Abstract
Public concerns regarding the use of copper-based algaecide for controlling problematic algae may arise due to the risks it creates to non-target algae. To examine this concern, a series of comparative algal toxicity experiments were conducted to study effects of prokaryotic and eukaryotic algal cell densities on their responses to exposures of copper sulfate and copper-ethanolamine (Cu-EA). Microcystis aeruginosa and Pseudokirchneriella subcapitata were cultured separately in BG 11 medium to three initial cell densities (5 × 104, 5 × 105, and 5 × 106 cells/mL). The 96-h EC50 values of copper sulfate for M. aeruginosa at the three cell densities were 9, 63, and 112 μg Cu/L, respectively; and were 192, 1873, and 4619 μg Cu/L for P. subcapitata. The 96-h EC50 values of Cu-EA were 101 and 2579 μg Cu/L for M. aeruginosa and P. subcapitata at 106 cells/mL. The margin of safety (MOS) for P. subcapitata at 104 cells/mL was 1.3, 0.9, and 0.8 when M. aeruginosa cell density was 104, 105, and 106 cells/mL. This laboratory study suggests that applying copper-based algaecides to control problematic algae at a relatively low cell density would inhibit their growth with minimum impacts on non-target algae; risks to non-target algae would increase with increases of problematic algal cell density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Excessive growth of blue-green algae or green algae can interfere with the uses of water resources (Chislock et al., 2013). Toxin-producing blue-green algae, particularly prokaryotic cyanobacteria Microcystis aeruginosa, in freshwater could grow up to a cell density of 109 cells/mL (Zohary and Pais-Madeira 1990) and often produce cyanotoxins (e.g., microcystin), which cause death in aquatic and terrestrial animals as well as creating problems for public drinking waters (Svrcek and Smith, 2004, Otten and Paerl, 2015). A provisional drinking water guideline of 1 μg/L (as total microcystin-LR, L: leucine, R: arginine) has been adopted by the World Health Organization (World Health Organization, 2011). Green algae, for example eukaryotic alga Pseudokirchneriella subcapitata, are usually relatively preferable to blue-green algae in water bodies since they are not commonly associated with toxin production problems and are a better base for aquatic food chains than blue-green algae (Paeri and Tucker 1995; Schrader et al., 1998). However, overgrowth of green algae can hamper or prevent usages of infested water resources such as discoloration and clogging of filters. When utilization of critical water resources is interfered by those problematic algae, immediate actions must be taken to control the growth of those algae in order to restore critical water resources.
Options for controlling problematic algae include mechanical, physical, biological, and chemical approaches (Timmons, 2005, Armellina et al., 1996, Gumbo et al., 2008; Calomeni et al., 2015a). Chemicals or algaecide applications are usually used to manage algal densities because of their effectiveness (Whitaker et al., 1978; Lam et al., 1995; Schrader and Dennis, 2005; Hoko and Makado, 2011). Copper is an active ingredient of many algaecides widely used to mitigate risks of toxin-producing cyanobacteria and problematic green algae in aquatic environments (Gibson, 1972; McKnight et al., 1983; Schrader and Dennis, 2005; Hoko and Makado, 2011). Toxicity of copper compounds to algae can be due to several forms, such as cupric ion hydroxy- or carbonate-Cu (Flemming and Trevors, 1989). Several studies have shown that exposures of different forms of copper-based algaecides cause different responses in target algal species (Murray-Gulde et al., 2002; Rodgers et al., 2010; Bishop and Rodgers, 2012). When copper compounds are used as aquatic algaecides, the cupric ion is usually bound by chelators (e.g., ethanolamine or citrate) to prevent precipitation or complexation from ions in water, which could have influences on the algaecide effectiveness for controlling algal growth.
Although copper-based algaecides have been used for decades, public concerns regarding their negative impacts on non-targeted species still persist. Target algae management in water resources is an adaptive technique to proactively inhibit problematic algal growth and minimize adverse effects on non-target algae. In order to effectively manage target algae in water through using copper-based algaecide, understanding the relative sensitivity of target and non-target algae at different densities to copper exposures is critical. Algal population sensitivity to chemical exposures depends on algal species. Given different algal species exposed to copper, the variation in algal sensitivity to the copper exposure could be as great as five orders of magnitude (Blanck et al., 1984; Kasai et al., 1993; Rojíčková and Maršálek, 1999; Ma, 2005). Cyanobacteria were found to be the most copper-sensitive phytoplanktonic to the application of copper sulfate (Le Jeune et al., 2006). Gregor et al. (2008) also observed that the cyanobacteria Aphanothece clathrata were more sensitive to copper sulfate than the green algae P. subcapitata.
Both target and non-target algal species may have broad ranges of cell densities in water bodies. Freshwater algal sensitivity to copper exposure decreases when algal cell density increases because greater adsorption by algal cells results in depletion of the equilibrium concentration of dissolved copper in the solution and provides a greater surface area which effectively dilutes the concentration of copper per algae cell (Franklin et al., 2002; Moreno-Garrido et al., 2000). In addition, the release of algal exudates into the solution could be responsible for reducing copper bioavailability in solutions (Franklin et al., 2002). Therefore, algal population with higher cell density may result in a higher exudate concentration in the solution compared to the situation of lower cell density, causing an increase in the complexation of copper in solution. Murray-Gulde et al. (2002) also found the amount of copper bound by each algal cell was related to the initial algal cell density; which indicates that as algal density increases, a concomitant decrease in the efficacy of algaecide in controlling algal growth would be expected at the same concentration of copper.
For target algal management with treatment of copper-based algaecide, it is essential to maximize effectiveness of algaecide and minimize risks for non-target algal species. Comprehensive knowledge regarding target and non-target algal species’ responses to copper exposures is needed to understand how to efficiently use copper-based algaecide. Predictions in responses of different algal species at different cell densities to copper formulations in laboratory experiments can provide useful information for selective control of algae using copper-based algaecides applied in site waters. Without initial laboratory experiments to guide the field algaecide application, excessive amounts of an effective algaecide or a less effective algaecide may be applied (Bishop and Rodgers, 2011). The specific objectives of this study were as follows: (1) to measure responses of M. aeruginosa and P. subcapitata cultured in BG11 medium to 96-h exposures of non-chelated copper compound (copper sulfate) and chelated copper compound (copper-ethanolamine) at three different initial cell densities; (2) to compare the sensitivity of M. aeruginosa and P. subcapitata to the exposures of copper sulfate and copper-ethanolamine; (3) to compare the effects of copper sulfate and copper-ethanolamine on M. aeruginosa and P. subcapitata; and (4) to assess potential risks for non-target algal species (P. subcapitata) when copper sulfate and copper-ethanolamine were used to control M. aeruginosa at different cell densities.
2 Materials and Methods
2.1 Algal Culture
M. aeruginosa UTEX 2385 and P. subcapitata UTEX 1648 (University of Texas at Austin, Austin, TX) were cultured in BG11 medium (Stanier et al., 1971) separately and maintained at a temperature of 24 ± 2 °C and a 12:12-h light-dark photoperiod illuminated by cool white fluorescent lighting at an intensity of 2100 lux. The three different initial cell densities for M. aeruginosa and P. subcapitata used in this study were 5 × 104, 5 × 105, and 5 × 106 cells/mL, which were represented as low, medium, and high initial cell density, respectively. At the initiation of experiment, water characteristics of the algal growth solution (BG11 medium) were as follows: temperature = 24 ± 2 °C; dissolved oxygen = 8.7-9.5 mg/L; pH = 7.8–8.0; conductivity = 1710–1730 μS/cm; alkalinity = 40 mg/L as CaCO3; hardness = 40 mg/L as CaCO3.
2.2 Preparation of Copper Compounds
Two copper compounds were used in this study: a copper salt (copper sulfate pentahydrate) (Sigma Chemical Co., St. Louis, MO, USA) and an ethanolamine-chelated copper compound (Cutrine®-Plus) (Lonza Inc., Germantown, WI, USA). The characteristics of copper sulfate and copper-ethanolamine (Cu-EA) are showed in Table 1. A stock solution (1000 mg Cu/L) was prepared 1 h prior to experiment initiation for each algaecide using NANOpureTM water (Thermo Fisher Scientific Inc., Waltham, MA). Algal toxicity experiments in this study were conducted using three replicates of treatment concentrations ranged from 5 to 20,000 μg Cu/L as copper sulfate, Cu-EA, and an untreated control. Experimental chambers consisted of static, nonrenewal 200 ml volumes of treated and untreated algae in culture solution contained in Erlenmeyer flasks. A 96-h exposure time was used in this study because statistically different temporal changes in cell densities were observed on the fourth day in preliminary experiments. The experimental conditions were the same for the algal cultures. Copper concentrations were measured as acid-soluble copper using a graphite furnace atomic absorption spectrometer (AA280FS, Agilent Technologies) (Method 3010-B; APHA, 2005). Water characteristics (pH, dissolved oxygen, conductivity, total alkalinity, total hardness, and temperature) were measured according to APHA (2005) methods.
2.3 Measurements of Responses of M. aeruginosa and P. subcapitata to Copper Compounds
Responses of M. aeruginosa and P. subcapitata to 96-h exposures of copper sulfate and Cu-EA were measured everyday by cell density and chlorophyll a concentration. Cell densities were measured using light microscopy with a Leitz Dialux 20 microscope (Leitz USA Scopes, Paramount, CA, USA) at ×400 magnification and an Improved Neubauer hemocytometer. Chlorophyll a concentrations were analyzed using a SpectraMax® 190 Gemini 96-well plate spectrofluorometer (Molecular Devices Corporation, Sunnyvale, CA) according to standard methods (APHA, 2005). The inhibition of chlorophyll a concentration and cell density after 96-h exposures of copper sulfate and Cu-EA was calculated as follows:
2.4 Assessment of Risks for P. subcapitata Exposed to Copper Required for Controlling M. aeruginosa at Different Cell Densities
For target algal management using copper-based algaecide, in addition to effectively control target algae, it is important to consider the margin of safety (MOS) for non-target algae, especially when they have different cell densities. Effective copper concentrations for controlling target algae (M. aeruginosa) were compared to toxicity data of non-target alga (P. subcapitata) to calculate margin of safety. The MOS was calculated as follows:
In this equation, the effective concentration for adverse effects on P. subcapitata was represented by the EC50 value (effective concentration for 50 % of the population), and the effective concentration for controlling M. aeruginosa was represented by the EC100 value (effective concentration for 100 % of the population). A MOS ≤ 1 indicates potential risks for P. subcapitata, while a MOS > 1 indicates less potential for adverse effects to P. subcapitata.
2.5 Statistical Analysis
A one-way analysis of variance (ANOVA) was used to determine differences in cell densities and chlorophyll a concentrations between the treatments and the untreated control. Differences were further discerned using Tukey’s test. The results were considered to be significantly different if the p value was less than 0.05. Ninety-six-hour EC50 and EC100 values were calculated using SAS 9.3 for probit analyses.
3 Results and Discussion
3.1 Sensitivity of Algae to Exposures of Copper Compounds at Different Cell Densities
Nominal copper concentrations of copper sulfate and Cu-EA for M. aeruginosa toxicity experiments ranged from 5 to 40, 40 to 120, and 80 to 160 μg Cu/L for the low, medium, and high initial cell density, respectively, and it ranged from 60 to 750, 500 to 10,000, and 2500 to 20,000 μg Cu/L, respectively, for the P. subcapitata toxicity experiments. There were no significant differences in nominal and measured copper concentrations; therefore, all results are reported as nominal concentrations.
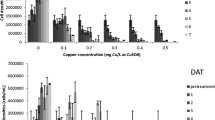
Cell densities consistently increased with time throughout the entire experiment in untreated controls, and cell densities were significantly higher than initial cell densities in the end of both algal toxicity experiments (Fig. 1). For untreated control in the end of experiment, cell density of M. aeruginosa was 1.8 × 105, 2.2 × 106, and 2.2 × 107 cells/mL for low, medium, and high initial cell density, respectively (Fig. 1a); and cell density of P. subcapitata was 7 × 105, 8 × 106, and 5 × 107 cells/mL, which was an order of magnitude higher than their respective initial cell density (Fig. 1b). M. aeruginosa cells usually occur at a low cell density (<102 cells/mL) in most surface water. Yen et al. (2012) found that M. aeruginosa cells in the reservoir could grow rapidly with doubling time of 2.38 days. Therefore, it is expected that formation of M. aeruginosa scum could (>106 cells/mL) occur in favorable environmental conditions if treatment for its growth inhibition is not involved.
Based on the Eqs. (1) and (2), inhibitions of algal cell density and chlorophyll a concentration after 96-h exposures of copper were calculated, and relationships in copper exposure concentration and inhibitions of chlorophyll a and cell density of M. aeruginosa and P. subcapitata were illustrated in Fig. 2. It showed that copper exposures caused different degrees of inhibition in algal chlorophyll a concentration and population. Noticeably, regardless of algal species in this study, the copper concentrations required to elicit the same algal responses increased with increases of initial cell density. For example, after 96-h exposures of copper sulfate, estimated EC50 values for M. aeruginosa as determined by chlorophyll a concentration increased from 9 to 112 μg Cu/L as cell density increased from low to high (Table 2). Similar results were also observed in the P. subcapitata toxicity experiment. These results imply that algal cell density could alter the distribution of copper to algal cells, and the minimum amount of copper required to achieve control of algae should be proportional to cell density (Murray-Gulde et al., 2002).
The World Health Organization (WHO) guidance values for the relative probability of acute health effects during recreational exposures to cyanobacteria and microcystin are based on cyanobacterial cell densities (Chorus and Bartram, 1999). According to the WHO guidance, cyanobacterial cell densities of 2 × 104 and 107 cells/mL represent guidelines for low and high health risk level in recreational waters, respectively. Tsai (2015) showed that if no treatment is implemented to reduce cell density of microcystin-producing M. aeruginosa, total microcystin concentration would increase with growing of M. aeruginosa population and potentially enhance the risks associated with exposures of microcystin (Pilotto et al., 1997). Since the minimum amount of copper required for controlling problematic algae is density-dependent, early treatments for controlling toxin-producing cyanobacteria at relatively low cell density with copper-based algaecide can not only minimize the amount of copper applied, but it would also reduce the risks related to exposures of cyanotoxins. However, if the treatment is not implemented at an early stage when algal cell density is low, as the algal population increases, the amount of copper required for controlling problematic algae may exceed the maximum label concentration of copper. This study showed that as cell density increased from low to high, the EC50 values for P. subcapitata following 96-h exposure of copper sulfate as determined by chlorophyll a concentration increased from 192 and 4619 μg Cu/L, which is higher than the maximum label concentration of 2000 μg Cu/L (Tables 1 and 2).
Inhibitions of M. aeruginosa and P. subcapitata after exposures of copper sulfate and Cu-EA in terms of cell density paralleled the inhibitions of chlorophyll a concentration (Fig. 2). Calomeni and Rodgers (2015b) also found that cell densities of viable M. aeruginosa and P. subcapitata were highly correlated to chlorophyll a concentrations with R 2 of 0.99 and 0.97, respectively. Blue-green algae and green algae may co-exist in natural waters; in order to discern the effects of copper-based algaecide on target and non-target algae, Calomeni and Rodgers (2015b) suggested that chlorophyll a concentration can be used as a response endpoint for algal toxicity experiments along with microscopic observations of algal assemblages in the sample as algal dynamics may change following exposures to chemicals. Importantly, measures of algal cell density and chlorophyll a concentration sometimes may not be adequate measurements to accurately determine the effectiveness of algaecide. For example, cell density measurements cannot reflect the cell’s morphology and physiology such as deformed cells and changes of cell volume, which would lead to underestimate the algaecide effectiveness (Chao and Chen, 2001). Measuring chlorophyll a concentration may have interferences by other pigments (e.g., pheophytins), resulting in overestimation of algal viability (APHA, 2005). In order to better understand algal responses to algaecide exposures, additional measures such as flow cytometers, electron microscopes, and fluorescent microscopes may be needed.
Using chlorophyll a concentration as a response endpoint of algal toxicity experiments for low initial cell density, 96-h estimated EC50 value for M. aeruginosa exposed to copper sulfate (9 μg Cu/L) was 20 times lower than the value for P. subcapitata (192 μg Cu/L) (Table 2). Likewise, regardless of copper compounds in this study, 96-h estimated EC50 values for M. aeruginosa were significantly lower than the values for P. subcapitata when their cell densities were medium or high. Similar results were also observed when cell density was used as a response endpoint (Table 2). These results showed that M. aeruginosa was an order of magnitude more sensitive to copper compounds than P. subcapitata when they had the same cell density. These significant differences could be due to the cell’s weight and size. In this study the initial average cell mass of M. aeruginosa and P. subcapitata was 15 and 32 pg/cell, and cell volume was 255 and 416 μm3/cell, respectively. Previous studies have showed that algal cells with larger weight and volume would result in lower loading of toxicant per unit of biomass and volume (Stratton and Gilles, 1990; Chao and Chen, 2000; Lin et al., 2007). Additionally, since algal cell walls are the most outer contact site for its interaction with copper, characteristics of algal cell wall surface could influence algal sensitivity to copper exposures. It was assumed that copper bounds more strongly to the mucopolysaccharide cell wall of cyanobacteria (e.g., M. aeruginosa) than to the cellulose cell wall of eukaryotic algae (e.g., P. subcapitata), resulting in stronger interference with cell surface properties and functions of cyanobacteria compared to eukaryotic algae (Brand et al., 1986). Furthermore, Hadjoudja et al. (2009) found that cyanobacteria Microcystis aeruginosa was more sensitive to copper than the green algae Chlorella vulgaris, which was likely due to a lower hydrophobic character and more carboxyl groups on the M. aeruginosa cell surface, which resulted in higher copper binding ability on M. aeruginosa than on C. vulgaris.
3.2 Comparative Toxicity of Copper Compounds to Algae
Comparative 96-h toxicity of copper sulfate and Cu-EA to M. aeruginosa and P. subcapitata at different cell densities was determined by EC50 values. Except for the EC50 values determined by chlorophyll a concentration at low initial cell density, Cu-EA showed significantly lower EC50 values to M. aeruginosa and P. subcapitata compared to copper sulfate (Table 2). For example, EC50 values of Cu-EA and copper sulfate as determined by chlorophyll a concentration for M. aeruginosa were 54 and 63 μg Cu/L at medium initial cell density, respectively. The results suggested that the 96-h toxicities of Cu-EA to both algae were generally greater than copper sulfate.
Toxicity of copper to algae occurs through several mechanisms, such as formation of stable complexes with non-copper proteins, interference with electron transport, and oxidative stress. Those mechanisms can result in different algal cell dysfunctions such as inhibition of photosynthesis and suppression of cell division (Stauber and Florence, 1987; Gledhill et al., 1997). Copper-based algaecides vary in their potency for a given algal species. Comparing the effectiveness of copper salt and chelated copper for the control of algae, chelated copper formulations can be uptaken by passive transport, resulting in more copper entering internal sites of action and achieving control with lower amounts of copper than copper sulfate (Stauber and Florence, 1987).
In addition to this study, Rodgers et al. (2010) also found that copper-ethanolamine complex was more effective for controlling the problematic alga Prymnesium parvum at 103–104 cells/mL than copper sulfate in laboratory studies. Importantly, copper concentration and formulation play important roles for controlling problematic algae. According to the study by Murray-Gulde et al. (2002), chelated copper-based algaecides could provide a higher concentration of copper in the water column for a longer period of time and loss to the sediment is slower than for a non-chelated copper-based algaecide such as copper sulfate. Currently, Calomeni et al. (2014) showed that the potency slope of chelated copper-based algaecide was roughly twice as potent as copper sulfate for P. subcapitata and Planktothrix agardhii (both cell densities were approximately 106 cells/mL). Although those results suggested that less copper could be applied as a chelated form to elicit the same results as copper sulfate, this study showed that relative effectiveness of copper compounds to algae could be cell density-dependent. When cell densities were low, the toxicity of Cu-EA to chlorophyll a concentration was not significantly higher than copper sulfate; but when cell densities were medium or high, the toxicity of Cu-EA to chlorophyll a concentration was significantly higher than copper sulfate.
3.3 Margins of Safety for P. subcapitata at Different Cell Densities
P. subcapitata is a widely distributed freshwater green alga and commonly used for algal toxicity experiments (Franklin et al., 2002; Tsai and Chen, 2007; Calomeni et al. 2014); therefore, it was also chosen as an example of non-target algal species in this study to estimate potential risks (i.e., margins of safety) after exposures of copper used for controlling M. aeruginosa. Results of this study showed that chlorophyll a concentration was a more sensitive response endpoint than cell density in P. subcapitata toxicity experiments at low and high initial cell densities (i.e., estimated EC50 values determined by chlorophyll a at low and high initial cell densities were lower than by cell density) (Table 2), Therefore, margins of safety for P. subcapitata at different cell densities after copper exposures were determined by effective copper concentrations on chlorophyll a concentration (Table 3).
Regardless of M. aeruginosa cell density, margins of safety for P. subcapitata after 96-h exposures of copper sulfate and Cu-EA were enhanced with increases of its cell density (Table 3). For example, when M. aeruginosa cell density was high, margin of safety for P. subcapitata exposed to copper sulfate increased from 0.8 to 19.8 as cell density increased from low to high. Similar results were also observed when M. aeruginosa cell density was low or medium. In spite of P. subcapitata cell density, the margins of safety for P. subcapitata decreased with increases of M. aeruginosa cell density. For example, when exposed to copper sulfate, margin of safety for P. subcapitata at low cell density declined from 1.3 to 0.9 and 0.8 when M. aeruginosa cell density increased from low to medium and high. Similar results were also observed when P. subcapitata cell density was medium or high.
According to the Eq. (3), a MOS ≤ 1 indicates potential for risks for P. subcapitata, while a MOS > 1 indicates less potential for adverse effects to P. subcapitata. When P. subcapitata cell density was equal to or higher than M. aeruginosa cell density (bold numbers in Table 3), there was less potential for adverse effects to P. subcapitata, since the margins of safety for P. subcapitata exposed to both copper compounds were greater than 1. On the contrary, when P. subcapitata cell density was lower than M. aeruginosa cell density, there could be potential for risks for P. subcapitata, since the margins of safety were less than 1 (numbers with underline in Table 3). The results were concomitant with the relationships of copper exposure concentration and growth inhibition of algae (Fig. 2), where overlapped responses of M. aeruginosa (at medium and high initial cell densities) and P. subcapitata (at low initial cell density) to copper exposures were observed. Therefore, these results suggested that controlling M. aeruginosa by using copper-based algaecide when its cell density is relatively lower than non-target algae could enhance the margin of safety for non-target species.
In the Eq. (3), it is noted that the minimum amount of copper required for controlling M. aeruginosa can significantly affect margins of safety for P. subcapitata. In this study, the minimum amount of copper as copper sulfate used for controlling M. aeruginosa at low cell density was 152 μg Cu/L, and the copper concentration causing 50 % inhibition of P. subcapitata at low cell density was 192 μg Cu/L as copper sulfate. In this case, the MOS for P. subcapitata was 1.3 (i.e., 192/152). Although M. aeruginosa can be controlled by any copper concentration greater than 152 μg Cu/L as copper sulfate, the MOS for P. subcapitata will decrease when M. aeruginosa is treated by any copper concentrations greater than 152 μg Cu/L. Furthermore, an excessive amount of copper applied for control of noxious algae in the field could also exacerbate adverse effects on non-target species. Tsai (2015) showed that applying an excessive amount copper for controlling microcystin-producing M. aeruginosa could result in the release of relatively more microcystin compared to applying a minimum amount of copper. Microcystin released from M. aeruginosa could have allelopathic effects on aquatic phytoplankton and creatures (Pflugmacher, 2002; LeBlanc et al., 2005; Ma et al., 2015).
The ultimate goal of target algal management through the use of copper-based algaecide is to effectively control problematic algae and at the same time maximize margins of safety for non-target species. Since P. subcapitata is less sensitive than M. aeruginosa to copper exposures, this study showed that the risks for P. subcapitata (i.e., margins of safety < 1) exposed to copper concentration required for controlling M. aeruginosa were only observed when its cell density was low. In the field, algal species in aquatic systems may have a wide range of sensitivity to copper exposures (Blanck et al., 1984). Besides, margins of safety for non-target species differ by forms of copper as well as species. Murray-Gulde et al. (2002) reported that Cutrine-Plus had a greater margin of safety for freshwater fish Pimephales promelas than copper sulfate. In some cases, non-target species may be more sensitive than target algae to copper exposures, resulting in a margin of safety that is less than 1. For example, noxious algae P. parvum has been related with fish kills in brackish inland waters (Lindholm et al., 1999; Roelke et al., 2010; Brooks et al., 2011; Umphres et al., 2012). Rodgers et al. (2010) reported that the margin of safety for P. promelas and freshwater invertebrate Ceriodaphnia dubia after 96-h exposure to Cutrine-Plus required for controlling P. parvum was 4.3 and 0.6, respectively. Under this circumstance, authorities considering applications of copper formulations for controlling problematic algae will have to consider the relative risks of controlling problematic algae.
An appropriate formulation and concentration of copper applied for controlling problematic algae differ from site to site and therefore need to be carefully determined, because it depends on several factors such as target algal cell density, target and non-target species sensitivity to copper, site water characteristics, and copper-based algaecide application history (Bishop and Rodgers, 2012; Calomeni et al., 2015a).
4 Conclusions
This bench-scale study demonstrates a conceptual model contrasting the responses of prokaryotic and eukaryotic algae at different cell densities to two copper compounds. At the same cell density, M. aeruginosa was more sensitive than P. subcapitata to copper exposures. Estimated 96-h EC50 values for M. aeruginosa and P. subcapitata after copper exposures are dependent on cell density, which suggests that the amount of copper required for controlling algae is positively related to cell density. Consequently, the minimum amount of copper required for controlling algae at high cell density such as algal scum may exceed maximum label copper concentration. The margin of safety for P. subcapitata decreases with the increase of M. aeruginosa cell density. Applying a minimum amount of copper required for controlling problematic algae can maximize margins of safety for non-target species. When problematic algal cell density is lower than non-target algal cell density, treatment with an appropriate amount of copper can maximize the efficacy for target species while minimize risks to non-target species. Under these experimental conditions, 96-h toxicities of Cu-EA to M. aeruginosa and P. subcapitata at medium and high cell densities are greater than copper sulfate, indicating that less copper could be applied as a chelated algaecide to elicit the same effect as a copper salt and potentially protect non-target species. Overall, this study suggests that applying copper-based algaecides to control problematic algae at a relatively low cell density would inhibit their growth with minimum impacts on non-target algae; risks to non-target algae would increase with increases of problematic algal cell density.
References
APHA (American Public Health Association). (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington, DC.
Armellina, A. D., Bezic, C. R., & Gajardo, O. A. (1996). Propagation and mechanical control of Potamogeton illinoensis Morong in irrigation canals in Argentina. Journal of Aquatic Plant Management, 34, 12–16.
Bishop, W. M., & Rodgers, J. H., Jr. (2011). Responses of Lyngbya magnifica Gardner to an algaecide exposure in the laboratory and field. Ecotoxicology and Environmental Safety, 74, 1832–1838.
Bishop, W. M., & Rodgers, J. H., Jr. (2012). Responses of Lyngbya wollei to exposures of copper-based algaecides: the critical burden concept. Archives of Environmental Contamination and Toxicology, 62, 403–410.
Blanck, H., Wallin, G., & Wängberg, S.-A. (1984). Species-dependent variation in algal sensitivity to chemical compounds. Ecotoxicology and Environmental Safety, 8, 339–351.
Brand, L. E., Sunda, W. G., & Guillard, R. R. L. (1986). Reduction of marine phytoplankton reproduction rate by copper and cadmium. Journal of Experimental Marine Biology and Ecology, 96, 225–250.
Brooks, B. W., Grover, J. P., & Roelke, D. L. (2011). Prymnesium parvum: an emerging threat to inland waters. Environmental Toxicology and Chemistry, 30, 1955–1964.
Calomeni, A.J., Iwinski, K.J., Kinley, C.M., McQueen, A.D., Rodgers, Jr. J.H., 2015a. Responses of Lyngbya wollei to algaecide exposures and a risk characterization associated with their use. Ecotoxicology and Environmental Safety. 116, 90-98.
Calomeni, A.J., Rodgers, Jr. J.H., 2015b. Evaluation of the utility of six measures for algal (Microcystis aeruginosa, Planktothrix agardhii and Pseudokirchneriella subcapitata) viability. Ecotoxicology and Environmental Safety. 111, 192-198.
Calomeni, A. J., Rodgers, J. H., Jr., & Kinley, C. M. (2014). Responses of Planktothrix agardhii and Pseudokirchneriella subcapitata to copper sulfate (CuSO4 · 5H2O) and a chelated copper compound (Cutrine-Ultra). Water, Air, and Soil Pollution, 225, 1–15.
Chao, M. R., & Chen, C. Y. (2000). Effects of inoculum’s mean cell volume on algal toxicity tests. Water Science and Technology, 42(7-8), 291–296.
Chao, M. R., & Chen, C. Y. (2001). Discrepancies between different response parameters in batch and continuous algal toxicity tests. Journal of Hazardous Materials, 82, 129–136.
Chislock, M. F., Doster, E., Zitomer, R. A., & Wilson, A. E. (2013). Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nature Education Knowledge, 4, 10.
Chorus, I., & Bartram, J. (1999). Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. London, United Kingdom: World Health Organization (WHO).
Flemming, C. A., & Trevors, J. T. (1989). Copper toxicity and chemistry in the environment: a review. Water, Air, and Soil Pollution, 44, 143–158.
Franklin, N. M., Stauber, J. L., Apte, S. C., & Lim, R. P. (2002). Effect of initial cell density on the bioavailability and toxicity of copper in microalgal bioassays. Environmental Toxicology and Chemistry, 21, 742–751.
Gibson, C. E. (1972). The algicidal effect of copper on a green and blue-green alga and some ecological implications. Journal of Applied Ecology, 9, 513–518.
Gledhill, M., Nimmo, M., Hill, S. J., & Brown, M. T. (1997). The toxicity of copper (II) to marine algae, with particular reference to macroalgae. Journal of Phycology, 33, 2–11.
Gregor, J., Jancula, D., & Marsaalek, B. (2008). Growth assays with mixed cultures of cyanobacteria and algae assessed by in vivo fluorescence: one step closer to real ecosystems? Chemosphere, 70, 1873–1878.
Gumbo, R. J., Ross, G., & Cloete, E. T. (2008). Biological control of Microcystis dominated harmful algal blooms. African Journal of Biotechnology, 7(25), 4765–4773.
Hadjoudja, S., Vignoles, C., Deluchat, V., Lenain, J. F., Le Jeune, A. H., & Baudu, M. (2009). Short term copper toxicity on Microcystis aeruginosa and Chlorella vulgaris using flow cytometry. Aquatic Toxicology, 94, 255–264.
Hoko, Z., & Makado, P. K. (2011). Optimization of algal removal process at Morton Jaffray water works, Harare, Zimbabwe. Physics and Chemistry of the Earth, 36, 1141–1150.
Kamrin, M. A. (1997). Pesticide profiles: toxicity, environmental impact, and fate. Boca Raton, FL: Lewis Publishers.
Kasai, F., Takamura, N., & Hatakeyama, S. (1993). Effects of simetryne on growth of various freshwater taxa. Environmental Pollution, 79, 77–83.
Lam, A. K. Y., Prepas, E. E., Spink, D., & Hrudey, S. E. (1995). Chemical control of hepatotoxic phytoplankton blooms: implications for human health. Water Research, 29, 1845–1854.
Le Jeune, A. H., Charpin, M., Deluchat, V., Briand, J. F., Lenain, J. F., Baudu, M., & Amblard, C. (2006). Effect of copper sulfate treatment on natural phytoplanktonic communities. Aquatic Toxicology, 80, 267–280.
LeBlanc, S., Pick, F. R., & Aranda-Rodriguez, R. (2005). Allelopathic effects of the toxic cyanobacterium Microcystis aeruginosa on duckweed, Lemna gibba L. Environmental Toxicology, 20(1), 67–73.
Lin, K. C., Lee, Y. L., & Chen, C. Y. (2007). Metal toxicity to Chlorella pyrenoidosa assessed by a short-term continuous test. Journal of Hazardous Materials, 142, 236–241.
Lindholm, T., Öhman, P., Kurki-Helasmo, K., Kincaid, B., & Meriluoto, J. (1999). Toxic algae and fish mortality in a brackish-water lake in Åland, SW Finland. Hydrobiologia, 397, 109–120.
Ma, J. (2005). Differential sensitivity of three cyanobacterial and five green algal species to organotins and pyrethroids pesticides. Science of the Total Environment, 341, 109–117.
Ma, Z., Fang, T., Thring, R. W., Li, Y., Yu, H., Zhou, Q., & Zhao, M. (2015). Toxic and non-toxic strains of Microcystis aeruginosa induce temperature dependent allelopathy toward growth and photosynthesis of Chlorella vulgaris. Harmful Algae, 48, 21–29.
McKnight, D. M., Chisholm, S. W., & Harleman, D. R. F. (1983). CuSO4 treatment of nuisance algal blooms in drinking water reservoirs. Environmental Management, 7, 311–320.
Moreno-Garrido, I., Lubian, L. M., & Soares, A. M. V. M. (2000). Influence of cellular density on determination of EC50 in microalgal growth inhibition tests. Ecotoxicology and Environmental Safety, 47, 112–116.
Murray-Gulde, C. L., Heatley, J. E., Schwartzman, A. L., & Rodgers, J. H., Jr. (2002). Algicidal effectiveness of Clearigate, Cutrine-Plus, and copper sulfate and margins of safety associated with their use. Archives of Environmental Contamination and Toxicology, 43, 19–27.
Otten, T. G., & Paerl, H. W. (2015). Health effects of toxic cyanobacteria in U.S. drinking and recreational waters: our current understanding and proposed direction. Curr Environ Health Reports, 2, 75–84.
Paeri, H. W., & Tucker, C. S. (1995). Ecology of blue-green algae in aquaculture ponds. Journal of the World Aquaculture Society, 26, 109–131.
Pflugmacher, S. (2002). Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environmental Toxicology, 17, 407–413.
Pilotto, L. S., Douglas, R. M., Burch, M. D., Cameron, S., Beers, M., Rouch, G. R., Robinson, P., Kirk, M., Cowie, C. T., Hardiman, S., Moore, C., & Attewell, R. G. (1997). Health effects of recreational exposure to cyanobacteria (blue-green algae) during recreational water-related activities. Australian and New Zealand Journal of Public Health, 21, 562–566.
Rodgers, J. H., Jr., Johnson, B. M., & Bishop, W. M. (2010). Comparison of three algaecides for controlling the density of Prymnesium parvum. Journal of American Water Resources Association, 46, 153–160.
Roelke, D. L., Grover, J. P., Brooks, B. W., Glass, J., Buzan, D., Southard, G. M., Fries, L., Gable, G. M., Schwwierzke-Wade, L., Byrd, M., & Nelson, J. (2010). A decade of fish-killing Prymnesium parvum blooms in Texas: roles of inflow and salinity. Journal of Plankton Research, 33, 243–254.
Rojíčková, R., & Maršálek, B. (1999). Selection and sensitivity comparisons of algal species for toxicity testing. Chemosphere, 14, 3329–3338.
Schrader, K. K., de Regt, M. Q., Tidwell, P. D., Tucker, C. S., & Duke, S. O. (1998). Compounds with selective toxicity towards the off-flavor metabolite-producing cyanobacterium Oscillatoria cf. chalybea. Aquaculture, 163, 85–99.
Schrader, K. K., & Dennis, M. E. (2005). Cyanobacteria and earthy/musty compounds found in commercial catfish (Ictalurus punctatus) ponds in the Mississippi Delta and Mississippi-Alabama Blackland Prairie. Water Research, 39, 2807–2814.
Stanier, R. Y., Kunisawa, R., Mandel, M., & Cohen-Bazire, G. (1971). Purification and properties of unicellular blue-green algae (Order Chroococcales). Bacteriological Reviews, 35, 171–205.
Stauber, J. L., & Florence, T. M. (1987). Mechanism of toxicity of ionic copper and copper complexes to algae. Marine Biology, 94, 511–519.
Stratton, G. W., & Gilles, J. (1990). Importance of bioassay volume in toxicity tests using algae and aquatic invertebrates. Bulletin of Environmental Contamination and Toxicology, 44, 420–427.
Svrcek, C., & Smith, D. W. (2004). Cyanobacteria toxins and the current state of knowledge on water treatment options: a review. Journal of Environmental Engineering and Science, 3, 155–185.
Timmons, F. L. (2005). A history of weed control in the United States and Canada. Weed Science, 53, 748–761.
Tsai, K. P. (2015). Effects of two copper compounds on Microcystis aeruginosa cell density, membrane integrity, and microcystin release. Ecotoxicology and Environmental Safety, 120, 428–435.
Tsai, K. P., & Chen, C. Y. (2007). An algal toxicity database of organic toxicants derived by a closed-system technique. Environmental Toxicology and Chemistry, 26, 1931–1939.
Umphres, G. D., Roelke, D. L., & Netherland, M. D. (2012). A chemical approach for the mitigation of Prymnesium parvum blooms. Toxicon, 60, 1235–1244.
Whitaker, J., Barica, J., Kling, H., & Buckley, M. (1978). Efficacy of copper sulfate in the suppression of Aphanizomenon flos-aquae blooms in prairie lakes. Environmental Pollution, 15, 185–194.
World Health Organization. (2011). Guidelines for drinking-water quality (4th ed.). Geneva, Switzerland: World Health Organization.
Yen, H. K., Lin, T. F., & Tseng, I. C. (2012). Detection and quantification of major toxigenic Microcystis genotypes in Moo-Tan reservoir and associated water treatment plant. Journal of Environmental Monitoring, 14, 687–696.
Zohary, T., & Pais-Madeira, A. M. (1990). Structural, physical, and chemical characteristics of Microcystis aeruginosa hyperscums from a hypertrophic lake. Freshwater Biology, 23, 339–352.
Acknowledgments
I would like to thank John H. Rodgers Jr., Wayne Chao, Ben E. Willis, Alyssa J. Calomeni, Ciera M. Kinley, and Andrew D. McQueen at Clemson University for their advice and assistance. I also thank Jennifer E. Untener for reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• M. aeruginosa and P. subcapitata respond differently to copper sulfate and Cu-EA.
• Target algal cell density could affect margin of safety for a non-target alga.
• Margin of safety for a non-target alga would depend on its cell density.
• Margin of safety for a non-target alga would depend on its sensitivity to copper.
Rights and permissions
About this article
Cite this article
Tsai, KP. Management of Target Algae by Using Copper-Based Algaecides: Effects of Algal Cell Density and Sensitivity to Copper. Water Air Soil Pollut 227, 238 (2016). https://doi.org/10.1007/s11270-016-2926-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2926-8