Abstract
Filamentous mat-forming cyanobacteria are increasingly impairing uses of freshwater resources. To effectively manage, a better understanding of control measures is needed. Copper (Cu)-based algaecide formulations are often applied to reactively control nuisance cyanobacterial blooms. This laboratory research assessed typical field exposure scenarios for the ability of Cu to partition to, and accumulate in Lyngbya wollei. Exposure factors (Cu concentration × duration) of 4, 8, 16, 24, 32 h were tested across three aqueous Cu concentrations (1, 2, 4 ppm). Results indicated that internally accumulated copper correlated with control of L. wollei, independent of adsorbed copper. L. wollei control was determined by filament viability and chlorophyll a concentrations. Similar exposure factors elicited similar internalized copper levels and consequent responses of L. wollei. Ultimately, a “concentration-exposure-time” (CET) model was created to assist water resource managers in selecting an appropriate treatment regime for a specific in-water infestation. By assessing the exposure concentration and duration required to achieve the internal threshold of copper (i.e., critical burden) that elicits control, water management objectives can be achieved while simultaneously decreasing the environmental loading of copper and potential for non-target species risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Nuisance and noxious algae (including cyanobacteria) have become increasing threats to freshwater ecosystems in the United States and globally (Paerl et al. 2016). Lyngbya wollei Farlow ex Gomont (Speziale and Dyck 1992) is a filamentous, mat-forming cyanobacterium that frequently causes devastating economic and ecological impacts such as impeding fish and wildlife habitat and obviating drinking water supplies (Bishop and Rodgers 2012). Lyngbya wollei can form dense biphasic mats, both benthic and floating, that displace desirable plant and algal species, alter food web structure, cause asphyxia from microbial decomposition of biomass and are aesthetically displeasing (Speziale and Dyck 1992; Bishop and Rodgers 2011). Lyngbya wollei is a prolific producer of aromatic compounds that cause undesirable tastes and odor in drinking water and off-flavor in aquaculture species (Schrader and Blevins 1993). Additionally, an array of toxins can be produced including: neurotoxins (BMAA, anatoxin-a, multiple saxitoxin analogues), hepatotoxins (cylindrospermopsins), dermatotoxins (aplysiatoxin, lyngbyatoxin) and other undescribed or new classes of toxins (anabaenopeptins, lyngbyaur-eidamides; Seifert et al. 2007; Foss et al. 2012; Hudon et al. 2014; Paerl et al. 2016). These toxins can, in part, elicit allelopathic risks to crops (Bhadha et al. 2014) and pose health risks to livestock, wildlife, and humans (Carmichael et al. 2001; Briand et al. 2003). Vijayavel et al. (2013) also found mats to commonly harbor fecal bacteria that can pose health risks to humans.
When L. wollei achieves problematic densities or produces secondary compounds that restrict water uses, aquatic algaecides are often the preferred management strategy due to their rapid activity and ability to at least temporarily alleviate the problem (Bishop and Rodgers 2012). Algaecides have been shown to restore water resource usages when used in accordance with designed management programs (Bishop et al. 2015). Copper (Cu)-based algaecides are commonly used to control problematic algal blooms in surface waters, although the efficacy of a specific treatment may be a function of several factors including: water characteristics, Cu formulation, initial concentration, exposure duration, and characteristics of the alga (Bishop et al. 2014). Laboratory experiments have been used to guide algal management decisions and characterize the algaecide exposure that induces a target alga to respond as desired (Bishop and Rodgers 2012). Accurate prediction of responses to Cu algaecide exposures is needed to increase the efficiency of Cu use, decrease risks to non-target species, comply with regulatory standards and achieve management objectives (Cairns and Pratt 1989; USEPA 2011).
Typically, algaecides are strategically applied directly to algae in water to restore certain water uses (e.g., drinking, irrigation, recreation). Localized spot treatments are also a common application method in ponds and lakes, especially for accumulated mats of algae. Surface spray applications are employed to target buoyant algal accumulations and injection or granular application is often used to target the benthic mats (Bishop et al. 2015). A similar treatment regime is used in canal and flowing water systems where liquid Cu applications are dripped or ‘slugged’ in the canal for an exposure duration that is usually <4 h (SePRO Corporation 2015). In these localized treatment scenarios, there are often large water-chemical dilution potentials from where the product is placed on the algae. Additionally, the United States Environmental Protection Agency (USEPA) approved Cu-based algaecide labels permit <1/2 of the water body to be treated in a single application. This type of treatment may not result in a constant exposure over a longer duration (e.g., 96 h), as used in many standard laboratory toxicity assays to assess organism effects (USEPA 2002). Rather a pulsed, short-term exposure (min to h) of maximum label concentrations may be encountered by the nuisance algae. The short-term interaction between aqueous Cu concentrations and the algal mass is critical in understanding potential for control.
Cu rapidly partitions to algae (Crist et al. 1990), however, only some binding sites may lead to internalization while others do not (Xue and Sigg 1990; Kiefer et al. 1997; Veglio and Beolchini 1997). Although Cu may exert some impacts to algae extracellularly (Sunda and Huntsman 1998), toxicity is typically manifested only after the transfer of Cu into the cell (Stauber and Florence 1987; Stauber and Davies 2000). Bishop et al. (2015) found toxicity to closely correlate to an internal threshold of Cu per unit mass of algae (i.e., a critical burden). The interaction of internalized Cu with cellular components is often irreversible (Yan and Pan 2002; Kaduková and Virčíková 2005) and exerts multiple toxicological responses including: free radical production, enzyme inhibition, and disruption of photosynthesis, respiration, and ATP production (Stauber and Florence 1987). The research presented herein advances the understanding of Cu adsorption and uptake (internalization) kinetics, as related to typical treatment scenarios in field applications with a common Cu-based algaecide. By evaluating the influence of initial Cu concentration and aqueous exposure duration; water resource managers and other decision makers can: (1) better predict the mass of Cu needed for targeted algal control at a specific site, (2) increase efficiency of Cu use, and (3) minimize off-site movement of Cu and potential for non-target organism exposure.
The overall aim of this research was to improve understanding of short-term Cu sorption kinetics and its influence on L. wollei viability. The specific objectives of this research were to: (1) measure Cu adsorption and accumulation in experiments with a series of Cu concentrations and exposure durations; (2) examine the relation between Cu adsorption and internalization to L. wollei chlorophyll a content and filament viability; (3) evaluate the relation between exposure and toxicity (i.e., control) thresholds of L. wollei; and (4) contrast the exposures required to reach the internal threshold of Cu to achieve control of L. wollei.
Materials and Methods
Lyngbya wollei used in experiments was collected by standard rake grabs from Pond 4 (36°02 × 49.3″N; 78°14 × 13.27″W) of the First Fruits Farm near Louisburg, NC. The composite samples were placed into sealable food-grade bags and transported with cool packs in a cooler to the SePRO Research and Technology Campus (SRTC) in Whitakers, NC for testing. Samples were cultured and tested at a constant temperature of 23 ± 1°C and a 16-h light/8-h dark photoperiod with fluorescent lighting (Spectralux T5/HO 6500K blue; 3000K red) at an intensity of 67.5 ± 2.7 µmol photons/m2/s (Lewis et al. 1994). Experiments were conducted with well water that had physicochemical characteristics of pH (8 ± 1), dissolved oxygen (8.5 ± 1 mg O2/L), temperature (22 ± 2°C), conductivity (230–390 μS/cm2), alkalinity (90–140 mg/L as CaCO3), hardness (90–130 mg/L as CaCO3), total phosphorus (90–110 μg/L) and total nitrogen (3.9–4.1 mg/L). Well water was similar to field site water with exception of increased conductivity, alkalinity and hardness. All water chemistry analyses were conducted according to standard methods [American Public Health Association (APHA) 2005]. Replicated experiments (i.e., samples treated similarly as described above) were conducted with samples taken at two time periods (April 5th and August 4th, 2016) from the same sampling site within the pond. Replicate tests were performed to evaluate the temporal consistency of laboratory results, assess potential Lyngbya strain alterations due to laboratory culture conditions, and reinforce predictive capability to the field condition (Lakeman et al. 2009).
During all Cu tests, the mass of L. wollei was held constant at 0.1 g ± 0.01 g wet weight. Six treatment replicates were allocated to each of three aqueous Cu concentrations [1, 2, 4 ppm Cu as Captain XTR (equivalent to 5, 10, 20 mg Cu/g algae)] in 500 mL borosilicate Erlenmeyer flasks. Stock algaecide solutions were prepared within 4 h of test initiation, and serial dilutions were used to obtain treatment Cu concentrations. Treatments were designed to have similar exposure factors [Cu concentration (ppm) × duration (h)] of 4, 8, 16, 24, and 32. The 1 ppm Cu concentration treatments had exposure durations of 4, 8, 16, 24, and 32 h. The 2 ppm Cu concentration treatments had exposure durations of 2, 4, 8, 12, and 16 h. The 4 ppm Cu concentration treatments had exposure durations of 1, 2, 4, 6, and 8 h. After the designated exposure time, the algal mat was gently lifted from the treatment flasks and immediately transferred to 500 mL of untreated water with similar quality and conditions. The control treatment replicate was also transferred similarly to untreated water at the end of each exposure duration.
Initial viability of filaments was assessed on five randomly collected samples prior to treatment initiation and ranged from 89% to 96%. Three treatment replicates were randomly selected for response measurements at 7-day post-treatment. Approximately 0.05 g of Lyngbya from each replicate was taken for filament viability analysis. The subsample was immersed in 5 mL of 0.1% methylene blue for approximately 10 min and trichomes that allowed entry of methylene blue were considered dead (Corradi and Gorbi 1993). Mortality was calculated as the percent of trichomes out of 100 randomly selected that were stained bright blue. The remainder of each treatment replicate was weighed (ww) and frozen at −12°C for a minimum of 24 h before analysis of chlorophyll a. These subsamples were then placed into 10 mL of buffered acetone and ground using a mortar and pestle and allowed to extract for an additional 12–48 h in the freezer prior to analysis (modified from APHA 2005). Chlorophyll a was measured fluorometrically with a Wallac Victor2 spectrofluorometer by correlating with a matrix-matched standard calibration curve (10–640 µg/L; Sigma C-5753). Samples were diluted in the same matrix in order to be encompassed by the standard curve. Chlorophyll a content was calculated as µg/g L. wollei ww.
The other three treatment replicates were used to assess adsorbed and accumulated Cu. Adsorbed Cu was measured by rinsing the L. wollei with 10 mL of 2 mM EDTA for 10 min to remove adhered metal ions from the surface, as EDTA does not penetrate the cell (Knauer et al. 1997). Samples were then filtered (0.22 µm pore size Whatman GF/F glass microfiber filter) and acidified (2% v/v trace-metal-grade nitric acid; Fisher Scientific, Inc. A509) to preserve for later analysis. Internal accumulated Cu was subsequently measured by digesting the rinsed algae in 2 mL of 70% trace-metal-grade nitric acid, 2 mL of 30% H2O2 (Fisher Scientific, Inc. BP2633), and 6 mL NanoPure™ water. The L. wollei was sonicated for 30 s to disperse filaments and then heated at 180°C, and repeated, to achieve a clear solution (Tripathi et al. 2006; USEPA 1996). The mass of accumulated Cu at which mortality (control) was achieved was defined as the critical burden. Initial target Cu concentrations were confirmed by treating separate flasks with the same water volume and type (without algae) with the target Cu concentrations. These were consistently between 94% and 102% of target Cu concentrations, therefore nominal were used in this study. To assess Cu that was depurated from the cyanobacteria, acid-soluble Cu concentrations were measured by taking 15 mL from each treatment, then acidifying to 2% v/v with trace metal grade nitric acid and filtering before analysis. Cu was measured using inductively coupled plasma-optical emission spectrometry (ICPE 9000; Shimadzu Corporation) with a matrix-matched calibration curve from serial dilution of a 1000 ppm Cu standard (Fisher Scientific, Inc. SC194; APHA 2005). The limit of detection for Cu was 5 µg/L (0.1 µg Cu/g cyanobacteria). Method blanks were analyzed with each analytical batch and there was no adventitious contamination detected.
A one-way analysis of variance (ANOVA) was used to discern differences in adsorbed Cu, accumulated Cu, and 7-day median lethal concentrations (LC50) and LC75 values for both chlorophyll a content and filament viability in each experiment. Data from each experiment were pooled for analysis because no significant treatment by experiment results were measured. Differences among treatments were identified using a Tukey’s post-hoc test (α = 0.05). Exposure–response relationships were evaluated using chlorophyll a and filament viability as the response variables. Toxicity values (7-day LC50/LC75) were based on the non-linear regression analyses using an exponential decay, single 3-parameter best fit line [f = y0 + a × exp(−b × x)] by comparison to untreated controls. If toxicity threshold values were similar between response parameters, the average was used to create the CET Model. A Shapiro–Wilk test was used to assess whether the data were normally distributed and had heterogeneous variance (F-test). All data were analyzed with Microsoft Excel (Microsoft 2010) and SigmaPlot® 12.5 (Systat Software 2014).
Results and Discussion
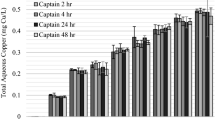
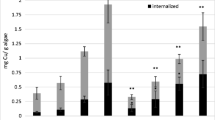
Cu in the 2 and 4 ppm Cu treatments was largely associated with the adsorbed fraction to L. wollei, whereas at 1 ppm, most partitioned Cu was associated with the internal accumulated fraction (Fig. 1). The amount of adsorbed Cu increased with increasing exposure duration or factor in the 2 and 4 ppm Cu treatments, although there were no measured differences in the amount of adsorbed Cu with the 1 ppm treatment at any exposure duration. Despite large differences in adsorbed Cu among all treatments, internal Cu was not significantly different between any treatment at the 4, 16, and 24 factor exposures. The 4 ppm treatment, however, had significantly more internal Cu than either the 1 or 2 ppm treatments at the factor 8 exposure, and the 1 ppm concentration showed significantly less internal Cu at the factor 32 exposure than at the 2 and 4 ppm concentrations. Because the 2 and 4 ppm concentrations at a factor of 32 were not significantly different, and continually increased over lower factor exposures, this suggests that the 1 ppm concentration had exhausted the internal chemical potential with the factor 32 exposure. The amount of Cu that depurated from the L. wollei at experiment completion was <3.2%, 3.8%, and 4.0% of the amended Cu in the 1, 2, and 4 ppm treatments, respectively.
Patterns of adsorbed copper (Cu) (lines) and accumulated internal Cu (bars) to Lyngbya wollei at 7-day following similar factor exposures. Exposure factors consisted of 1, 2, and 4 ppm Cu concentrations over a series of exposure durations. The dashed line represents the critical internal threshold of copper required to elicit the 7-day LC75 toxicity value. Error bars represent 95% confidence intervals around the mean
Depending on actual field application site characteristics (e.g., flow, proportion of area treated, wind intensity and direction, stratification, algal biomass), the exposure duration (i.e., contact time) with the target nuisance algae can be altered. Environmental exposures are dynamic in time and space due to site-specific conditions. It is crucial to understand how exposure times and durations impact sorption of Cu and subsequent control of target nuisance algae. This research was designed to enhance predictions of Cu fate and impact to the target nuisance algal infestation based on representative exposures of many typical field applications scenarios of Cu-based algaecides (i.e., spot treatment, benthic injection, canal drip/slug, partial surface application). Based on the results from these controlled laboratory tests, we found that the required lethal Cu (as Captain XTR) threshold partitions to the cyanobacteria from the water within a brief time frame (e.g., 1.9–14.9 h), depending on the magnitude of the exposure factor, with minimal depuration potential.
Both percent viable filaments and chlorophyll a content decreased as the magnitude of exposure increased (Fig. 2). Chlorophyll a content had a greater variance than percent viable filaments. This finding supports previous research in which percent viable filaments have been observed to be a more sensitive measure of L. wollei response to Cu-based algaecides (Bishop et al. 2015). There were no observed trends with L. wollei response and adsorbed Cu. The quantity of adsorbed Cu was much greater than the internal Cu at the 2 and 4 ppm concentrations, with no increase in efficacy (Fig. 1). Adsorbed Cu at the 1 ppm concentration was similar with all factor exposures tested, although the internal Cu increased with factor and was proportional with Lyngbya responses. Our results indicate that internal accumulated Cu correlated with control of L. wollei and adsorbed Cu was not representative of Captain XTR efficacy. This internal Cu correlation has similarly been found in a different accession of L. wollei as well as the green alga, Pithophora varia (Bishop 2016). In other algae, Cu exclusion from the cell was considered the primary tolerance mechanism (Twiss et al. 1993; Yan and Pan 2002). Because Cu has many critical internal modes of toxic action (Stauber and Florence 1987) and external factors (e.g., mucilage) can sorb Cu without inciting toxicity (Tien et al. 2005); this further supports our finding of a critical internal Cu threshold level in which control is achieved independent of adsorbed Cu.
The 7-day LC50 and LC75 values, using the factor exposure, were similar for all treatments based on chlorophyll a content (p < 0.05; Table 1). The 7-day LC50 and LC75 values, using the factor exposure, were similar for the 1 and 2 ppm concentrations based on filament viability, though significantly less for the 4 ppm concentration (p < 0.05; Table 1). The 4 ppm treatment had significantly decreased (p < 0.05) filament viability at 4 and 8 factor exposures compared to the 1 and 2 ppm concentrations and at the 16 factor exposure compared to the 2 ppm treatment. The 1 and 2 ppm treatments had similar response curves at all factor exposures. Filament viability has been shown to be a more sensitive response measurement, with lower variance, in the laboratory with short exposure durations compared with chlorophyll a content (Bishop and Rodgers 2011; Bishop et al. 2015). Despite differences in exposure factors required to elicit 7-day LC50 and LC75 values within filament viability analyses, no significant differences in exposure factors were discerned between the filament viability and chlorophyll a content analyses.
Using data from this research, a “concentration-exposure-time” model was created to assist water resource managers in selecting an appropriate treatment regimen to attain the desired level of control for a specific site (Fig. 3). As illustrated in the CET model, when the exposure duration, or “contact time” is shortened, the magnitude of exposure can be increased to achieve control. This concept is often practiced for L. wollei management when applicators use equipment such as trailing hoses to inject the algaecide directly onto the benthic mats, spray directly onto floating mats, or by altering the metered amount and/or duration in a canal or flowing water treatment. On the opposite end of the application spectrum when the contact time is expected to last longer than a few hours, lesser amounts of algaecide could be used to achieve control. When translating information on the CET model to L. wollei treatment in a lake or pond it is imperative to adjust exposure on a mass to mass relationship according to the critical burden concept (Bishop and Rodgers 2012). In this research, the physical model used concentrations of 1–4 ppm Cu (5–20 mg Cu/g cyanobacteria), these concentrations would translate to 0.2 g L. wollei per liter of water in treatment area. Because L. wollei biomass is often patchy and differs by site, the mass of algaecide required to achieve control will differ as well. By accounting for the mass of algae and algaecide contact-time, as we have done in this study, more accurate predictions of efficacy can be accomplished to aid decisions for L. wollei management. Future research will include characterizing copper exposures in field treatment programs and comparing field efficacy with laboratory results.
Control efficacy diagram for Lyngbya wollei based on mean toxicity threshold values. Control areas defined by a specified copper (Cu) concentration over a specific exposure duration (h). Exposures incorporated 0.1 g wet weight of cyanobacteria in 500 mL volumes. The 1, 2, and 4 ppm Cu concentrations were equivalent to 5, 10 and 20 mg Cu/g cyanobacteria exposures
There were obvious trends associated with normalizing the exposure based on factor. The general trend observed was that the L. wollei response increased proportionally with factor. Cu readily sorbs to algae at the exterior of the cell, though both inert binding sites (Crist et al. 1990) and at sites that lead to internalization are present (Yan and Pan 2002; Kaduková and Virčíková 2005). Prior research has determined that internal Cu is of particular importance in attaining effective control, especially with species like L. wollei which has a thick protective mucilaginous sheath (Bishop and Rodgers 2012; Tien et al. 2005). This concept was reinforced in our study, as the internal Cu was relatively similar across treatment concentrations (based on factor exposures). In contrast, the adsorbed Cu was significantly greater with increasing Cu concentration treatments. Even with adsorption far in excess of the designated critical burden threshold, control was not achieved until the internal Cu threshold was attained (Fig. 1). In this study, we selected a chelated Cu formulated with an added surfactant to maximize the amount of Cu that was likely to bind and accumulate in a short time period; this formulation has also been used to successfully treat and control L. wollei in large-scale management programs (Bishop et al. 2015).
As Lyngbya infestations are continuing to spread in range distribution throughout the U.S., including the Laurentian Great Lakes basin, and can elicit severe economic and ecological impacts, understanding the effectiveness of management strategies becomes even more crucial for attaining control and maintaining current water resource uses. Our research findings aid in these management strategies and specifically present a treatment concentration and duration model that predicts the exposure necessary to achieve control of a known biomass of cyanobacteria, based on site-specific field characteristics. By advancing the general understanding of Cu exposure and algal and cyanobacterial control relationships, management costs and timeline can be better predicted. Moreover, enhanced Cu use efficiency can decrease overall environmental inputs and potential for non-target organism risks.
References
American Public Health Association (APHA) (2005) Standard methods for the examination of water and wastewater. 21st edn. American Public Health Association, Washington, DC
Bhadha JH, Lang TA, Alvarez OM, Giurcanu MC, Johnson JV, Odero DC, Daroub SH (2014) Allelopathic effects of Pistia stratiotes and Lyngbya wollei Farlow ex Gomont on seed germination and root growth. Sustain Agric Res 3:121–130
Bishop WM (2016) A Risk-based decision information system for selecting an algal management program (Doctoral dissertation, North Carolina State University)
Bishop WM, Rodgers Jr JH (2011) Responses of Lyngbya magnifica Gardner to an algaecide exposure in the laboratory and field. Ecotoxicol Environ Saf 74:1832–1838
Bishop WM, Rodgers JH Jr (2012) Responses of Lyngbya wollei to exposures of copper-based algaecides: the critical burden concept. Arch Environ Contam Toxicol 62:403–410
Bishop WM, Johnson BM, Rodgers JH Jr (2014) Comparative responses of target and non-target species to exposures of a copper-based algaecide. J Aquat Plant Manag 52:65–70
Bishop WM, Willis BE, Horton CT (2015) Affinity and Efficacy of copper following an algicide exposure: application of the critical burden concept for Lyngbya wollei control in Lay Lake, AL. Environ Manag 55:983–990
Briand J-F, Jacquet S, Bernard C, Humbert J-F (2003) Health hazards for terrestrial vertebrates from toxic cyanobacteria in surface water ecosystems. Vet Res 34:361–377
Cairns J, Pratt JR (1989) The scientific basis of bioassays. Hydrobiologia 188–189:5–20
Carmichael WW, Azevedo SM, An JS, Molica RJ, Jochimson EM, Lau S, Rinehart KL, Shaw GR, Eaglesham GK (2001) Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ Health Perspect 109:663–668
Corradi MG, Gorbi G (1993) Chromium toxicity on two linked trophic levels. II. Morphophysiological effects on Scenedesmus acutus. Ecotoxicol Environ Saf 25:72–78
Crist RH, Martin JR, Guptill PW, Eslinger JM, Crist DR (1990) Interaction of metals and protons with algae. 2. Ion exchange in adsorption and metal displacement by protons. Environ Sci Technol 24:337–342
Foss AJ, Phlips EJ, Yilmaz M, Chapman A (2012) Characterization of paralytic shellfish toxins from Lyngbya wollei dominated mats collected from two Florida springs. Harmful Algae 16:98–107
Hudon C, De Sève M, Cattaneo A (2014) Increasing occurrence of the benthic filamentous cyanobacterium Lyngbya wollei: a symptom of freshwater ecosystem degradation. Freshw Sci 33(2):606–618
Kaduková J, Virčíková E (2005) Comparison of differences between copper bioaccumulation and biosorption. Environ Int 31:227–232
Kiefer E, Sigg L, Schosseler P (1997) Chemical and spectroscopic characterization of algae surfaces. Environ Sci Technol 31:759–764
Knauer K, Behra R, Sigg L (1997) Adsorption and uptake of copper by the green algae Scenedesmus subspicatus (Chlorophyta). J Phycol 33:596–601
Lakeman MB, von Dassow P, Cattolico RA (2009) The strain concept in phytoplankton ecology. Harmful Algae 8:746–758
Lewis PA, Klemm DJ, Lazorchak JM, Norberg-King TJ, Peltier TJ, Peltier WH, Heber MA (1994) USEPA Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms (Green alga, Selenastrum capricornutum, Growth 1003.0). 3rd ed. EPA/600.4–91/002, Cincinnati, OH
Microsoft (2010) Microsoft Excel [computer software]. Redmond, WA
Paerl HW, Gardner WS, Havens KE, Joyner AR, McCarthy MJJ, SE Newell, Qin B, Scott JT (2016) Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 54:213–222
Schrader KK, Blevins WT (1993) Geosmin-producing species of Streptomyces and Lyngbya from aquaculture ponds. Can J Microbiol 39:834–840
Seifert M, McGregor G, Eaglesham G, Wickramasinghe W, Shaw G (2007) First evidence for the production of cylindrospermopsin and deoxy-cylindrospermopsin by the freshwater benthic cyanobacterium, Lyngbya wollei (Farlow ex Gomont) Speziale and Dyck. Harmful Algae 6:73–80
Speziale BJ, Dyck LA (1992) Comparative taxonomy of Lyngbya wollei Comb. Nov. (Cyanobacteria). J Phycol 28:693–706
Stauber JL, Davies CM (2000) Use and limitations of microbial bioassays for assessing copper bioavailability in the aquatic environment. Environ Rev 8:255–301
Stauber JL, Florence TM (1987) Mechanism of toxicity of ionic copper and copper complexes to algae. Mar Biol 94:511–519
Sunda WG, Huntsman SA (1998) Processes regulating cellular metal accumulation and physiological effects: Phytoplankton as model systems. Sci Total Environ 219:165–181
Systat Software, Inc. (2014) SigmaPlot version 12.5, San Jose, CA
Tien CJ, Sigee DC, White KN (2005) Copper adsorption kinetics of cultured algal cells and freshwater phytoplankton with emphasis on cell surface characteristics. J Appl Phycol 17:379–389
Tripathi BN, Mehta SK, Amar A, Gaur JP (2006) Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu+ 2 and Zn+ 2. Chemosphere 62:538–544
Twiss MR, Welbourn PM, Schwartzel E (1993) Laboratory selection for copper tolerance in Scenedesmus acutus (Chlorophyceae). Can J Bot 71:333–338
USEPA (1996) Microwave assisted acid digestion of siliceous and organically based matrices, method 3052. EPA SW-846, Ch. 3.2, US EPA. Washington, DC
USEPA (2002) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms (Green Alga, Selenastrum capricornutum, Growth 1003.0). 4th ed. EPA-821-R-02-012, Washington, DC 20460
USEPA (2011) National pollutant discharge elimination system (NPDES). Pesticide general permit (PGP) for discharges from the application of pesticides. Washington, DC
Veglio F, Beolchini F (1997) Removal of metals by biosorption: a review. Hydrometallurgy 44(3):301–316
Vijayavel K, Sadowsky MJ, Ferguson JA, Kashian DR (2013) The establishment of the nuisance cyanobacteria Lyngbya wollei in Lake St. Clair and its potential to harbor fecal indicator bacteria. J Great Lakes Res 39(4):560–568
Xue HB, Sigg L (1990) Binding of Cu(II) to algae in a metal buffer. Water Res 24:1129–1136
Yan H, Pan G (2002) Toxicity and bioaccumulation of copper in three green microalgal species. Chemosphere 49:471–476
Acknowledgements
The authors thank SePRO Corporation for use of their accredited laboratory to conduct this research. We also thank Mr. Jason Brown of the First Fruits Farm for facilitating this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bishop, W.M., Lynch, C.L., Willis, B.E. et al. Copper-Based Aquatic Algaecide Adsorption and Accumulation Kinetics: Influence of Exposure Concentration and Duration for Controlling the Cyanobacterium Lyngbya wollei . Bull Environ Contam Toxicol 99, 365–371 (2017). https://doi.org/10.1007/s00128-017-2134-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2134-2