Abstract
Environmental pollution by pentachlorophenol (PCP) is a critical concern worldwide, and microbial bioremediation could constitute an ecologically friendly solution. The main objectives of this study were at first to clarify the factors, affecting the ability and efficiency of PCP biodegradation by the bacterium isolate P6, and secondly to optimize the condition of using P6 for PCP bioremediation. The PCP mineralizing bacterium was isolated from the contaminated forest soil of Tunisia, and it was identified as Citrobacter freundii (C. freundii), by using conventional and molecular characteristics. The HPLC and spectroscopic analysis were used to investigate the PCP degradation and the biomass formation by this isolate P6. The main results showed that P6 was able to degrade or to transform more than 98 % of 640 mg/l PCP afterwards 168 h in mineral salt medium (MSM). As well, the optimal aerobic growth conditions of P6 in MSM include essentially the range of pH (4 ≤ pH ≤ 9) and of temperature (25 °C < temperature < 30 °C). The addition of glucose as extra carbon sources has an effect to enhance the PCP biodegradation. On the other side, this isolate of C. freundii is capable to remove or transform around 95.33 % of PCP added in the sterilized soil suspension supplemented with PCP and adjusted to a final concentration of around 400 mg/l during 2 weeks of incubation at 25 °C. This last result argues in favor of the use of this strain P6 of C. freundii as a microbial tool of remediation of PCP-contaminated site.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The application of pentachlorophenol (PCP) as a pesticide generally leads to some important environmental problems in the sites of application, by toxicant emissions and soil and groundwater contamination (Qi et al. 2015, Guoqiang et al. 2014). PCP (C6Cl5OH) industries is the most toxic environmental pollutant that will be brought about in thousands of tons annually by the pulp paper and agrochemical (Yuancai et al. 2014; Singh et al. 2007). So, this xenobiotic and harmful element is a hydrophobic and ionisable organic contaminant (Menghua et al. 2015.,Yang et al. 2008). For all these reasons, PCP has been listed and considered as a priority pollutant by the US Environmental Protection Agency (Damsa et al. 2007; Santosh et al. 2010a, b), since this pollutant is reported as carcinogenic (Xiaomin et al. 2015), mutagenic, acute pancreatitis, endocrinal agents, and in general as a significant toxic element to humans (Yuancai et al. 2014; Xu et al. 2012; Chandra et al. 2008). PCP is one of the most widely used biocide compounds (Tripathi and Kumar 2013), herbicide, algaecide, fungicide, insecticide (Susanne et al. 2014), germicide, molluscicide, and wood preservative (US-EPA 1978; ATSDR 1994; Yu-Heng et al. 2016). Also, it is expected to be recalcitrant to aerobic biodegradation because it is an aromatic highly chlorinated compounds (Tripathi and Kumar 2013).
In Tunisia, serious research on PCP contamination has been almost confined to a NATO Science for Peace project (SfP 981674) conducted between 2006 and 2009 in Tunisian cork oak forests (Quercus suber) following suspicions of PCP contamination in cork stoppers. The implementation and achievement of this project made significant improvements in the understanding of PCP contamination in Tunisian oak forests in the region of Tabarka (northwestern Tunisia). Registered PCP soil contents showed fluctuation during the 3 years of study and varied between 4 and 17 mg/kg of dry soil. Environmental pollution byxenobiotics is a worldwide problem and the development of the bioremediation technologies by the use of the microbesto clean contaminated sites is of a substantial interest (Zhong et al. 2016; Damsa et al. 2007). Santosh et al. (2010a, b)) reported three strains of Bacillus sp. as CL3, CL5, and CL11 competent to degrade the PCP at high rates. Strains CL3 and CL11 were able to remove slightly a smaller amount of PCP (up to 80 %) compared to strain CL5, which was able to eliminate around 91 % of PCP when they all grow at 600 mg/l. In the same way, these authors have isolated another bacterium identified as Pseudomonas stutzeri CL7 that is competent to mineralize more than 90 % of 600 mg/l PCP. In previous study, it has been shown that Citrobacter freundii has the ability to degrade 77 % of PCP in soil containing 100 ppm of PCP at 40 % moisture (Gautam et al. 2003). This strain could grow at high concentrations 5 % (w/v) of tannic acid and produced extracellular tannase (Kumar et al. 1999) and biphenyl (150 mg/ml) (Grishchenkov et al. 2002). Therefore, these prominent properties make C. freundii attractive candidates for use in bioremediation.
Bioremediation process is relatively slow and requires keeping the experimental conditions conductive to the viability, the growth, and the activity of the microorganisms (Kao et al. 2005; Tripathi and Garg 2010). The application of statistical experimental design techniques in bioremediation studies could result in an improved biodegradation of toxicants, thereby reducing development time and process cost. Optimization of the biodegradation conditions is a prerequisite for large-scale applications of microbial biodegradation processes.
The main objectives of this study were focused (1) to isolate a bacterial strain able to degrade PCP at high rates, (2) to study the main factors affecting the ability and efficiency of PCP biodegradation by the isolate P6 of C. freundii, (3) to optimize the use of this bacterium as microbial tool for PCP bioremediation, and (4) to study the PCP degradation in soil at determined optimal conditions.
2 Materials and Methods
Soil samples were collected in February 2011 at the first surface soil layer of 0–20 cm from the Tunisian Quercus suber (cork oak) forest of Tabarka situated at northwestern Tunisia, by using the international standards (ISO 2002). This soil is known as contaminated by PCP according to Hechmi et al. (2013). Soil sample was kept up in a sterilized bag at 4 °C until use. The different physico-chemical and biological characteristics were investigated using atomic absorption and spectrophotometric and microbiological methods as described in Table 1.
For the selection of bacteria degrading PCP, 10 g of soil was suspended in 90 ml of MSM adjusted to different concentrations of PCP, ranging between 20 and 700 mg/l. The MSM was composed as follows (mg/l): KH2PO4, 800; Na2HPO4, 800; MgSO47H2O, 200; CaCl22H2O, 10; and NH4Cl, 500. Each mixture MSM-PCP will be added with 1 ml of trace metal solution that included the following (mg/l): FeSO47H2O, 5; ZnSO4H2O, 4; MnSO44H2O, 0.2; NiCl6H2O, 0.1; H3BO3, 0.1; CoCl26H2O, 0.5; ZnCl2, 0.25; and EDTA, 2.5 (Kao et al. 2005; Sharma et al. 2009). The pH solution was accustomed to 7.3 ± 0.2. PCP was added to the sterile medium after autoclaving. The mixture MSM-PCP was maintained under shaking at 150 rpm in a shaker (Zhicheng) for 2 h. Decimal dilutions were prepared from 10−1 to 10−7 (Tessa et al. 2006; Hatimi and Tahrouch 2007), and the bacterial count (colony forming unit or CFU) was performed in each PCP concentration (20, 50, 80, 100, 150, 200, 250, 300, 400, and 500 mg/l) on solid MSM (added with 16 g of agar-agar) at 28 °C during 48 h under shaking. From the bacterial growth medium adjusted to the high PCP content of around 400 mg/l, the bacterium P6 was isolated, purified, and used to study the PCP degradation in liquid medium via HPLC and spectrophotometric analysis (Melina et al. 2013; Santosh et al. 2010a, b).
The biochemical characterization of the isolate P6 was done in accordance with the Bergey’s Manual of Systematic Bacteriology (Holt et al. 1994). This isolate was exposed to microscopic examination for morphological analysis, and capsule stain was determined with 20 % of copper sulfate. Catalase activity test was confirmed after the bubble formation in the presence of 3 % of H2O2 solution, and oxidase test was performed on paper disks using tetramethyl-pphenylenediamine. Antimicrobial susceptibility of this isolate P6 was performed by using the disk diffusion method on Mueller Hinton agar according to the CLSI criteria. Motility, indole, and urease activity test were determined by using Api 20E System.
Genomic DNA was extracted according to Kapley et al. (2001) from overnight-grown culture. PCR amplification was performed using the following primers: forward primer (5′-AGAGTTTGATCCTGGCTCAG-3′) and reverse primer (3′-ACGGGCGGTGTGTTC-5′) for 16S rRNA, as described by Weisburg et al. (1991). PCR products were purified with a Promega PCR purification kit and sequenced using an Applied Biosystems sequencer. The BLAST database of the National Center for Biotechnology Information (NCBI) was used to compare resolved sequence with 16S rDNA sequence data deposited in GenBank website. The NCBI accession numbers for the 16S rRNA gene sequence of the P6 isolate determined in the present study was KT906215.
The study of the PCP degradation was performed by inoculating the strain P6 at 1 % of inoculum (106 CFU ml/l) in 250-ml Erlenmeyer flasks, containing 50 ml of MSM supplemented with 20 to 700 mg/l of PCP. The flasks were incubated in a shaker at 30 °C and 150 rpm for up to 168 h. Bacterial growth was assessed by the OD600nm measure, and PCP lessening was quantified by HPLC, by sampling 1 ml of culture at 24-h intervals. HPLC analysis was carried out on a Perkin Elmer Series YL9100 system fitted on Symmetry C18 column and detector UV at 280 nm. The cell suspension was centrifuged, at 8000 rpm for 5 min, and the supernatant was filtered through a 0.22-mm cellophane filter. The column was eluted in an isocratic mode using mobile phase (acetonitrile/ortho-phosphoric acid) at a flow rate of 1 ml/min (Liu et al. 2007). The readings were integrated by the Empower Software System. The PCP concentrations were calculated based on the peak area measurements by comparison to an external standard of known concentration of PCP prepared in methanol. All analyses were carried out in triplicates.
Glucose effects and the environmental growth conditions (mainly pH, temperature, PCP concentration) of the strain P6 on the PCP biodegradation were evaluated in this study. PCP concentration effect on the growth of P6 and on their PCP-degrading ability was also examined. The isolate was inoculated to 250-ml Erlenmeyer flask containing 100 ml of MSM supplemented with different concentrations of PCP, namely 20, 50, 100, 200, 300, 400, 500, 600, 640, and 700 mg/l. The growth of the bacterial cells was evaluated at OD600nm and the degradation of PCP in the culture by HPLC as described above.
The pH effect on the PCP degradation was studied by culturing the strain P6 at a varied pH of 4, 7, 7.5, 8.5, or 9 in MSM supplemented with 100 mg/l of PCP as the sole carbon source. The pH value of the medium was adjusted if necessary with NaOH (0.5 M) or HCl (0.2 M).
In the temperature control experiment, three different incubation temperatures (25, 30, and 37 °C) were chosen to evaluate the effect of temperature on the PCP biodegradation or transformation. The flasks for different treatments were incubated in darkness for 168 h at 30 °C in an orbital shaker at 150 rpm, and all analyses were carried in triplicate.
Soil Bioremedation Procedure
One milliliter of the bacterial suspension of P6, containing a round 107 CFU/ml, was inoculated in 100 ml of MSM in closing 10 g of sterilized soil supplemented with 100 mg/l PCP (Laine and Jorgensen 1997). The mixture was incubated at 30 °C under shaking at a rate of 150 rpm for 2 weeks ,and the PCP was extracted from the soil, according to Chandra et al. (2006). The soil was sonicated and acidified to pH 2.0 with 1 N HCl. PCP was then extracted for three times with an equal volume of ethyl acetate by intermittent shaking for 30 min in separating funnels. The organic layer was dried over anhydrous sodium sulfate. Filtered samples were evaporated under vacuum at 40 °C, subsequently resuspended in 1 ml of methanol, and injected on an HPLC to quantify the residual of PCP.
3 Results and Discussion
The role PCP biodegradation in soil was studied during 7 days by a saprophytic isolate C. freunddi under aerobic conditions. The nutritional (glucose) and physical (pH, temperature, PCP concentration) factors are important environmental determinants which regulate bioremediation strategies in polluted ecosystems. In this study, bioaccumulation methods by C.freundii (P6) were used to clean up forest soil contaminated with pesticide (PCP).
A series of experiments were carried out in a liquid suspension of forest soil with increasing PCP application rates (Fig. 1). Therefore, soil enumeration cells at low concentration of 20 mg/l of PCP, the number of the cultivable bacterial cells is around 9 × 105 CFU/g dry soil. This number decreased at around1.8 × 105 CFU/g dry soil in the case of 50 mg/l of PCP, and it gradually decreased to 0.18 × 105 CFU/g dry soil in the case of 400 mg/l of PCP. With the concentration of 520 mg/l of PCP, the bacterial growth appeared totally inhibited since we registered a total absence of the bacterial colonies in the different plates used for the enumeration. So, the case of 400 mg/l of PCP was selected to study and to assess the optimal conditions of PCP degradation by the isolate P6 in liquid medium and by using HPLC.
The morphological and biochemical characterization of the bacterial isolate P6 was presented in Table 2. This isolate is a Gram-negative strain, with catalase-positive test, aerobic and non-motile bacteria. Besides, the susceptibility to various antibiotics (such as amikacin, cefepine…) was studied because it is needed in the selection of robust strain and more competitive and able to survive in unfavorable environmental conditions. Api 20E system confirmed that the strain P6 presented 99 % of similarity with C. freundii. Sequence analysis using Blast (NCBI database) assigned the isolate P6, with a high degree of confidence (>97 %), to C. freundii (GeneBank accession number, KT906215). This isolate showed a high efficiency of degrading the PCP, with values around 98 % of 640 mg/l PCP in liquid mineral medium and after 7 days.
A PCP biodegradation study using the strain P6 showed that the isolate P6 of C. freundii was capable to grow in all mixtures of PCP tested, by operating PCP as a source of carbon and energy for all the tested concentrations ranging between 20 and 640 mg/l. HPLC was applied to detect the PCP peak in the liquid mineral medium and spectrophotometer to measure the growth of the functional bacterium P6 at 600 nm (the optical density at 600 nm orOD600nm). Figure 2 taken as an example shows the bacterial growth and the PCP degradation at 640 mg/l of PCP during 168 h. The OD600nm showed a notable increase from 48 h up to 72 h and slowly declined starting from 96 h to reach a stationary phase up to 168 h. Degradation of PCP showed a net increase over the period of incubation. Thereby, it was observed that the isolate P6 employed more than 9.89 % of PCP after 24 h and above 14.56 % after 48 h of incubation. The PCP was completely not detected subsequently to the microbial removal or transformation in the medium within 72 h of incubation (Figs. 2 and 3). These results show that P6 isolate has the ability to degrade the PCP at high concentrations (640 mg/l).
A large variety of bacteria were known that could utilize chlorophenols as a source of carbon and energy under aerobic conditions. Also, it was well known that there are two main strategies for using chlorophenols by aerobic bacteria, as a source of carbon firstly, and/or secondly, as a source of energy (Solyanikova and Golovleva 2004). Lower chlorinated phenols (with one to two chlorine substituents) are initially attacked by monooxygenases, providing chlorocatechols as the first intermediate molecules (chlorocatecholpathway), which are subject to ring cleavage prior to dechlorination. On the other hand, polychlorinated phenols (three to five chlorines) are converted to chlorohydroquinones as the initial intermediate molecules (hydroquinone pathway). Successive reactions progressively remove chlorines from the ring prior to ring cleavage.
The batch culture experiments revealed that PCP was biodegraded with the stoichiometry release of chloride anions by the strains P6. Therefore, simultaneous release of chloride ions corroborated the previous findings of dechlorination during PCP degradation, as described by Mohn and Kennedy (1992).
Chandra et al. (2006)) reported that Bacillus cereus ITRCS6 degraded 67 % of 300 mg/l of PCP after 168 h of incubation. The aerobic bacterial strain of Serratia marcescens could utilize up to 300 mg/l of PCP within 168 h (Singh et al. 2007). Shah and Thakur (2002) have observed that Pseudomonas sp. could degrade PCP in liquid medium. The strains of Bacillus CL3, CL5, and CL11 and P. stutzeri CL7 isolated from the secondary sludge of a pulp paper mill could tolerate around 600 mg/l of PCP and also degraded more than 90 % of this quantity of PCP (Santosh et al. 2010a, b). Similar to our results, numerous works have also stated that Citrobacter sp. has good potential toxic pollutant degradation (Wang et al. 2009). In the present study, the isolate P6 of C. freundii could remove more than 98 % of the amount of 640 mg/l of PCP. Therefore, the removal efficiency of PCP achieved by the isolate P6 of C. freundii used in this study appeared more efficient than those reported earlier in the literature (Santosh et al. 2010a, b; Kao et al. 2005; Crawford and Mohn 1985).
Carbon Sources
Figure 4 showed the effect of adding an extra source of carbon like glucose on the PCP biodegradation by the isolate P6. So, glucose was used as the primary substrate in this experiment, and a complete PCP removal was observed within 168 h of microcosm incubation, while having glucose as an extra source of carbon, and the toxic effects caused by pollutant could be mitigated and lessened by available carbon sources in an aerobic environment. Although, a shorter lag period of 48 h showed that the addition of glucose appeared as significantly enhancing and increasing the PCP biodegradation. The use of glucose as a source of carbon is considered in this experimentation as an indicator of P6 growth and PCP degradation. The other possible reason of using glucose by P6 is to confirm the occurrence of the co-metabolism as the dominant mechanism of PCP biodegradation by this bacterium. Similarly, Radehaus and Schmidt (1992) found that the glucose addition to Pseudomonas sp. RA2 culture has an effect to stimulate the PCP mineralization. Indeed, in a dual-substrate experiment (80 mg/l glucose/80 mg/l PCP), Pseudomonas sp. R2A isolated from polluted soil could mineralize simultaneously almost 90 % of glucose and 95 % of PCP (Petra and Steven 1992). Besides, the study of Top and Hanson (1990) reported that the strain of Flavobacterium sp. is competent to rapidly mineralize 50 mg/l of PCP in the presence of 100 mg/l of glucose. The addition of another carbon source is not always considered and taken as a stimulating and enhancing factor for PCP degradation, since it is not often affecting the PCP degradation rate (Wang et al. 2012). This idea was proved and confirmed by Kao et al. (2005) who studied the effect of glucose and the sodium acetate on the PCP degradation by Pseudomonas mendocina NSYSU.
PCP Rates
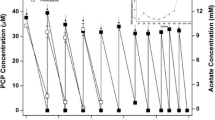
The application of PCP at different rates, ranging between 20 and 700 mg/l as a carbon source for growth and development in a liquid MSM medium of this bacterium P6 of C. freundii and under aerobic conditions, was piloted in this study. Therefore, Fig. 6a showed that the PCP consumed or transformed by the strainP6 within 168 h of incubation is used as the sole carbon source and as primary substrate. In addition, the results revealed that PCP in microcosms and in the range of concentrations around 20 to 640 mg/l was rapidly dechlorinated after 168 h. Thereby, the strain P6 of C. freundii was able to degrade around 70 % of PCP added at the rate of 200 mg/l in the medium. The optimal PCP concentration, corresponding to 99 % of P6 growth, was around 400 mg/l. On the other side, no PCP removal was detected in all control microcosm experiments (data not shown). Approximately 99 % of PCP were removed within 7 days of incubation in these microcosm experiments. No PCP removal (or transformation) was detected in microcosms treating with PCP concentrations beyond 640 mg/l and after 168 h of incubation. This last result indicated that the isolate P6 of C. freundii was able to degrade the PCP at high concentration of around 640 mg/l. In the literature, it was reported that P. mendocina NSYSU could not degrade PCP starting from 320 mg/l (Kao et al. 2005). However, three Bacillus CL3, CL5, and CL11 and Pseudomonas sp. CL7 could degrade around 90 % of all tested PCP rates added and ranging between 20 and 600 mg/l. Starting from 600 mg/l of PCP, the growth of these three strains of Bacillus was completely inhibited (Santosh et al. 2010a, b).
To sum up, the results obtained in this study indicated that the strain P6 identified as C. freundii is an important bacterium capable of degrading or transforming PCP by using it as a sole source of carbon. This strain could be applied or operated in the future process of remediation of PCP-contaminated sites.
pH
Figure 5b showed the pH effects on the PCP biodegradation by the strain P6. The removal or degradation of PCP by P6 was tested at pH values ranging between 4 and 9 and at an initial concentration of 100 mg/l of PCP. The isolate P6 could remove more than 90 % of PCP at all tested pH ranging between 4 and 9. The PCP degradation efficiency of strain P6 was higher at pH 8.5 and 7.5 compared to pH 4.
Bacterial degradation of PCP is strongly affected by the pH value of the medium (Luciano et al. 2015), and it was seemingly reduced and lessened when the pH was less than 6.0 (Barbeau et al. 1997). Sphingomonas chlorophenolica removed around 90 % of PCP when the initial pH was around 9.2, and on the contrary, it could not remove PCP when the pH was below 6.0 (Yang et al. 2008). In another work, Edgehill (1994) found that the growth rate for Arthrobacter species at pH 7.4 was higher than that under acidic conditions in the presence of PCP. Wolski et al. (2005) reported the degradability of PCP at less pH range value of 6.3 and 8.0 and with a maximum rate of PCP degradation by Pseudomonas species at pH 6.3. Thus, Santosh et al. (2010a, b)) reported that the strain of Pseudomonas sp. CL7 could degrade PCP at a high level, around 96.5 and 94.5 %, and in the range of pH between 7.5 and 8.5, respectively. Biodegradation of PCP was not inhibited in relatively acidic (pH 4) and alkaline (pH 9) conditions, inversely to the result reported by Kao et al. (2005) that PCP degradation was inhibited in acidic (pH 5) and alkaline conditions (pH 10). The removal of PCP by three isolates of Bacillus CL3, CL5, and CL11 was also tested at pH values of 7.5, 8.5, and 9.5 and at an initial concentration of 100 mg/l PCP. The growth of strain CL5 was significantly important in all the pH levels tested as compared to strains CL3 and CL11. All isolates removed around 90 % of PCP in the pH range of 7.5 and 8.5, and the PCP removal efficiency was significantly reduced when the initial pH of the medium was around 9.5 with CL3 and CL11. The PCP degradation efficiency of strain CL5 appeared important at pH 8.5 and 9.5 compared to the one obtained at 7.5 (Santosh et al. 2010a, b). Our results appeared more important than those found and cited in the literature indicating mainly that PCP degradation was significant for pH values ranging between 4 and 9.
Temperature
The effect of temperature on the PCP degradation by the isolate P6 of C. freundii was illustrated in Fig. 5c. The optimal temperature of the PCP degradation by the isolate P6 was observed at around 30 °C and with no PCP removal at around 37 °C. The PCP degradation was greater at 25 and 30 °C but the removal was inhibit at 37 °C. Temperature is another important environmental factor that may influence the rates at which pollutants will be degraded or removed (Trevors 1982), through altering both microbial activity and the physical and chemical properties of pollutants (Providenti et al. 1993; Miller et al. 2004). Numerous authors reported in the literature the optimal temperature for PCP degradation by some bacterial species that were as follows: range of 25–35 °C for Acinetobacter species (Sharma et al. 2009), 30 °C for P. mendocina NSYSU (Kao et al. 2005), 37 °C for Pseudomonas sp. strain CL7 (Santosh et al. 2010a, b), and 30 and 37 °C for three Bacillus isolates CL3, CL5, and CL11 (Santosh et al. 2010a, b). In addition, Crawford and Mohn (1985) showed significant removal of PCP between 24 and 35 °C by Flavobacterium sp., nevertheless, the removal was ineffective below 12 °C or above 40 °C. Our results also showed that at a high temperature of 30 °C, the PCP removal looked less efficient.
Efficient Bioaugmediation of PCP Contaminated Soil
The isolate P6 of C. freundii used in this study has been shown to degrade PCP in MSM at high rate of up to 640 mg/l. Therefore, this strain could be used as a bioremediation microorganism for PCP processing of contaminated sites. The survival of the isolate P6 was monitored by the growth determination as colony forming units (CFU). The soil inoculation by the P6 was checked at the exponential stage of bacteria growth with 5 ml of bacterial suspension in 100 g of soil. The growth of the bacterial strain increased from the initial CFU of 346 102 to 698 103 after 2 weeks. The HPLC study (Fig. 6) revealed that the tested bacterium was capable to remove up to 95.33 % of 400 mg/l of PCP added in the soil and incubated at controlled conditions of temperature and pH and within a period of 2 weeks. P6 is able to remove up to 95.33 % PCP from soil suspension.
Remediation of PCP existing and prevailing in the natural contaminated environment may occur by transformation or biodegradation through the action of natural chemical or biological processes. Biodegradation is one of the significant processes that acts under aerobic conditions. PCP is stable to chemical hydrolysis and oxidation, but some microorganisms could metabolize this compound. Treatment of PCP-contaminated wastewater has been performed successfully in pilot scale and field studies by aerobic organisms such as Flavobacterium species and Rhodococcus species (Salonen et al. 1983; Apajalahti and Salonen 1986). Aerobic PCP biodegradation products include both oxidized residues and less-chlorinated derivatives (Davis et al. 1994). In the present study, the bacterium P6 of C. freundii tested has shown a very good potential to mineralize around 93.33 % of PCP within 2 weeks of incubation in controlled conditions, and with a concentration slightly above 400 mg/l of PCP. Our latest result appeared more important than the one found by Santosh et al. (2010a, b)) since these authors showed for both that the strain of Pseudomonas sp. CL7 could degrade around 66.8 % of 100 mg/l of PCP and the Bacillus consortia (CL3, CL5 and CL11) could reduce around 77 % of the same concentration for 2 weeks in secondary sludge of pulp and paper mill. The biological treatment procedures using some specific microbes are effectively utilized to clean up environmental matrix contaminated with chlorinated phenols (Mrozik et al. 2010; Cea et al. 2010; Yuancai et al. 2014; Zhong et al. 2016). All these evidence indicated that the species C. freundii had a potential to be used in high concentration of PCP-contaminated field in liquid or solid material.
4 Conclusion
This paper investigates the optimal bacterial growth conditions of the strain P6 and its ability to use PCP as a sole source of carbon. The P6 was isolated during this study from the PCP-contaminated soil of Tabarka and it was identified as C. freundii. This study showed that this bacterium was competent to degrade or to transform PCP present at the high concentration of around 640 mg/l in the medium. This degradation or transformation could reach around 98 % of PCP after 72 h and 100 % after 96 h, respectively. This result contributes in clarifying and elucidating the main factors affecting the ability and the efficiency of PCP biodegradation by this isolate C. freundii (P6). Optimization of definite physico-chemical parameters of growth and of PCP degradation by P6 showed that the optimal degradation happened at around temperature of 25 °C and pH ranging from 4 to 9. These informations provided by the present could be used as an efficient tool to optimize degradation conditions in the natural contaminated area, by adjusting the pH and the temperature in order to have a better degradation outcome. Bioremediation of pentachlorophenol-contaminated soil could be enhanced with an additional bacterial strain to native and autochthonsoilones. Also, this strain C. freundii (P6) was competent to degrade around 95.33 % of PCP added in the soil microcosms within 2 weeks of treatment. This result plaids and acts in favor of future applications of this bacterium in a real and practical remedial task.
Change history
20 November 2017
One of the bibliographic reference entry of this article had the author name and family name switched by mistake.
References
Agency for toxic substances and disease registry (ATSDR). (1994). Toxicological profile for acetone prepared by syracuse research corp for U.S. Departement of health and human services.
Apajalahti, J., & Salonen, S. (1986). Degradation of polychlorinated phenols by Rhodococcuschlorophcnolicus. Applied and Environmental Microbiology, 25, 62–65.
Barbeau, C., Deschenes, L., Karamanev, D., Comeau, Y., & Samson, R. (1997). Bioremediation of pentachlorophenol-contaminated soil by bioaugmentation using activated soil. Applied Microbiology and Biotechnology, 48, 745–752.
Cea, M., Jorquera, M., Rubilar, O., Langer, H., Tortella, G., & Diez, M. C. (2010). Bioremediation of soil contaminated with pentachlorophenol by Anthracophyllum discolor and its effect on soil microbial community. Journal of Hazardous Materials, 181, 315–323.
Chandra, R., Ghosh, A., Jain, R. K., & Singh, S. (2006). Isolation and characterization of two potential pentachlorophenol degrading aerobic bacteria from pulp paper effluent sludge. Journal of General and Applied Microbiology, 52, 125–130.
Chandra, R., Raj, A., Yadav, S., & Patel, D. K. (2008). Reduction of pollutants in pulp paper mill effluent treated by PCP degrading bacterial strains. Environmental Monitoring Assessment, 155, 1–11.
Crawford, R. L., & Mohn, W. W. (1985). Microbiological removal of pentachlorophenol from soil using a Flavobacterium. Enzyme and Microbial Technology, 7, 617–620.
Damsa, R., Patonb, G., & Killhamb, K. (2007). Bioaugmentation of pentachlorophenol in soil and hydroponic systems. InternationalBiodeterioration& Biodegradation, 60, 171–177.
Davis, A., Campbell, J., Gilbert, C., Ruby, M. V., Bennett, M., & Tobin, S. (1994). Attenuation and biodegradation of chlorophenols in ground water at a former wood treating facility. Ground Water, 32, 248–257.
Edgehill, R. U. (1994). Pentachlorophenol removal from slightly acidic mineral salts, commercial sand, and clay soil by recovered Arthrobacter strain ATCC 33790. Applied Microbiology and Biotechnology, 41, 142–148.
Edward, T., & Richard, S. H. (1990). Degradation of pentachlorophenol by Flavobacterium species grown in continuous culture under various nutrient limitation. Applied and Environmental Microbiology, 56, 2.
Gautam, S. K., Rjeev, S., Ahmad, A. H., & Indu, S. T. (2003). Biodegradation of pentachlorophenol potentiality of pseudomonas sp. in soil microcosm. Journal of Microbiology and Biotechnology, 19, 73–78.
Grishchenkov, V., Slepen’kin, A., & Borodin, A. (2002). Anaerobic degradation of biphenyl by the facultative anaerobic strain Citrobacter freundii BS2211 volume 38. Applied Biochemistry and Microbiology, 2, 125–128.
Guoqiang, S., Panpan, W., Shenguang, G., Lei, G., Jinghua, Y., Mei, Y. (2014). Photo electrochemical sensor for pentachlorophenol on microfluidic paper-based analytical device based on the molecular imprinting technique. Biosensors and Bioelectronics, 5697–103
Hatimi, A., & Tahrouch, S. (2007). Caractérisations chimique, botanique et microbiologique du sol des dunes littorales du Souss-Massa. Biomatec Echo, 2, 85–97.
Hechmi, H., Ben Aissa, N., Hassen, A., & Jedidi, N. (2013). Phytoremediation potential of maize (zea mays l) in co-contaminated soils with pentachlorophenol and cadmium. Connection with or arising out of the use of this material. International Journal of Phytoremediation, 15, 703–713.
Holt, J., Krieg, N., Sneath, P., Staley, J., & Williams, S. (1994). Bergey’s maual of determinativebacteriology. Williams & Wilkins, Baltimore. Ninth Edition.
ISO (2002) Soil quality-part 1: guide on the design of sampling procedures. International organisation of standarisation. http://www.iso.org/iso/catalogue_detail.htm?csnumber=32423. Accessed 31 March 2016.
Kao, C. M., Liu, J. K., Chen, Y. L., Chai, C. T., & Chend, S. C. (2005). Factors affecting the biodegradation of PCP by Pseudomonas mendocina NSYSU. Journal of Hazardous Materials, 124, 68–73.
Kapley, A., Lampel, K., & Purohit, H. J. (2001). Rapid detection of salmonella in water samples by multiplex polymerase chain reaction. Water Enveronmental Research, 73, 461–465.
Kumar, R., Gunasekaran, P., & Lakshmanan, M. (1999). Biodegradation of tannic acid by Citrobacter freundii isolated from a tannery effluent. Journal of Basic Microbiology, 39(3), 161–168.
Laine, M. M., & Jorgensen, K. S. (1997). Effective and safe composting of chlorophenol contaminated soil in pilot scale. EnvironmentalScience and Technology, 3, 371–378.
Liu, F., Hong, M., Liu, D., Li, Y., Shou, P., Yan, H., & Shi, G. (2007). Biodegradation ofmethyl parathion by Acinetobacter radioresistens USTB-04. Journal of EnvironmentalScience, 19, 1257–1260.
Luciano, B., Federica, L., Gennaro, C., Mara, C., Maria, C., & Olga, R. (2015). Biosorption of pentachlorophenol by Anthracophyllum discolor in the form of live fungal pellets. New Biotechnology, 32(1), 1871–6784.
Melina, N., Gonzalo, H. S., Gaston, A. C., Marcelo, S., Jorge, F. G., & Silvia, E. M. (2013). Hydrocarbon biodegradation and dynamic laser speckle for detecting chemotactic responses at low bacterial concentration. Journal of Environmental Sciences, 25(3), 613–625.
Menghua, C., Li, W., Zhihui, A., & Lizhi, Z. (2015). Efficient remediation of pentachlorophenol contaminated soil with tetrapolyphosphate washing and subsequent ZVI/air treatment efficient remediation of pentachlorophenol contaminated soil with tetrapolyphosphate washing and subsequent ZVI/air treatment. Journal of Hazardous Materials, 29(2), 27–33.
Miller, M. N., Stratton, G. W., & Murray, G. (2004). Effects of nutrient amendments and temperature on the biodegradation of pentachlorophenol contaminated soil. Water, Air, and Soil Pollution, 151, 87–101.
Mohn, W. W., & Kennedy, K. J. (1992). Reductive dehalogenation of chlorophenol by Desulfomonite tiedjei DCB-1. Applied andEnvironmental Microbiology, 58, 1367–1370.
Mrozik, A., & Piotrowska-Seget, Z. (2010). Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiological Research, 165, 363–375.
Petra, M., & Steven, K. (1992). Characterization of a novel Pseudomonas sp. that mineralized high concentrations of pentachlorophenol. Applied and Environmental Microbiology, 58, 9.
US EPA (Environmental Protection Agency) (1978). Integrated risk information system on pentachlorophenol. Washington, DC: National Centre for Environmental Assessment, Office of Research and Development.
Providenti, M. A., Lee, H., & Trevors, J. T. (1993). Selected factors limiting the microbial degradation of recalcitrant compounds. Journal of Industrial Microbiology, 12, 379–395.
Qi, F., Xiongjie, S., Liping, Z., Qiangwei, W., Xianfeng, W., Yong yong, G., & Bingsheng, Z. (2015). Effect of titanium dioxide nanoparticles on the bioavailability, metabolism, and toxicity of pentachlorophenol in zebra fish larvae. Journal of Hazardous Materials, 283, 897–904.
Radehaus, P. M., & Schmidt, S. K. (1992). Carracterization of novel Pseudomonas sp. that mineralize high concentration of Pentachlorophenol. Applied and Environmental Microbiology, 58, 2879–2885.
Salonen, S. M. S., Hakulinene, M. S. R., Valo, R., & Apajalahti, J. H. A. (1983). Biodegradation of recalcitrant organochlorine compounds in fixed film reactors. Water Science and Technology, 14, 309–390.
Santosh, K., Chakrabarty, S. K., & Sudhakara, R. M. (2010a). (a). Characterization of pentachlorophenol degrading Bacillus strains from secondary pulp-and-paper-industry sludge. International Biodeterioration & Biodegradation, 64, 609–613.
Santosh, K., Santosh, K., Chakrabarty, S. K., & Sudhakara, R. M. (2010b). (b). Pentachlorophenol degradation by Pseudomonas stutzeri CL7 in the secondary sludge of pulp and paper mill. Journal of Environmental Sciences, 22(10), 1608–1612.
Shah, S., & Thakur, I. S. (2002). Enrichment and characterization of microbial community from tannery effluent for degradation of pentachlorophenol. World Journal of Microbiology and Biotechnology, 18(6), 693–698.
Shail, S. R., Chandra, D. K., Patel, M. M. K., & Vibhuti, R. (2007). Investigation of the biotransformation of pentachlorophenolandpulp, paper mill effluent decolorisation by the bacterial strains in a mixed culture chlorophenolicum ATCC 39723. Chemosphere, 68, 864–870.
Sharma, A., Thakur, I. S., & Dureja, P. (2009). Enrichment, isolation and characterization of pentachlorophenol degrading bacterium Acinetobacter sp ISTPCP-3 from effluent discharge site. Biodegradation, 20, 643–650.
Singh, S., Chandra, R., Patel, K., & Rai, V. (2007). Isolation and characterization of novel Serratiamarcescens (AY927692) or pentachlorophenol degradation from pulp and paper mill waste. World Journal of Microbiology and Biotechnology, 23, 1747–1754.
Solyanikova,I.P., Golovleva, L.A. (2004). Bacterial degradation of chlorophenols pathways biochemical and geniticaspects. Journal of environmental Science and Health, 39, 333-351.
Susanne, S., Jan, N., Olaf, G., & Barbara, G. (2014). Cytotoxic effects exerted by pentachlorophenol by targeting nodal pro-survival signaling pathways in human pancreatic cancer cells. Toxicology Reports, 1, 1162–1174.
Tessa, M., Barbara, A., Siva, S., Grant, N., Iris, V., Brett, R., Cara, N., & Doris, L. (2006). Phytoremediation and long-term site management of soil contaminated with pentachlorophenol (PCP) and heavy metals. Journal of environment Management, 79, 232–241.
Top, E., & Hanson, R. S. (1990). Degradation of pentachlorophenol by a Flavobacterium species grown in continuous culture under various nutrient limitations. Applied and Environmental Microbiology, 56, 541–544.
Trevors, J. T. (1982). Effect of temperature on the degradation of pentachlorophenolby Pseudomonas species. Chemosphere, 11, 471–475.
Tripathi, M., & Garg, S. K. (2010). Studies on selection of efficient bacterial strain simultaneously tolerant to hexavalent chromium and pentachlorophenol isolated from treated tannery effluent. Research Journal of Microbiology, 5(8), 707–716.
Tripathi, M., & Kumar, G. S. (2013). Co-remediation of pentachlorophenol and Cr6+ by free and immobilized cells of native Bacillus cereus isolate: spectrometric characterization of PCP dechlorination products, bioreactor trial and chromate reductase activity. Process Biochemistry, 48, 496–509.
Wang, Y., Gu, B., & Xu, W. (2009). Electro-catalytic degradation of phenol on several metal-oxide anodes. Journal Hazardous Materials, 162, 1159–1164.
Wang, S., Huang, L., Gan, L., Quan, X., Li, N., Chen, G., Lu, L., Xing, D., & Yang, F. (2012). Combined effects of enrichment procedure and non-fermentable or fermentable co-substrate on performance and bacterial community for pentachlorophenol degradation in microbial fuel cells. Bioresource Technology, 6, 120.
Weisburg, W. G., Barns, S. M., Pelletier, D. A., & Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology, 173, 697–703.
Wolski, A., Murialdo, E., & Gonzalez, F. (2005). Effect of pH and inoculums size on pentachlorophenol degradation by Pseudomonas sp. Water SA, 32, 1–6.
Xiaomin, L., Zhong, L., Chunling, L., Jing, B., Yingtao, S., & Yongtao, L. (2015). Enhanced microbial degradation of pentachlorophenol from soil in the presence of earthworms: evidence of functional bacteria using DNA stable isotope probing. Soil Biology & Biochemistry, 81, 168–177.
Xu, T., Lou, L., Luo, L., Cao, R., Duan, D., & Chen, Y. (2012). Effect of bamboo biocharon pentachlorophenolleachability and bioavailability in agricultural soil. Science of the Total Environment, 414, 727–731.
Yang, C., Cao, G., Li, Y., Zhang, X., Ren, H., Wang, X., Feng, J., Zhao, L., & Xu, P. (2008). A constructed alkaline consortium and its dynamics in treating alkaline black liquor with very high pollution load. PloS One, 3(11), 3777.
Yuancai, L., Yuancai, C., Wenzhe, S., & Yongyou, H. (2014). Enhanced selection of micro-aerobic pentachlorophenol degradinggranular sludge. Journal of Hazardous Materials, 280, 134–142.
Yu-heng, O., Chau-Yuan, W., & Yang-hsin, S. (2016). Short-chain organic acids increase the reactivity of zero valentironnanoparticles toward polychlorinated aromatic pollutants. Chemical Engineering Journal, 284, 372–379.
Zhong, L., Zhen, Z., Zhihao, W., Jiewen, Y., Laiyuan, Z., Hanqiao, H., Chunling, L., Jing, B., Yongtao, L., & Dayi, Z. (2016). The impact on the soil microbial community and enzyme activity of two earthworm species during the bioremediation of pentachlorophenol-contaminated soils. Journal of Hazardous Materials, 301, 35–45.
Acknowledgment
The authors would like to thank the University of Tunis El Manar for granting a PhD studentship to Ms. Rim Werheni. This research was part funded by the Tunisian Ministry of Higher Education and Scientific Research (CERTE) and by the NATO, Science for Peace, Project ESP. MD. SFPP981674, with thanks to all members of the project, particularly to Professor Cristina Silva Pereira (IBET), Oeiras, Portugal, and to Professors Hassnaoui I. and Aouni L. of Sylvo-pastoral Institute of Tabarka, Tunisia, for granting permission to sample.
Author information
Authors and Affiliations
Corresponding author
Additional information
A correction to this article is available online at https://doi.org/10.1007/s11270-017-3619-7.
Rights and permissions
About this article
Cite this article
WerheniAmmeri, R., MokniTlili, S., Mehri, I. et al. Pentachlorophenol Biodegradation by Citrobacter freundii Isolated from Forest Contaminated Soil. Water Air Soil Pollut 227, 367 (2016). https://doi.org/10.1007/s11270-016-2959-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2959-z