation on double surfactant emulsion liquid membrane technology. In IOP Conference Series: Materials Science and Engineering 722(1):012076. IOP Publishing, Bristol
Rajamohana N, Rajasimmanb M, Al Qasmia F (2019) Parametric studies on the removal of nickel using emulsion liquid membrane. Desalin Water Treat 141:89–94
Reis MTA, Carvalho JMR (1994) Recovery of heavy metals by a combination of two processes: cementation and liquid membrane permeation. Miner Eng 7(10):1301–1311
Goto M, Yamamoto H, Kondo K, Nakashio F (1991) Effect of new surfactants on zinc extraction with liquid surfactant membranes. J Membr Sci 57(2–3):161–174
Lorbach D, Marr R (1987) Emulsion liquid membranes Part II: Modelling mass transfer of zinc with bis (2-ethylhexyl) dithiophosphoric acid. Chem Eng Process 21(2):83–93
Fouad EA, Bart HJ (2008) Emulsion liquid membrane extraction of zinc by a hollow-fiber contactor. J Membr Sci 307(2):156–168
Tahmasebizadeh P, Javanshir S, Ahmadi A (2021) Zinc extraction from a bioleaching solution by emulsion liquid membrane technique. Sep Purif Technol 276:119394
Ghorbanpour P, Jahanshahi M (2021) Removal of zinc by emulsion liquid membrane using lecithin as biosurfactant. J Dispers Sci Technol 1–9
Rajasimman M, Rajamohan N, Sujatha S (2021) Recovery of zinc from electroplating wastewater using green emulsion liquid membrane. Water Supply 21(5):2008–2018
Gasser MS, El-Hefny NE, Daoud JA (2008) Extraction of Co (II) from aqueous solution using emulsion liquid membrane. J Hazard Mater 151(2–3):610–615
Strzelbicki J, Charewicz W (1978) Separation of cobalt by liquid surfactant membranes. Sep Sci Technol 13(2):141–152
Parbat SA, Bhanvase BA, Sonawane SH (2020) Investigation on liquid emulsion membrane (LEM) prepared with hydrodynamic cavitation process for cobalt (II) extraction from wastewater. Sep Purif Technol 237:116385
Bhowal A, Datta S (2001) Studies on transport mechanism of Cr (VI) extraction from an acidic solution using liquid surfactant membranes. J Membr Sci 188(1):1–8
Goyal RK, Jayakumar NS, Hashim MA (2011) Chromium removal by emulsion liquid membrane using [BMIM] +[NTf2] − as stabilizer and TOMAC as extractant. Desalination 278(1):50–56
Nosrati S, Jayakumar NS, Hashim MA (2011) Extraction performance of chromium (VI) with emulsion liquid membrane by Cyanex 923 as carrier using response surface methodology. Desalination 266(1–3):286–290
Salazar E, Ortiz MI, Urtiaga AM, Irabien JA (1992) Kinetics of the separation-concentration of chromium (VI) with emulsion liquid membranes. Ind Eng Chem Res 31(6):1523–1529
Hasan MA, Selim YT, Mohamed KM (2009) Removal of chromium from aqueous waste solution using liquid emulsion membrane. J Hazard Mater 168(2–3):1537–1541
Chiha M, Samar MH, Hamdaoui O (2006) Extraction of chromium (VI) from sulphuric acid aqueous solutions by a liquid surfactant membrane (LSM). Desalination 194(1–3):69–80
Rajasimman M, Sangeetha R, Karthik P (2009) Statistical optimization of process parameters for the extraction of chromium (VI) from pharmaceutical wastewater by emulsion liquid membrane. Chem Eng J 150(2–3):275–279
Rajasimman M, Karthic P (2010) Application of response surface methodology for the extraction of chromium (VI) by emulsion liquid membrane. J Taiwan Inst Chem Eng 41(1):105–110
Kumbasar RA (2010) Selective extraction of chromium (VI) from multicomponent acidic solutions by emulsion liquid membranes using tributhylphosphate as carrier. J Hazard Mater 178(1–3):875–882
Kumbasar RA (2008) Selective separation of chromium (VI) from acidic solutions containing various metal ions through emulsion liquid membrane using trioctylamine as extractant. Sep Purif Technol 64(1):56–62
Kumbasar RA (2010) Selective extraction and concentration of chromium (VI) from acidic solutions containing various metal ions through emulsion liquid membranes using Amberlite LA-2. J Ind Eng Chem 16(5):829–836
Kumbasar RA (2009) Extraction of chromium (VI) from multicomponent acidic solutions by emulsion liquid membranes using TOPO as extractant. J Hazard Mater 167(1–3):1141–1147
Mori Y, Uemae U, Hibino S, Eguchi SE (1990) Proper condition of the surfactant liquid membrane for the recovery and concentration of chromium (VI) from aqueous sulfuric acid solution. Int Chem Eng 30:124–133
Garcia MG, Acosta AO, Marchese J (2013) Emulsion liquid membrane pertraction of Cr (III) from aqueous solutions using PC-88A as carrier. Desalination 318:88–96
Othman N, Noah NFM, Sulaiman RNR, Jusoh N, Tan WT (2021) Emulsion liquid membrane modeling for chromium removal from electroplating wastewater using TOMAC as a carrier. Water Environ Res 93(9):1669–1679
Perumal M, Soundarajan B, Thazhathuveettil Vengara N (2019) Extraction of Cr (VI) by pickering emulsion liquid membrane using amphiphilic silica nanowires (ASNWs) as a surfactant. J Dispersion Sci Technol 40(7):1046–1055
Bolne PC, Ghodke SA, Bhanvase BA (2021) Intensified hydrodynamic cavitation-based process for the production of liquid emulsion membrane (LEM) for the extraction of chromium (VI) ions. Int J Environ Res 15(2):313–320
Anarakdim K, Gutiérrez G, Cambiella Á, Senhadji-Kebiche O, Matos M (2020) The effect of emulsifiers on the emulsion stability and extraction efficiency of Cr (VI) using emulsion liquid membranes (ELMs) formulated with a green solvent. Membranes 10(4):76
Anarakdima K, Gutiérrezb G, Matosb M, Sidia H, Hammania L, Senhadji-Kebichea O (2019) Selective separation and recovery of Cr (VI) in the presence of other metal ions, especially Fe (III), by green emulsion liquid membrane. Presented at the International Conference on Sustainable Water Treatment Technologies and Environment (SUST-WATER 2019) (Vol. 14, p. 16)
Kumbasar RA (2009) Extraction and concentration study of cadmium from zinc plant leach solutions by emulsion liquid membrane using trioctylamine as extractant. Hydrometallurgy 95(3–4):290–296
Kumbasar RA (2010) Extraction of cadmium from solutions containing various heavy metal ions by Amberlite LA-2. J Ind Eng Chem 16(2):207–213
Li Q, Liu Q, Li K, Tong S (1997) Separation study of cadmium through an emulsion liquid membrane. Talanta 44(4):657–662
Li QM, Liu Q, Zhang QF, Wei XJ, Guo JZ (1998) Separation study of cadmium through an emulsion liquid membrane using triisooctylamine as mobile carrier. Talanta 46(5):927–932
Urtiaga AM, Alonso A, Ortiz I, Daoud JA, El-Reefy SA, de Ortiz SP, Gallego T (2000) Comparison of liquid membrane processes for the removal of cadmium from wet phosphoric acid. J Membr Sci 164(1–2):229–240
Basualto C, Poblete M, Marchese J, Ochoa A, Acosta A, Sapag J, Valenzuela F (2006) Extraction of cadmium from aqueous solutions by emulsion liquid membranes using a stirred transfer cell contactor. J Braz Chem Soc 17(7):1347–1354
Sznejer G, Marmur A (1999) Cadmium removal from aqueous solutions by an emulsion liquid membrane: The effect of resistance to mass transfer at the outer oil–water interface. Colloids Surf A 151(1–2):77–83
Abd Khalil AT, Buddin MS, Mokhtar NF, Puasa SW (2020) Performance evaluation of emulsion liquid membrane for simultaneous copper and cadmium removal: dispersion tool comparison. In IOP Conference Series: Earth and Environmental Science, 616(1):012077. IOP Publishing, Bristol
Benderrag A, Haddou B, Daaou M, Benkhedja H, Bounaceur B, Kameche M (2019) Experimental and modeling studies on Cd (II) ions extraction by emulsion liquid membrane using Triton X-100 as biodegradable surfactant. J Environ Chem Eng 7(3):103166
Kankekar PS, Wagh SJ, Mahajani VV (2010) Process intensification in extraction by liquid emulsion membrane (LEM) process: A case study; enrichment of ruthenium from lean aqueous solution. Chem Eng Process 49(4):441–448
Noah NFM, Othman N, Jusoh N (2016) Highly selective transport of palladium from electroplating wastewater using emulsion liquid membrane process. J Taiwan Inst Chem Eng 64:134–141
Manzak A, Tutkun O (2011) The extraction of lactic acid by emulsion type of liquid membranes using alamine 336 in escaid 100. Can J Chem Eng 89(6):1458–1463
Zheng HD, Chen JJ, Wang BY, Zhao SY (2013) Recovery of copper ions from wastewater by hollow fiber supported emulsion liquid membrane. Chin J Chem Eng 21(8):827–834
Derkach SR (2009) Rheology of emulsions. Adv Coll Interface Sci 151(1–2):1–23
Laki S, Kargari A (2016) Extraction of silver ions from aqueous solutions by emulsion liquid membrane. J Membr Sci Res 2(1):33–40
Kumar A, Thakur A, Panesar PS (2019) A comparative study on experimental and response surface optimization of lactic acid synergistic extraction using green emulsion liquid membrane. Sep Purif Technol 211:54–62
Manzak A, Sonmezoglu M (2010) Extraction of acetic acid from aqueous solutions by emulsion type liquid membranes using Alamine 300 as a carrier. Indian J Chem Technol 17:441–445
Bhatti I, Qureshi K, Kamarudin KSN, Bazmi AA, Bhutto AW, Ahmad F, Lee M (2016) Innovative method to prepare a stable emulsion liquid membrane for high CO2 absorption and its performance evaluation for a natural gas feed in a rotating disk contactor. J Nat Gas Sci Eng 34:716–732
Chakraborty M, Bhattacharya C, Datta S (2010) Emulsion liquid membranes: Definitions and classification, theories, module design, applications, new directions and perspectives. In Liquid Membranes (pp. 141–199). Elsevier
Kulkarni PS, Mukhopadhyay S, Bellary MP, Ghosh SK (2002) Studies on membrane stability and recovery of uranium (VI) from aqueous solutions using a liquid emulsion membrane process. Hydrometallurgy 64(1):49–58
Moyo F, Tandlich R (2015) Optimisation of the emulsion liquid membrane composition and demulsification for rhodium extraction. Waste Treatment and Recovery 1(1)
Floury J, Legrand J, Desrumaux A (2004) Analysis of a new type of high pressure homogeniser. Part B. study of droplet break-up and recoalescence phenomena. Chem Eng Sci 59(6):1285–1294
Wan Y, Zhang X (2002) Swelling determination of W/O/W emulsion liquid membranes. J Membr Sci 196(2):185–201
Kargari A, Kaghazchi T, Soleimani M (2004) Mass transfer investigation of liquid membrane transport of gold (III) by methyl isobutyl ketone mobile carrier. Chemical Engineering & Technology: Industrial Chemistry-Plant Equipment-Process Engineering-Biotechnology 27(9):1014–1018
Kargari A, KaghazchiSohrabi TM, Soleymani A (2006) Application of experimental design to emulsion liquid membrane pertraction of gold (III) ions from aqueous solutions. Iran J Chem Eng 3:77–91
Kumbasar RA (2009) Cobalt–nickel separation from acidic thiocyanate leach solutions by emulsion liquid membranes (ELMs) using TOPO as carrier. Sep Purif Technol 68(2):208–215
Mikucki BA, Osseo-Asare K (1986) Effects of the emulsifier in copper extraction by LIX64N-span 80 liquid surfactant membranes. Solvent Extr Ion Exch 4(3):503–530
Mikucki BA, Osseo-Asare K (1986) The liquid surfactant membrane process: Effect of the emulsifier type on copper extraction by LIX65N-LIX63 mixtures. Hydrometallurgy 16(2):209–229
Sary R, Hafez A, Khedr M, El-Hassanin A (2007) Removal of lead by an emulsion liquid membrane. Desalination 212:165–175
Goyal RK, Jayakumar NS, Hashim MA (2011) A comparative study of experimental optimization and response surface optimization of Cr removal by emulsion ionic liquid membrane. J Hazard Mater 195:383–390
Abbassian K, Kargari A (2016) Modification of membrane formulation for stabilization of emulsion liquid membrane for extraction of phenol from aqueous solutions. J Environ Chem Eng 4(4):3926–3933
Tang B, Yu G, Fang J, Shi T (2010) Recovery of high-purity silver directly from dilute effluents by an emulsion liquid membrane-crystallization process. J Hazard Mater 177(1–3):377–383
Sengupta B, Sengupta R, Subrahmanyam N (2006) Process intensification of copper extraction using emulsion liquid membranes: Experimental search for optimal conditions. Hydrometallurgy 84(1–2):43–53
Hasan MA, Aglan RF, El-Reefy SA (2009) Modeling of gadolinium recovery from nitrate medium with 8-hydroxyquinoline by emulsion liquid membrane. J Hazard Mater 166(2–3):1076–1081
Raikar NB, Bhatia SR, Malone MF, McClements DJ, Henson MA (2011) Predicting the effect of the homogenization pressure on emulsion drop-size distributions. Ind Eng Chem Res 50(10):6089–6100
Kumar A, Thakur A, Panesar PS (2018) Stability analysis of environmentally benign green emulsion liquid membrane. J Dispersion Sci Technol 39(10):1510–1517
Djenouhat M, Hamdaoui O, Chiha M, Samar MH (2008) Ultrasonication-assisted preparation of water-in-oil emulsions and application to the removal of cationic dyes from water by emulsion liquid membrane: Part 1: Membrane stability. Sep Purif Technol 62(3):636–641
Wasewar KL (2012) Reactive extraction: an intensifying approach for carboxylic acid separation. Int J Chem Eng Appl 3(4):249
Kondo K, Kita K, Nakashio F (1981) Extraction kinetics of copper with liquid surfactant membranes in a stirred transfer cell. J Chem Eng Jpn 14(1):20–25
Frankenfeld JW, Cahn RP, Li NN (1981) Extraction of copper by liquid membranes. Sep Sci Technol 16(4):385–402
Kulkarni PS, Mahajani VV (2002) Application of liquid emulsion membrane (LEM) process for enrichment of molybdenum from aqueous solutions. J Membr Sci 201(1–2):123–135
Ma H, Kökkılıç O, Waters KE (2017) The use of the emulsion liquid membrane technique to remove copper ions from aqueous systems using statistical experimental design. Miner Eng 107:88–99
Ma H, Kökkılıç O, Marion CM, Multani RS, Waters KE (2018) The extraction of nickel by emulsion liquid membranes using Cyanex 301 as extractant. Can J Chem Eng 96(7):1585–1596
Ma H, Kökkılıç O, Langlois R, Song X, Qin Y, Waters KE (2019) Selective separation of copper and nickel ions from aqueous solutions containing calcium by emulsion liquid membranes using central composite design. Can J Chem Eng 97(6):1881–1893
Raghuraman B, Tirmizi N, Wiencek J (1994) Emulsion liquid membranes for wastewater treatment: equilibrium models for some typical metal-extractant systems. Environ Sci Technol 28(6):1090–1098
Teramoto M, Sakai T, Yanagawa K, Miyake Y (1983) Modeling of the permeation of copper through liquid surfactant membranes by continuous operations. Sep Sci Technol 18(11):985–997
Völkel W, Halwachs W, Schügerl K (1980) Copper extraction by means of a liquid surfactant membrane process. J Membr Sci 6:19–31
Hu SYB, Wiencek JM (1998) Emulsion-liquid-membrane extraction of copper using a hollow-fiber contactor. AIChE J 44(3):570–581
Valenzuela FR, Basualto C, Sapag J, Tapia C (1997) Membrane transport of copper with LIX-860 from acid leach waste solutions. Miner Eng 10(12):1421–1427
Lazarova Z, Boyadzhiev L (1993) Kinetic aspects of copper (II) transport across liquid membrane containing LIX-860 as a carrier. J Membr Sci 78(3):239–245
Chauhan S, Patel T (2014) A review on solvent extraction of nickel. Int J Eng Res Technol 3(9):1315–1322
Sarma PB, Reddy BR (2002) Liquid–liquid extraction of nickel at macro-level concentration from sulphate/chloride solutions using phosphoric acid based extractants. Miner Eng 15(6):461–464
Sarangi K, Reddy BR, Das RP (1999) Extraction studies of cobalt (II) and nickel (II) from chloride solutions using Na-Cyanex 272: Separation of Co (II)/Ni (II) by the sodium salts of D2EHPA, PC88A and Cyanex 272 and their mixtures. Hydrometallurgy 52(3):253–265
Mahmoodi R, Mohammadi T, Moghadam M (2014) Separation of Cd (II) and Ni (II) ions by supported liquid membrane using D2EHPA/M2EHPA as mobile extractant. Chem Pap 68(6):751–756
Tait BK (1993) Cobalt-nickel separation: the extraction of cobalt (II) and nickel (II) by Cyanex 301, Cyanex 302 and Cyanex 272. Hydrometallurgy 32(3):365–372
Bourget C, Jakovljevic B, Nucciarone D (2005) CYANEX® 301 binary extractant systems in cobalt/nickel recovery from acidic sulphate solutions. Hydrometallurgy 77(3):203–218
Grigorieva NA, Pashkov GL, Fleitlikh IY, Nikiforova LK, Pleshkov MA (2010) Nickel extraction from sulfate media with Cyanex 301 in the presence of electron donor additives. Hydrometallurgy 105(1):82–88
Guan QJ, Wei S, Zhou GY, Liu JP, Yin ZG (2016) Recovery of cobalt and nickel in the presence of magnesium and calcium from sulfate solutions by Versatic 10 and mixtures of Versatic 10 and Cyanex 301. Trans Nonferrous Met Soc China 26(3):865–873
Gharabaghi M, Irannajad M, Azadmehr AR (2013) Separation of nickel and zinc ions in a synthetic acidic solution by solvent extraction using D2EHPA and Cyanex 272. Physicochem Probl Miner Process 49(1):233–242
Sole KC, Hiskey JB (1995) Solvent extraction of copper by Cyanex 272, Cyanex 302 and Cyanex 301. Hydrometallurgy 37(2):129–147
Biswas RK, Banu RA, Islam MN (2003) Some physico-chemical properties of D2EHPA: Part 2. Distribution, dimerization and acid dissociation constants in n-hexane/1 M (Na+, H+) SO42− system, interfacial adsorption and excess properties. Hydrometallurgy 69(1):157–168
Joo SH, Shin SM, Shin D, Oh C, Wang JP (2015) Extractive separation studies of manganese from spent lithium battery leachate using mixture of PC88A and Versatic 10 acid in kerosene. Hydrometallurgy 156:136–141
Younas M, Druon-Bocquet S, Sanchez J (2010) Kinetic and dynamic study of liquid–liquid extraction of copper in a HFMC: Experimentation, modeling, and simulation. AIChE J 56(6):1469–1480
Bhakhar MS (2012) Kinetic Studies on the Extraction of Heavy Metals from Aqueous Stream into Emulsion Liquid Membranes. Ph.D. Thesis, Maharaja Sayajirao University of Baroda, Vadodara, India
Jääskeläinen E, Paatero E (1999) Properties of the ammonium form of Versatic 10 in a liquid–liquid extraction system. Hydrometallurgy 51(1):47–71
Chemicals Inspection and Testing Institute (1992) Biodegradation and Bioaccumulation Data of Existing Chemicals Based on the CSCL Japan. Japan Chemical Industry Ecology - Toxicology and Information Center, Tokyo, Japan
Yalkowsky SH, Dannenfelser RM (1992) The AQUASOL dATAbASE of Aqueous Solubility, 5th edn. University of Arizona, Tucson, AZ, USA
Chakraborty M, Bhattacharya C, Datta S (2003) Effect of drop size distribution on mass transfer analysis of the extraction of nickel (II) by emulsion liquid membrane. Colloids Surf A 224(1–3):65–74
Chakraborty M, Bhattacharya C, Datta S (2003) Mass transfer analysis of the extraction of nickel (II) by emulsion liquid membrane. Indian J Chem Technol 10:311–320
Eyupoglu V, Kumbasar RA (2014) Selective and synergistic extraction of nickel from simulated Cr-Ni electroplating bath solutions using LIX 63 and D2EHPA as carriers. Sep Sci Technol 49(16):2485–2494
Kumbasar RA, Kasap S (2009) Selective separation of nickel from cobalt in ammoniacal solutions by emulsion type liquid membranes using 8-hydroxyquinoline (8-HQ) as mobile carrier. Hydrometallurgy 95(1–2):121–126
Kumbasar RA, Tutkun O (2008) Separation of cobalt and nickel from acidic leach solutions by emulsion liquid membranes using Alamine 300 (TOA) as a mobile carrier. Desalination 224(1–3):201–208
Li LQ, Wang C, Li YD (1997) Separation of cobalt and nickel by emulsion liquid membrane with the use of EDTA as masking reagent. J Membr Sci 135(2):173–177
Hachemaoui A, Belhamel K, Bart HJ (2010) Emulsion liquid membrane extraction of Ni (II) and Co (II) from acidic chloride solutions using bis-(2-ethylhexyl) phosphoric acid as extractant. J Coord Chem 63(13):2337–2348
Kakoi T, Ura T, Kasaini H, Goto M, Nakashio F (1998) Separation of cobalt and nickel by liquid surfactant membranes containing a synthesized cationic surfactant. Sep Sci Technol 33(8):1163–1180
Yamashita K, Kakoi T, Kosaka H, Goto M, Nakashio F (1998) Synergistic extraction of nickel by liquid surfactant membranes. Sep Sci Technol 33(3):369–385
Mickler W, Reich A, Uhlemann E, Bart HJ (1996) Liquid membrane permeation of zinc, cadmium and nickel with 4-acyl-5-pyrazolones and β-diketones. J Membr Sci 119(1):91–97
Serga VE, Kulikova LD, Purin BA (2000) Extraction of nickel by liquid membranes in an electric field. Sep Sci Technol 35(2):299–313
Ruppert M, Draxler J, Marr R (1988) Liquid-membrane-permeation and its experiences in pilot-plant and industrial scale. Sep Sci Technol 23(12–13):1659–1666
Acknowledgements
The work is financially supported by the Ministry of Science and Technology of China through the Foreign Youth Talent Program (QN2021074001L). The anonymous reviewers are greatly appreciated for their dedicated work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, H., Waters, K.E. & Wang, H. A Review of Copper and Nickel Extraction from Wastewater by Emulsion Liquid Membrane (ELM). Mining, Metallurgy & Exploration 40, 13–39 (2023). https://doi.org/10.1007/s42461-022-00726-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-022-00726-6