Abstract
Application of copper-based algaecide formulations is commonly conducted to control nuisance cyanobacterial blooms. Most field application scenarios have a rapid decline in external aqueous copper concentrations. Copper partitioned to algae can remain bound in external state, transition into the cell, or desorb back into solution. Understanding short-term fate of applied copper-based algaecides is critical in risk assessment for non-target species as well as achieving desired efficacy of target nuisance algae. This research assessed the ability of copper from different algaecide formulations to partition to Lyngbya wollei and the subsequent internalization and desorption of copper following cessation of the aqueous exposure. Following a 6-h exposure, there were no significant differences in total partitioned copper between copper sulfate and an ethanolamine chelated copper formulation (Captain® XTR). Four days after cessation of the aqueous copper exposure, all chelated copper and copper sulfate (except 2 mg Cu/L) exposures had significantly decreased adsorbed copper to L. wollei. However, chelated copper had significantly more internalized copper (P < 0.05) at the 0.5, 1, and 2 mg Cu/L treatments compared with the 6-h measurements and higher internalized copper than copper sulfate at the 2 and 4 mg Cu/L treatments. Average desorbed copper was lower in most chelated copper treatments compared with copper sulfate, although no statistically significant differences were measured between formulations. This information will allow water resource managers to select the most efficient algaecide formulation for desired algal control, with a better understanding of depuration potential, offsite movement, and risks to non-target organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Reactive management of nuisance algae (including cyanobacteria) often involves application of copper-based algaecides to restore water resource uses (Mastin et al. 2002). Copper-based algaecides are typically only applied to a portion of a water resource (e.g., localized band/spot, benthic injection) or metered into a flowing canal over a certain time period. There are often large dilution potentials from where the product is applied, which may decrease the aqueous copper exposures realized by the target alga. Additionally, algal/sediment sorption occurs rapidly (Crist et al. 1990) and can further decrease the exposure duration. Angel et al. (2015) found copper that adsorbed to the diatom, Phaeodactylum tricornutum, may desorb back into aqueous solution although some copper is still retained in the cell. The transient nature of adsorbed copper needs to be further understood since binding is mostly reversible and subject to equilibrium conditions with aqueous copper concentrations (Knauer et al. 1997; Veglio and Beolchini 1997). The secondary transition of copper to become internalized, remain bound in external state, or desorb is critical in understanding fate of applied copper-based algaecides, especially when the external aqueous exposure is ceased. By measuring the short-term binding, uptake, and desorption of copper with the target alga, potential for adverse impacts to non-target species can be better predicted. This information will allow water resource managers to select the most efficient algaecide formulation for desired algal control, while concomitantly decreasing depuration potential, offsite movement, and risks to non-target organisms.
Controlling algae with copper is a function of achieving the required amount of copper at the toxic sites of action (i.e., dose; Bishop and Rodgers 2012). Copper algaecide formulations are designed differently and this may alter the adsorption or transfer of copper into the algal cell. Since control of many mat-forming algae in field scenarios is a function of exceeding an internalized copper threshold (i.e., critical burden; Bishop et al. 2015; Bishop 2016), copper-based algaecide formulations that primarily adsorb to algae may not elicit the desired level of control. Additionally, copper in an externally bound state will have increased opportunity to desorb back into surrounding water, especially if the copper concentration in surrounding water is decreased due to dilution or binding (Angel et al. 2015; Bishop et al. 2017). Chelated copper is typically designed to enhance formulation stability, decrease water chemistry interferences, promote the interaction of copper with algae, and ultimately increase efficiency of control (Straus and Tucker 1993; Mastin and Rodgers 2000). Chelated copper has also been shown to be innately less toxic to non-target freshwater fish (e.g., Salvelinus fontinalis and Pimephales promelas) in toxicity testing (Wagner et al. 2017) likely due to decreased interactivity of the cupric ion with gill-binding sites that impact ion transfer (Playle et al. 1993). Copper ions from copper sulfate, as a charged polar species, have lesser ability to penetrate the algal cell membrane as they are restricted to active transport (Sunda and Huntsman 1998). Whereas, organic chelating agents (e.g., monoethanolamine, triethanolamine, and ethylenediamine) are incorporated into copper algaecide formulations to alter the cupric ion properties allowing passive transport across biological membranes (Stauber and Florence 1987; Straus and Tucker 1993; Mastin and Rodgers 2000) and increased control of target nuisance algae (Calomeni et al. 2014; Bishop et al. 2015). Surfactants are often added to formulations to further enhance interactivity of these formulations with algae and penetration of the copper into the algal cell (Closson and Paul 2014). Since internalized copper correlates to control of nuisance algae (Bishop 2016) and is less likely to desorb than externally adsorbed (Kuyucak and Volesky 1989; Knauer et al. 1997; Kaduková and Virčíková 2005), this provides an avenue to assess the efficiency of copper algaecide formulations.
Non-target organism toxicity is primarily manifested from dissolved aqueous copper exposures in aquatic systems (Playle et al. 1993; Meyer et al. 1999). Sorption to organic matter (including algae) has been found to decrease copper toxicity to non-target organisms (Ma et al. 1999; Bishop 2016). Important factors in assessing effectiveness and toxicity of copper formulations include (1) efficiency of sorption to algal cells, (2) internalization to achieve control, and (3) ability to desorb and add to the available copper to non-target species. Sorption to algae often occurs rapidly, although there are numerous inert binding sites on the cell surface that may not lead to internalization as well as sites that lead to internalization (Xue and Sigg 1990; Veglio and Beolchini 1997). Much of copper bound in pulsed exposures may rapidly desorb back into solution especially under high concentrations that saturate binding sites or transport proteins (Angel et al. 2015). Loosely adsorbed copper is more likely to be desorbed than copper that transfers into the cell (Knauer et al. 1997; Kaduková and Virčíková 2005). Since desorbed copper poses increased risks to non-target organisms, understanding short-term sorption and desorption potential is critical in predicting algaecidal efficacy and potential unintended impacts.
Lyngbya wollei (Farlow ex Gomont) Speziale and Dyck is a freshwater, filamentous cyanobacterium common throughout the southeastern USA (Speziale and Dyck 1992) and spreading in range distribution across the USA including the Laurentian Great Lakes basin (Bridgeman and Penamon 2010). L. wollei can have significant negative economic and ecological impacts to water resources through production of thick mats (habitat destruction, impede recreation, and property values), toxin and taste/odor compounds (off flavor, irrigation integrity, and human safety), and housing fecal bacteria (Lagos 1998; Bridgeman and Penamon 2010; Mihali et al. 2011; Manganelli et al. 2012; Vijayavel et al. 2013). Large infestations are often very difficult and expensive to control with many management options showing limited to no efficacy. Select USEPA-registered algaecides have shown potential for control in large water resources (Bishop et al. 2015), though a better understanding of their activity is needed. L. wollei possesses a thick extracellular mucilaginous sheath primarily composed of fibrils, made from a cellulose-like homoglucan polymer, cross linked with multiple monosaccharides (Hoiczyk 1998; Hoiczyk and Hansel 2000). The sheath of L. wollei can comprise over 55% of dry biomass and provides numerous advantages, in part, including predation deterrence, UV protection, nutrient acquisition, mobility, and desiccation avoidance (Hoiczyk and Hansel 2000; Camacho and Thacker 2006). Another function of the mucilage is to decrease metal toxicity to allow for growth in polluted environments (Reynolds 2007), though also applicable to tolerance of applied copper from algaecides. Li et al. (2001) observed a strong affinity of copper for mucilaginous sheaths and Tien et al. (2005) estimated that the surface mucilage accounted for 40% of total adsorbed copper. An infestation of L. wollei in Lake Gaston, on the NC/VA border, provided an opportunity to assess formulation ability to penetrate or depurate from this nuisance cyanobacterium. In this research, we tested a copper salt and chelated copper with added surfactants in order to assess efficiencies of formulations at transferring to toxic sites of action and conversely the ability of copper to desorb from ligands in/on L. wollei. A constant mass of L. wollei was tested with a range of copper concentrations as a scaled representation of the field site. Increased biomass of Lyngbya is predicted to proportionately alter the amount of copper needed for control (Franklin et al. 2002; Bishop and Rodgers 2012; Tsai 2016).
This research compared copper algaecide formulations for efficiency of adsorption and subsequent desorption as well as internalization of copper. By selecting the most efficient formulation that achieves the internal threshold for control, with the lowest aqueous exposure concentration, more effective control of nuisance algal blooms can be achieved while increasing the margin of safety for non-target organisms. Specific objectives were (1) to measure copper adsorbed to and internalized in Lyngbya wollei following a 6-h exposure of 0.5, 1, 2, and 4 mg Cu/L as copper sulfate pentahydrate and a chelated copper ethanolamine complex, (2) to measure adsorbed, internalized, and desorbed copper 4 days after transfer to untreated water, and (3) to compare copper mass adsorbed, internalized, and desorbed between formulations at different exposures. Exposure factors (Cu concentration times duration) were selected to span sublethal (less than 50% control), moderate (50–75% control), and complete (> 75%) control of L. wollei based on efficacy trials in prior research (Bishop et al. 2017).
2 Methods
2.1 Culture and Testing Conditions
Lyngbya wollei accessions were collected from two locations in Lake Gaston, NC/VA: (1) 36° 29′ 36″ N; 77° 53′ 54″ W and (2) 36° 33′ 06″ N; 78° 00′ 50″ W by dragging a thatch rake. Benthic mats were transferred on the day of collection to the SePRO Research and Technology Campus (SRTC) in Whitakers, NC, for testing. Replicated experiments were conducted using field samples taken at two time periods (5.26.17 from Pretty Creek, prior to Captain XTR treatment and 7.10.17 from Reference site). This was done to evaluate the temporal consistency of laboratory results and reinforce predictive capability to the field site (Lakeman et al. 2009). Samples were cultured under and tested with a constant temperature of 23 ± 1 °C and a 16-h light/8-h dark photoperiod with fluorescent lighting (Spectralux T5/HO 6500K blue; 3000K red) at an intensity of 67.5 ± 2.7 μmol photons/m2/s (Lewis et al. 1994). Studies were conducted using well water which had characteristics of pH (8 ± 1.), dissolved oxygen (8.5 ± 1 mg O2/L), temperature (22 ± 2 °C), conductivity (230–390 μS/cm2), alkalinity (90–140 mg/L as CaCO3), hardness (90–130 mg/L as CaCO3), total phosphorus (90–110 μ/L), and total nitrogen (3.9–4.1 mg/L). Well water had increased alkalinity, hardness, and conductivity than Lake Gaston field water. Well water was used due to its ability to consistently support L. wollei growth while not containing any known constituents that may compromise testing. All analyses were measured according to Standard Methods (American Public Health Association [APHA] 2005).
2.2 Experimental Design
The mass of L. wollei was held at a constant 0.1 g ± 0.01 g wet weight. Six treatment replicates were exposed to four aqueous copper concentrations 0.5, 1, 2, and 4 mg Cu/L of each copper formulation (chelated copper ethanolamine complex as Captain XTR; SePRO Corporation Carmel, IN, and copper sulfate pentahydrate; C489-1 Fisher Scientific, Inc.) in a 500-mL Erlenmeyer flask with 250 mL exposure water. Stock algaecide solutions were made within 4 h of test initiation and serial dilutions were used to obtain target copper concentrations. All treatments were maintained in the treated copper solution for a 6-h duration. Three replicates were randomly taken after the 6-h copper exposure and measured for adsorbed and internalized copper. The other three replicates were gently removed, using acid-washed forceps, from the flask containing copper and transferred to a clean flask containing 250 mL well water without any algaecide added. Adsorbed, internalized, and desorbed copper were measured 4 days after transfer to the clean flask. Untreated controls were also moved in a similar manner after 6 h.
2.3 Copper Analyses
The entire algal mass in each treatment was used to assess adsorbed and internalized copper. Adsorbed copper was measured by rinsing the L. wollei samples with 10 mL of 2 mM EDTA for 10 min to remove adhered metal ions from the surface as EDTA does not penetrate the cell (Knauer et al. 1997). Samples were then acidified (1% v/v trace metal grade nitric acid; Fisher Scientific, Inc. A509) to preserve and filtered (0.22 μm pore size Whatman GF/F glass microfiber filter) prior to analysis. Internalized copper was subsequently measured by digesting the rinsed algae in 2 mL 70% trace-metal-grade nitric acid, 2 mL of 30% H2O2 (Fisher Scientific, Inc. BP2633), and 6 mL NanoPure™ water. The mass was then sonicated for 30 s to disperse filaments and then heated to 180 °C, and repeated to achieve a clear solution (USEPA 1996; Tripathi et al. 2006). To assess copper depurated from the algae, both total and acid-soluble copper concentrations were measured. Total copper was measured on a 15 mL subsample of water from the homogenized flask, this was acidified (1% v/v trace metal grade nitric acid; Fisher Scientific, Inc. A509) and filtered (0.22 μm pore size Whatman GF/F glass microfiber filter) prior to analysis. To ensure all depurated copper was analyzed, total copper was also measured on a subset of samples by acidifying the entire flask to 1% nitric acid to account for all copper that may have adhered to the flask and particulates. Soluble copper (filtered then acidified) was measured on one replicate of each experiment. To calculate percentage of copper that desorbed, the average aqueous copper mass measured 4 days after transfer was divided by the average mass of copper transferred at the 6-h measurement (adsorbed plus internalized). All copper samples were measured using inductively coupled plasma-optical emission spectrometry (ICPE 9000; Shimadzu Corporation) with a matrix-matched calibration curve from serial dilution of a 1000 mg Cu/L standard (Fisher Scientific, Inc. SC194; APHA 2005). The limit of detection for copper was 5 μg Cu/L (0.1 μg Cu/ g algae). Method blanks were analyzed with each run to ensure that there was no contamination by the materials used in sample preparation.
2.4 Statistics
Data from each experiment were pooled for analysis since no significant treatment by experiment results was measured. A two-tailed T test was used to assess differences (α = 0.05) between 6 h after treatment (HAT) and 4 days after treatment (DAT) internalized and adsorbed copper with each treatment concentration and copper formulation as well as between formulations at corresponding exposures. A Shapiro-Wilk test was used to assess normality of the data, and all data were tested for constant variance (F test; McDonald 2014). All data were analyzed using Microsoft Excel® (Microsoft 2010).
3 Results
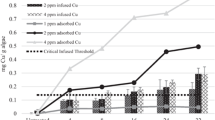
Initial aqueous copper concentrations in copper sulfate treatments were between 94 and 105% of targeted values and between 91 and 106% for chelated copper exposures; therefore, nominal copper concentrations were used in data graphs and analyses. Since results of total flask acidification and the homogenized 15 mL acid-soluble Cu samples were similar (< 5% variation) in replicates for each copper chemistry, this confirms negligible binding of copper to exposure vessels. Therefore, the 15 mL aliquot was used for determining desorbed copper. Acid-soluble copper analyses on desorbed copper showed an average of 85% of measured copper in soluble form. There were increasing amounts of copper adsorbed and internalized with increasing concentration of both copper formulations. At 6 HAT, the majority of copper partitioned to L. wollei was adsorbed (Figs. 1 and 2). There were no significant differences in internalized or adsorbed copper between the formulations at the 6 HAT sampling point (P > 0.05). Captain XTR had significantly decreased (P < 0.05, n = 6) adsorbed copper in all treatments from the 6 HAT to the 4 DAT measurements. Captain XTR had significantly increased (P < 0.05) internalized copper in the 0.5, 1, and 2 mg Cu/L treatments from the 6 HAT to the 4 DAT measurements (Fig. 1). Copper sulfate had significantly decreased (P < 0.05, n = 6) adsorbed copper in the 0.5, 1, and 4 mg Cu/L treatments from the 6 HAT to the 4 DAT measurements. Copper sulfate did not have any statistically significant differences in internalized copper from the 6 HAT to the 4 DAT measurements (Fig. 2; P > 0.05, n = 6).
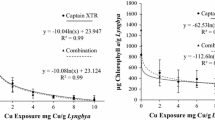
Following transfer to clean water for 4 days, Captain XTR had significantly more copper adsorbed to L. wollei at the 0.5 mg Cu/L treatment, whereas copper sulfate had significantly more adsorbed copper at the 2 mg Cu/L treatment. Captain XTR had significantly more internalized copper at both the 2 and 4 mg Cu/L treatments compared with copper sulfate. Although average desorbed copper was lower in most Captain XTR treatments compared with copper sulfate, no statistically significant differences were measured for desorbed copper (Fig. 3). Total partitioned copper (internalized plus adsorbed) was significantly higher in the 4 mg Cu/L as Captain XTR treatment than copper sulfate, though not different at any other treatment concentration.
Depurated copper following transfer to clean water was 22.1, 21.9, 22.3, and 20.0% of the transferred copper amount following the 6-h exposure duration in Captain XTR treatments of 0.5, 1, 2, and 4 mg/L, respectively. Copper that depurated following transfer to clean water was 16.8, 19.6, 30.6, and 28.2% of the transferred copper amount in copper sulfate treatments of 0.5, 1, 2, and 4 mg/L, respectively. Amount of initial copper amended to exposure vessels that depurated was 6.9, 5.0, 4.9, and 3.8% in Captain XTR treatments of 0.5, 1, 2, and 4 mg/L, respectively. Amount of initial copper amended to exposure vessels that depurated was 6.5, 5.2, 6.2, and 4.5% in copper sulfate treatments of 0.5, 1, 2, and 4 mg/L, respectively.
4 Discussion
Copper is an essential micronutrient at low levels (e.g., components of many enzymes and proteins; Sunda 1989) and can increase algal growth in low, pulsed exposures (Angel et al. 2017). Copper has many modes of action but most are internal to the cell and exceeding a toxic threshold (in excess of depuration or amelioration) is needed to cause toxicity to target algae (Knauer et al. 1997; Campbell et al. 2002; Angel et al. 2015; Bishop et al. 2015). Typical field applications of copper algaecides often elicit short aqueous exposure durations (< 1 day) due to rapid binding and removal of copper from the water. We found similar initial binding of copper to algae with both copper formulations tested in this research. Other research has confirmed this rapid interaction of copper with the cell (Crist et al. 1990; Levy et al. 2007; Bishop et al. 2017). However, initial interaction is not necessarily correlated with effectiveness. With other algal species, exclusion of copper from the cell was the primary mechanism of copper tolerance. For example, Yan and Pan (2002) found exclusion of copper in the green alga, Closterium lunula, to provide less internal copper and increase in tolerance. Twiss et al. (1993) also found a copper tolerant strain of Scenedesmus acutus to exclude copper. Whereas, Butler et al. (1980) reported a sensitive strain of Chlorella vulgaris to accumulate over seven times more copper than a tolerant strain. Higher adsorption at the cell surface may not directly lead to internalization nor have a significant algaecidal impact. The thick mucilaginous sheath of L. wollei may further increase inert, external binding sites and render copper non-bioavailable to the cell (Li et al. 2001; Yee et al. 2004; Tien et al. 2005). Following initial binding, understanding the subsequent transfer of copper is critical to assess formulation effectiveness as well as desorption ability.
Similar desorption was measured between copper formulations tested in this work, and both had decreased adsorbed copper after transfer to untreated water. This suggests that some inert, external binding sites that had interacted with both formulations were able to be transferred back into surrounding water. However, Captain XTR had significantly increased internalized copper at multiple treatment concentrations tested compared with copper sulfate following the 4-day depuration period, whereas copper sulfate did not significantly change. Even with rapid cessation of the aqueous external copper concentrations, there was still an influx of copper from Captain XTR into the cells. Copper sulfate maintained the same level of internal copper supporting prior research on decreased ability of internalized copper to desorb (Knauer et al. 1997; Kaduková and Virčíková 2005). The greatest change in copper partitioning was removal of adsorbed copper back into solution. This suggests different uptake mechanisms between the chelated formulations and copper sulfate. The free cupric ion, as primarily results from copper sulfate exposures, may be restricted to active transport across the cellular membrane due to its charge characteristics (Sunda 1989; Knauer et al. 1997). Passive diffusion is another route of copper internalization (Khummongkol et al. 1982), and chelated metals have shown increased ability to passively move across biological membranes (Stauber and Florence 1987).
Copper sulfate and chelated copper exposures have resulted in similar adsorbed copper amounts, though not resulted in the same degree of control (Bishop and Rodgers 2012; Bishop et al. 2017). Internalized copper often differs by formulation and has shown to be significantly correlated with control (Bishop 2016). In general, chelated formulations have more routes of entry, other than active transport alone, to achieve internal copper thresholds (Stauber and Florence 1987). Results of prior works also showed increased effectiveness with chelated formulations over copper sulfate (Bishop and Rodgers 2012; Calomeni et al. 2014; Iwinski et al. 2016). Although we did not directly measure effectiveness in this work, since internalized copper correlates with control and Captain XTR exposures continued to increase in internalized levels and were significantly higher in the highest treatments tested, we would hypothesize increased efficacy. Enhanced control with chelated copper over copper sulfate with L. wollei has been shown in other works with static exposures (Bishop and Rodgers 2012; Bishop 2016) and pulsed exposures at similar internalized levels (Bishop et al. 2017).
Since the internalized copper remained unchanged or increased, this aligns with prior research showing the enhanced transient nature of adsorbed copper (Gonzalez-Davila et al. 1995; Knauer et al. 1997) as opposed to internalized copper that is not readily released (Yan and Pan 2002; Kaduková and Virčíková 2005). With many field applications of copper algaecides, a pulsed exposure scenario is realized due to label restrictions on a small portion of water volume that can be treated as well as localized area (e.g., band, benthic injection) or flowing water applications. It is predicted the reversible binding nature of adsorbed copper would be accentuated by pulse exposures as this provides less contact time with the algal surface and rapid decrease in external aqueous concentrations. Angel et al. (2015) did find rapid desorption/release back into solution following a pulse copper sulfate exposure. With increased inert binding sites due to the large external mucilaginous sheath in L. wollei, a greater amount of adsorbed copper and potentially desorbed copper is predicted with this species. Future work will further assess the form and bioavailability of desorbed copper, especially since chelated copper, if maintained in this state following sorption, may still be innately less toxic to many non-target species (Wagner et al. 2017). Despite similar desorption from both copper formulations, the continued internalization with Captain XTR is more likely to attain the internal threshold for desired control, thereby decreasing need for subsequent treatments. Selecting appropriate copper formulations can better achieve desired management objectives for a site-specific algal infestation.
5 Conclusion
Similar initial copper sorption was measured between two copper algaecide formulations on L. wollei with a thick mucilaginous sheath. Desorption of copper following transfer to clean water was also similar between the formulations, although significant increases in internalized copper were measured with the chelated copper algaecide. Internalized copper was less transient and did not desorb to the same extent as adsorbed copper. Increasing internalized amounts are also needed to achieve the threshold for control of target nuisance algal species, and the chelated copper algaecide provided an increased movement of copper into cells. This research provides a better understanding of short-term copper fate following copper-based algaecide applications. With increased regulatory scrutiny regarding copper use, these data can allow water resource managers to make more informed decisions on effectiveness and risks associated with copper formulations.
References
American Public Health Association. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington, DC.
Angel, B. M., Simpson, S. L., Chariton, A. A., Stauber, J. L., & Jolley, D. F. (2015). Time-averaged copper concentrations from continuous exposure predicts pulsed exposure toxicity to the marine diatom, Phaeodactylum tricornutum: importance of uptake and elimination. Aquatic Toxicology, 164, 1–9.
Angel, B. M., Simpson, S. L., Granger, E., Goodwyn, K., & Jolley, D. F. (2017). Time-averaged concentrations are effective for predicting chronic toxicity of varying copper pulse exposures for two freshwater green algae species. Environmental Pollution, 230, 787–797.
Bishop, W.M. (2016). A Risk-based decision information system for selecting an algal management program (doctoral dissertation). Retrieved from North Carolina State University Libraries.
Bishop, W. M., & Rodgers Jr., J. H. (2012). Responses of Lyngbya wollei to exposures of copper-based algaecides: the critical burden concept. Archives of Environmental Contamination and Toxicology, 62, 403–410.
Bishop, W. M., Willis, B. E., & Horton, C. T. (2015). Affinity and efficacy of copper following an algicide exposure: application of the critical burden concept for Lyngbya wollei control in Lay Lake, AL. Journal of Environmental Management, 55, 983–990.
Bishop, W. M., Lynch, C. L., Willis, B. E., & Cope, W. G. (2017). Copper-based aquatic algaecide adsorption and accumulation kinetics: influence of exposure concentration and duration for controlling the cyanobacterium Lyngbya wollei. Bulletin of Environmental Contamination and Toxicology, 99(3), 365–371.
Bridgeman, T. B., & Penamon, W. A. (2010). Lyngbya wollei in western Lake Erie. Journal of Great Lakes Research, 36(1), 167–171.
Butler, M., Haskew, A.E.J., & Young, M.M. (1980). Copper tolerance in the green alga, Chlorella vulgaris. Plant, Cell and Environment, 3, 119–126.
Calomeni, A., Rodgers Jr., J. H., & Kinley, C. M. (2014). Responses of Planktothrix agardhii and Pseudokirchneriella subcapitata to copper sulfate (CuSO4·5H2O) and a chelated copper compound (Cutrine-Ultra). Water, Air, and Soil Pollution, 225, 2231.
Camacho, F. A., & Thacker, R. W. (2006). Amphipod herbivory on the freshwater cyanobacterium Lyngbya wollei: chemical stimulants and morphology defense. Limnology and Oceanography, 51, 1870–1875.
Campbell, P. G., Errécalde, O., Fortin, C., Hiriart-Baer, V. P., & Vigneault, B. (2002). Metal bioavailability to phytoplankton-applicability of the biotic ligand model. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 133, 189–206.
Closson, K. R., & Paul, E. A. (2014). Comparison of the toxicity of two chelated copper algaecides and copper sulfate to non-target fish. Bulletin of Environmental Contamination and Toxicology, 93, 660–665.
Crist, R. H., Martin, J. R., Guptill, P. W., Eslinger, J. M., & Crist, D. R. (1990). Interaction of metals and protons with algae. 2. Ion-exchange in adsorption and metal displacement by protons. Environmental Science and Technology, 24, 337–342.
Franklin, N. M., Stauber, J. L., Apte, S. C., & Lim, R. P. (2002). Effect of initial cell density on the bioavailability and toxicity of copper in microalgal bioassays. Environmental Toxicology and Chemistry, 21, 742–751.
Gonzalez-Davila, M., Santana-Casiano, J. M., Perez-Pena, J., & Millero, F. J. (1995). Binding of Cu(II) to the surface and exudates of the alga Dunaliella tertiolecta in seawater. Environmental Science and Technology, 29, 289–301.
Hoiczyk, E. (1998). Structural and biochemical analysis of the sheath of Phormidium uncinatum. Journal of Bacteriology, 180, 3923–3932.
Hoiczyk, E., & Hansel, A. (2000). Cyanobacterial cell walls: news from an unusual prokaryotic envelope. Journal of Bacteriology, 182(5), 1191–1199.
Iwinski, K. J., Calomeni, A. J., Geer, T. D., & Rodgers, J. H. (2016). Cellular and aqueous microcystin-LR following laboratory exposures of Microcystis aeruginosa to copper algaecides. Chemosphere, 147, 74–81.
Kaduková, J., & Virčíková, E. (2005). Comparison of differences between copper bioaccumulation and biosorption. Environmental International, 31, 227–232.
Khummongkol, D., Canterford, G. S., & Fryer, C. (1982). Accumulation of heavy metals in unicellular algae. Biotechnology and Bioengineering, 14, 2643–2660.
Knauer, K., Behra, R., & Sigg, L. (1997). Adsorption and uptake of copper by the green alga Scenedesmus subspicatus (Chlorophyta). Journal of Phycology, 33, 596–601.
Kuyucak, N., & Volesky, B. (1989). The mechanism of cobalt biosorption. Biotechnology and Bioengineering, 33, 823–831.
Lagos, N. (1998). Microalgal blooms: a global issue with negative impact in Chile. Biological Research, 31, 375–386.
Lakeman, M. B., von Dassow, P., & Cattolico, R. A. (2009). The strain concept in phytoplankton ecology. Harmful Algae, 8, 746–758.
Levy, J. L., Stauber, J. L., & Jolley, D. F. (2007). Sensitivity of marine microalgae to copper: the effect of biotic factors on copper adsorption and toxicity. Science of the Total Environment, 387, 141–154.
Lewis, P. A., Klemm, D. J., Lazorchak, J. M., Norberg-King, T. J., Peltier, T. J., Peltier, W. H., et al. (1994). Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms (green algae, Selenastrum capricornutum, growth 1003.0) (3 rd ed.). Cincinnati, OH: USEPA.
Li, P., Liu, Z., & Xu, R. (2001). Chemical characterization of the released polysaccharide from the cyanobacterium Aphanothece halophytica GR02. Journal of Applied Phycology, 13, 71–77.
Ma, H., Kim, S. D., Cha, D., & Allen, H. E. (1999). Effect of kinetics of complexation by humic acid on toxicity of copper to Ceriodaphnia dubia. Environmental Toxicology and Chemistry, 18, 828–837.
Manganelli, M., Scardala, S., Stefanelli, M., Palazzo, F., Funari, E., Vichi, S., Buratti, F. M., & Testai, E. (2012). Emerging health issues of cyanobacterial blooms. Annali dell'Istituto Superiore di Sanità, 48(4), 415–428.
Mastin, B. J., & Rodgers Jr., J. H. (2000). Toxicity and bioavailability of copper herbicides (Clearigate, Cutrine-Plus, and copper sulfate) to freshwater animals. Archives of Environmental Contamination and Toxicology, 39, 445–451.
Mastin, B. J., Rodgers Jr., J. H., & Deardorff, T. L. (2002). Risk evaluation of cyanobacteria-dominated algal blooms in a North Louisiana reservoir. Journal of Aquatic Ecosystem Stress and Recovery, 9, 103–114.
McDonald, J. H. (2014). Handbook of biological statistics (3rd ed.). Baltimore: Sparky House Publishing.
Meyer, J. S., Santore, R. C., Bobbitt, J. P., DeBrey, L. D., Boese, C. J., Paquin, P. R., et al. (1999). Binding of nickel and copper to fish gills predicts toxicity when water hardness varies, but free-ion activity does not. Environmental Science and Technology, 33, 913–916.
Microsoft. (2010). Microsoft excel [computer software]. Redmond: Microsoft.
Mihali, T. K., Carmichael, W. W., & Neilan, B. A. (2011). A putative gene cluster from a Lyngbya wollei bloom that encodes paralytic shellfish toxin biosynthesis. PLoS One. https://doi.org/10.1371/journal.pone.0014657.
Playle, R. C., Dixon, D. G., & Burnison, K. (1993). Copper and cadmium binding to fish gills: modification by dissolved organic carbon and synthetic ligands. Canadian Journal of Fisheries and Aquatic Sciences, 50, 2667–2677.
Reynolds, C. S. (2007). Variability in the provision and function of mucilage in phytoplankton: facultative responses to the environment. Hydrobiologia, 578, 37–45.
Speziale, B. J., & Dyck, L. A. (1992). Comparative taxonomy of Lyngbya wollei. comb. nov. (cyanobacteria). Journal of Phycology, 28, 693–706.
Stauber, J. L., & Florence, T. M. (1987). Mechanism of toxicity of ionic copper and copper complexes to algae. Marine Biology, 94, 511–519.
Straus, D. L., & Tucker, C. S. (1993). Acute toxicity of copper sulfate and chelated copper to channel catfish Ictalarus punctatus. Journal of the World Aquaculture Society, 24, 390–395.
Sunda, W. G. (1989). Trace metal interactions with marine phytoplankton. Biological Oceanography, 6, 411–442.
Sunda, W. G., & Huntsman, S. A. (1998). Processes regulating cellular metal accumulation and physiological effects: phytoplankton as model systems. Science of the Total Environment, 219, 165–181.
Tien, C. J., Sigee, D. C., & White, K. N. (2005). Copper adsorption kinetics of cultured algal cells and freshwater phytoplankton with emphasis on cell surface characteristics. Journal of Applied Phycology, 17, 379–389.
Tripathi, B. N., Mehta, S. K., Amar, A., & Gaur, J. P. (2006). Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu+2 and Zn+2. Chemosphere, 62, 538–544.
Tsai, K. P. (2016). Management of target algae by using copper-based algaecides: effects of algal cell density and sensitivity to copper. Water, Air Soil Pollut, 227, 1–11.
Twiss, M. R., Welbourn, P. M., & Schwartzel, E. (1993). Laboratory selection for copper tolerance in Scenedesmus acutus (Chlorophyceae). Canadian Journal of Botany, 71, 333–338.
United States Environmental Protection Agency. (1996). SW-846 test method 3052: microwave assisted acid digestion of siliceous and organically based matrices Ch. 3.2. Washington, DC: USEPA.
Veglio, F., & Beolchini, F. (1997). Removal of metals by biosorption: a review. Hydrometallurgy, 44(3), 301–316.
Vijayavel, K., Sadowsky, M. J., & Ferguson, J. A. (2013). The establishment of the nuisance cyanobacteria Lyngbya wollei in St. Clair and its potential to harbor fecal indicator bacteria. Journal of Great Lakes Research, 39(4), 560–568.
Wagner, J. L., Townsend, A. K., Velzis, A. E., & Paul, E. A. (2017). Temperature and toxicity of the copper herbicide (Nautique™) to freshwater fish in field and laboratory trials. Cogent Environmental Science. https://doi.org/10.1080/23311843.2017.1339386.
Xue, H. B., & Sigg, L. (1990). Binding of Cu(II) to algae in a metal buffer. Water Research, 24, 1129–1136.
Yan, H., & Pan, G. (2002). Toxicity and bioaccumulation of copper in three green microalgal species. Chemosphere, 49, 471–476.
Yee, N., Benning, L. G., Phoenix, V. R., & Ferris, F. G. (2004). Characterization of metal-cyanobacteria sorption reactions: a combined macroscopic and infrared spectroscopic investigation. Environmental Science and Technology, 38, 775–782.
Acknowledgements
The authors thank SePRO Corporation for use of their accredited laboratory to conduct this research and for use of equipment to collect field samples. Thanks to Hasan Hasan and Clayton Lynch for analytical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bishop, W.M., Villalon, G.V. & Willis, B.E. Assessing Copper Adsorption, Internalization, and Desorption Following Algaecide Application to Control Lyngbya wollei from Lake Gaston, NC/VA, USA. Water Air Soil Pollut 229, 152 (2018). https://doi.org/10.1007/s11270-018-3801-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3801-6