Abstract
Background and objectives
Recent studies have shed light on the potential role of curcumin in mitigating inflammation in patients with chronic kidney disease (CKD). This study aimed to evaluate the effects of curcumin supplementation on plasma levels of markers of inflammation and oxidative stress in patients with CKD undergoing hemodialysis (HD).
Methods
These are secondary exploratory analyses from a previous double-blind, randomized controlled pilot study registered under ClinicalTrials.gov Identifier no. NCT00123456. It included 28 hemodialysis patients from a previous study divided into two groups: curcumin group (receiving juice with 2.5 g of turmeric 3×/week for 12 weeks) and a control group. The TNF-α, IL-6 and Ox-LDL plasma levels were measured by sandwich enzyme immunoassays ELISA; lipid peroxidation was measured by the reaction between malondialdehyde (MDA) and thiobarbituric acid.
Results
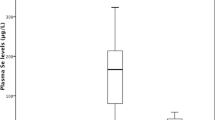
After 12 weeks of supplementation with curcumin, the TNF-α plasma levels were significantly reduced [from 15.0 (8.23–73.3) to 6.17 (1.11–55.0) pg/mL, p = 0.01].
Conclusion
12 weeks of treatment with curcumin in HD patients resulted in a reduction in the biomarker of inflammation (TNF-α), confirming our previous hypothesis that curcumin has an anti-inflammatory effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Botanically, Curcuma Longa is related to ginger (Zingiberaceae family); it is a perennial plant with a short stem and large oblong leaves; it also features oval, piriform, or oblong rhizomes, which are often branched and brownish-yellow in color [1]. This striking color is the bioactive compound curcumin, the main component of this plant [2]. Curcuma Longa contains a class of curcuminoids, composed of curcumin, demethoxycurcumin, and bisdemethoxycurcumin, but curcumin is the most studied compound in the literature [3, 4]. Curcuma Longa is a medicinal plant widely used for thousands of years by Asian countries as a food additive, preservative, and coloring.
According to several studies, curcumin has a therapeutic potential as an anti-inflammatory, antioxidant, anti-diabetic, anticancer, and anti-aging agent [5,6,7]. Regarding its anti-inflammatory function, curcumin can decrease the production of proinflammatory cytokines such as tumor of necrose factor-alpha (TNF-α), C-reactive protein (CRP), and interleukin (IL) 6 [8, 9]. Curcumin is a potent antioxidant that reduces the generation of reactive oxygen species (ROS) through its hydrogen-donating antioxidant activity, consequently reducing the cell damage. In addition, curcumin facilitates nuclear translocation of factor 2 related to nuclear erythroid factor-2 (Nrf2) by uncoupling of its binding protein Kelch-like ECH-associated protein 1 (Keap 1) and increases the expression of antioxidant enzymes, thus preventing lipid peroxidation and other damage to cellular proteins and DNA [10]. Also, curcumin seems to reduce the activation of nuclear factor κB (NF-κB), which is involved with cytokines synthesis and inflammation. Indeed, our previous study showed that curcumin reduced the expression of NF-κB [11] as well, decreased the plasma levels of p-cresyl sulfate (a uremic toxin produced by the gut microbiota involved with inflammation) in patients with chronic kidney disease on hemodialysis (HD) [12].
Chronic kidney disease (CKD) is a known worldwide public health problem [13] and is involved with many complications such as inflammation and oxidative stress [14], which are important risk factors for developing cardiovascular disease and accelerate kidney disease progression [15]. Regarding the activation of NF-κB in these patients, studies have shown overregulation, consequently increasing the production of proinflammatory cytokines [16].
Into the concept of food as medicine for CKD patients [17], several nutritional strategies have been proposed to these patients [11, 18,19,20,21], including the use of curcumin to mitigate inflammation as well oxidative stress [22].
Currently, some studies have shown the positive effects of curcumin supplementation in vivo models in animals and humans [23,24,25]. These studies demonstrated that curcumin positively regulates Nrf2 with a consequent increase in antioxidant enzymes and a reduction in inflammatory markers in CKD [23,24,25,26]. Furthermore, curcumin was also efficient in improving intestinal architecture by increasing tight junction proteins and alkaline intestinal phosphatase (IAP), leading to lower intestinal permeability and better circulation of inflammatory factors in the systemic circulation [27]. Previously we showed a reduction in NF-κB expression after curcumin treatment. Thus, we hypothesized here that curcumin supplementation decreases oxidative stress and inflammation biomarkers in hemodialysis patients. This study aims to evaluate the plasma levels of inflammation and oxidative stress markers after curcumin supplementation in patients with CKD on HD.
Methods and patients
These are secondary exploratory analyses from a previous study [11], that involved 28 HD patients from Renalcor Clinic, RJ, Brazil from March to December 2018, previously included in a study from our research group. For the sample calculation, the test power of 80% was used, with a type I error probability, and a null hypothesis test of 0.05 [11].
The Ethical Committee approved this project (number: 2346933). This study is registered in ClinicalTrials.Gov under the number NCT 03475017. Inclusion criteria were CKD patients on HD for 6 months, over the age of 18 years, and with access through an arteriovenous fistula (AVF). Exclusion criteria were pregnant patients, smokers, patients who received antibiotics in the last 3 months, antioxidant supplements and usual intake of turmeric, patients with autoimmune and infectious diseases, cancer, liver disease, and AIDS. The dialysis treatment was performed with fibre cellulose acetate dialyzers for 3.5–4.5 h/session 3×/week with 500 ml/min of dialysate flow and blood flow greater than 250 ml/min.
Patients were instructed to maintain ingested medications, as prescribed by the physician, such as simvastatin, beta receptor blockers, diuretics, metformin, and insulin, which were not changed during the study [11].
The primary outcome of the present study is the decrease in plasma levels of inflammatory markers such as IL-6, and TNF-α plasma levels and the secondary outcome is the decrease in oxidative stress markers, which are represented by oxidized low-density lipoprotein-LDL (LDL-ox) and malondialdehyde (MDA) plasma levels.
Experimental design
Patients were randomized in double-blind conditions into two groups: curcumin group, in which patients were given a juice containing 100 mL of orange juice, 12 g of carrots, and 2.5 g of turmeric extract (95% curcumin) three times per week at the end of the HD session, for 12 weeks, and the control group, which received the same juice without curcumin (Fig. 1). Adherence to the intervention was made by the researchers who checked whether the juice had been offered and consumed by the patients at the end of each dialysis session.
A trained professional performed the allocation into groups, curcumin and control, through a list of codes. The inclusion and exclusion criteria were previously applied to carry out the randomization. After this step, the patients were allocated in 1:1 branches according to the groups. Details about the study design and randomization have been published previously [11].
General data, analysis of food intake, and anthropometric data
Demographic, clinical, and biochemical data were obtained by analyzing the medical record, interviewing during consultations/reconsults, and collecting biological material (blood). The details were previously described in [11]. Food intake was assessed at the beginning and end of the intervention through 24-h food recall, and data were analyzed using NutWin® software [11] and analyzed according to KDOQI, 2020. As previously described in [11], the body mass index (BMI) was calculated.
Sample preparation and oxidative stress and inflammation markers
Blood samples were collected in the morning, before the start of the HD session, and midweek, with patients fasting for 12 h. For plasma preparation, blood tubes were centrifuged at 2500 rpm for 10 min at 4 °C, which was distributed in identified 1.5-ml polypropylene Eppendorf, and stored at −80 °C for later analysis [11].
The markers of inflammation, IL-6, and TNF-α, were measured via ELISA sandwich enzyme immunoassays using commercially available kits (PeproTech®, Rocky Hills, NJ, USA), following the manufacturer's recommendations for the development of the experiment. The Optical Density (OD) at 405 nm was measured using a Synergy II Microplate Reader (Biotek®) [28]. LDL-ox plasma levels were measured using the Human Ox-LDL ELISA kit (Elabscience Biotechnology Co., Ltd, USA) according to the manufacturer's protocol [29]. The reaction between MDA and thiobarbituric acid was measured according to the modified Ohkawa method to evaluate the lipid peroxidation [30].
Statistical analysis
The Shapiro Wilk test was used to verify the distribution of the variables. Normally distributed variables were expressed as mean ± standard deviation, and non-normally distributed variables were expressed as median (min–max). Comparisons between the baseline of the groups were performed using the two-tailed unpaired Student t-Test for parametric variables, and the Mann–Whitney test for the nonparametric variables. A mixed linear model was performed, and sex and age were added as influence potentials. The statistical analyses were performed through Jamovi 1.6.3 software.
Results
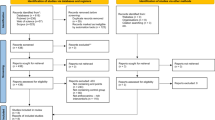
Sixty-three patients were assessed for eligibility, but 32 dropped out. Then, 31 patients were randomized to the curcumin and control group. A total of 28 patients completed the 3 months of curcumin supplementation as shown in the CONSORT diagram (Fig. 2), 14 patients in the curcumin group (54.0 ± 15 years, 7 women) and 14 in the control group (53.0 ± 12 years, 7 women). No side effects were observed in any of the groups during the intervention.
There was no statistical difference in any biochemical, anthropometric, or food intake parameters between the groups at the baseline, as shown in Table 1. There was no statistical difference concerning these data before and after the intervention [11].
Table 2 shows the inflammation and oxidative stress markers in both groups. There was no difference between the baseline values in the groups, IL-6 (p = 0.32), TNF-α (p = 0.94), MDA (p = 0.085) and ox-LDL (p = 0.17).
Regarding oxidative stress markers, no statistical difference was observed concerning ox-LDL and MDA plasma levels after 12 weeks of intervention, and there was also no statistical difference in the concentration of IL-6 plasma levels (Table 2). Figure 3 demonstrates that plasma levels of TNF-α were significantly reduced after curcumin intervention.
Discussion
This secondary analysis of our previous randomized controlled trial [11] showed that curcumin supplementation for 12 weeks in HD patients reduced the plasma levels of TNF-α, an inflammatory biomarker. However, this supplementation did not change oxidative stress markers such as MDA and ox-LDL. Our previous study demonstrated that curcumin supplementation in HD patients decreased the expression of NF-κB mRNA in peripheral blood mononuclear cell (PBMC) and also the plasma levels of ultrasensitive C-reactive protein (hs-CRP), but the Nrf2 mRNA expression was not changed [11], which corroborate with the current study since there was no change in oxidative stress markers after curcumin supplementation. On the other hand, the reduction of TNF-α plasma levels may be justified due to the downregulation of NF-κB by the curcumin intervention [11].
There are some hypotheses for this TNF-α plasma levels reduction. Curcumin acts as a TNF-α suppressor mainly at the transcriptional level. Curcumin can mediate TNF-α expression by inhibiting p300/Cyclic AMP-responsive element-binding protein (CREB)-specific acetyltransferase, generating repression of histone protein acetylation and histone acetyl transferase-dependent chromatin transcription [31, 32]. Furthermore, methylation of TNF promoters by curcumin decreases their expression. The receptors involved in TNF activation, toll-like receptors (TLRs), may also have their responsiveness decreased by curcumin treatment [33]. Also, it is also well known that curcumin can decrease NF-κB activation and lead to down-regulation of this transcription factor [34]. Experimental studies in cells and animals have confirmed that curcumin decreases the inflammatory cytokines such as TNF-α [35,36,37]. Curcumin inhibits NF-κB activation directly by inhibiting the degradation of IκB-α and reacting with NF-kB itself [38, 39]. Consequently, the production of proinflammatory mediators, including TNF-α, is suppressed by curcumin [39, 40]. Furthermore, TNF-α can be lowered by curcumin through IKK suppression, which prevents the NF-κB translocation to the nucleus and activation [10].
In addition, TNF-α can be inhibited by binding directly to curcumin. Curcumin docks to TNF-α receptor binding sites, including Leu89, Asn90, Asp105, Asn106 and Cys129. This binding is due to non-covalent and covalent interactions, interrupting the signal transduction between TNF-α and its receptor by direct binding and suppressing the inflammation induced by this cytokine [41]. Thus, one of the hypotheses that can be raised in this study is that curcumin inhibited TNF-α by direct action, a fact that does not happen with IL-6.
Randomized clinical studies in CKD patients that evaluated the effects of curcumin supplementation on inflammation corroborate our result [24, 42,43,44]. Samadian et al.[24] showed that 1500 mg/day of turmeric extract (66,3 mg of curcumin) in a capsule for 12 weeks in hemodialysis patients reduced TNF-α levels, hs-PCR, and IL-6 plasma levels. Another study with patients in hemodialysis showed that 120 mg of nano curcumin for 12 weeks also decreased TNF-α and IL-6 plasma levels [43]. Thus, as in the present study, Rodrigues et al. [44] did not find a reduction in the MDA concentration before and after the 1.0 g/day of curcumin after 12 weeks.
In addition, a recent systematic review on the effects of curcumin supplementation in CKD patients demonstrated that curcumin has particularly favorable effects on inflammation and oxidative stress and also is associated with a reduction in proteinuria [45]. Inflammation plays an essential role in CKD progression, mainly due to its ability to improve the regulation of intrarenal microcirculation and perfusion distribution [46]. Furthermore, chronic inflammation in CKD patients is associated with several factors, including dysregulation of intestinal microbiota, complications associated with uremia, a diet low in fruits, vegetables, and legumes [22, 46]. In this context, curcumin appears to be a good nutritional strategy to reduce inflammation in hemodialysis patients [22, 46].
The results presented in this study must be interpreted with some limitations. We did not assess the absorption of curcumin in the plasma of patients, and its absorption may have been insufficient to find a significant difference in the concentration of MDA, Ox-LDL and IL-6. Curcumin is a polyphenol with rapid metabolism and systemic elimination, which may contribute to low plasma and tissue levels of curcumin. Despite this, according to Aggarwal et al. [34], curcumin can still manifest its effect in vivo. Some approaches could be used to improve the bioavailability of curcumin, such as the use of adjuvants that interfere with glucuronidation, the use of liposomal curcumin, nanoparticles; the use of curcumin phospholipid complex; or use of structural analogs of curcumin [47]. Unlike previously published studies, this study uses curcumin supplementation through a juice, thus preserving the concept of "foodome," the bioactive compound can be potentiated due to its ingredients and chemical structure [11, 48]. Therefore, further studies in HD patients with a larger number of participants should be encouraged.
In conclusion, curcumin supplementation for 12 weeks in hemodialysis patients decreases the plasma concentration of TNF-α and can be a good strategy to mitigate inflammation in CKD patients.
References
Chattopadhyay KB, Bandyopadhyay U, Banerjee RA (2004) Turmeric and curcumin: biological actions and medicinal applications. Curr Sci 87:44–53
Kotha RR, Luthria DL (2019) Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 24:2930. https://doi.org/10.3390/molecules24162930
Hewlings SJ, Kalman DS (2017) Curcumin: a review of its effects on human health. Foods 6:92. https://doi.org/10.3390/foods6100092
Soleimani V, Sahebkar A, Hosseinzadeh H (2018) Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother Res 32:985–995. https://doi.org/10.1002/ptr.6054
Patel SS, Acharya A, Ray RS, Agrawal R, Raghuwanshi R, Jain P (2020) Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit Rev Food Sci Nutr 60:887–939. https://doi.org/10.1080/10408398.2018
Pivari F, Mingione A, Brasacchio C, Soldati L (2019) Curcumin and type 2 diabetes mellitus: prevention and treatment. Nutrients 11:1837. https://doi.org/10.3390/nu11081837
Mirzaei H, Bagheri H, Ghasemi F, Khoi JM, Pourhanifeh MH, Heyden YV, Mortezapour E, Nikdasti A, Jeandet P, Khan H, Sahebkar A (2021) Anti-cancer activity of curcumin on multiple myeloma. Anticancer Agents Med Chem 21:575–586. https://doi.org/10.2174/1871520620666200918113625
Liu J, Liu H, Xiao C, Fan H, Huang Q, Liu Y, Wang Y (2014) Curcumin protects neurons against oxygen-glucose deprivation/reoxygenation-induced injury through activation of peroxisome proliferator-activated receptor-c function. J Neurosci Res 92:1549–1559. https://doi.org/10.1002/jnr.23438
Rahimi HR, Mohammadpour AH, Dastani M et al (2016) The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed 6:567–577
Zia A, Farkhondeh T, Pourbagher-Shahri AM, Samarghandian S (2021) The role of curcumin in aging and senescence: molecular mechanisms. Biomed Pharmacother 134:111–119. https://doi.org/10.1016/j.biopha.2020.111119
Alvarenga L, Salarolli R, Cardozo LFMF, Santos RS, de Brito JS, Kemp JA, Reis D, de Paiva BR, Stenvinkel P, Lindholm B, Fouque D, Mafra D (2020) Impact of curcumin supplementation on the expression of inflammatory transcription factors in hemodialysis patients: a pilot randomized, double-blind, controlled study. Clin Nutr 39:3594–3600. https://doi.org/10.1016/j.clnu.2020.03.007
Salarolli RT, Alvarenga L, Cardozo LFMF, Teixeira KTR, de Moreira SGL, Lima JD, Rodrigues SD, Nakao LS, Fouque D, Mafra D (2021) Can curcumin supplementation reduce plasma levels of gut-derived uremic toxins in hemodialysis patients? A pilot randomized, double-blind, controlled study. Int Urol Nephrol 53:1231–1238. https://doi.org/10.1007/s11255-020-02760-z
Ng JK, Li PK (2018) Chronic kidney disease epidemic: how do we deal with it? Nephrology (Carlton) 23:116–120. https://doi.org/10.1111/nep.13464
Rapa SF, Di Iorio BR, Campiglia P, Heidland A, Marzocco S (2019) Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int J Mol Sci 21:263. https://doi.org/10.3390/ijms21010263
Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B (2019) Oxidative stress in chronic kidney disease. Pediatr Nephrol 34:975–991. https://doi.org/10.1007/s00467-018-4005-4
Pedruzzi LM, Cardozo LF, Medeiros RF, Stockler-Pinto MB, Mafra D (2015) Association between serum ferritin and lipid peroxidation in hemodialysis patients. J Bras Nefrol 37:171–176. https://doi.org/10.5935/0101-2800.20150028
Mafra D, Borges NA, Lindholm B, Shiels PG, Evenepoel P, Stenvinkel P (2021) Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat Rev Nephrol 17:153–171. https://doi.org/10.1038/s41581-020-00345-8
Alvarenga L, Cardozo LFMF, Borges NA, Chermut TR, Ribeiro M, Leite M Jr, Shiels PG, Stenvinkel P, Mafra D (2021) To bee or not to bee? The bee extract propolis as a bioactive compound in the burden of lifestyle diseases. Nutrition 83:111094. https://doi.org/10.1016/j.nut.2020.111094
de Paiva BR, Esgalhado M, Borges NA, Kemp JA, Alves G, Leite PEC, Macedo R, Cardozo LFMF, de Brito JS, Mafra D (2020) Resistant starch supplementation attenuates inflammation in hemodialysis patients: a pilot study. Int Urol Nephrol 52:549–555. https://doi.org/10.1007/s11255-020-02392-3
Esgalhado M, Kemp JA, Paiva BR, Brito JS, Cardozo LFMF, Azevedo R, Cunha DB, Nakao LS, Mafra D (2020) Resistant starch type-2 enriched cookies modulate uremic toxins and inflammation in hemodialysis patients: a randomized, double-blind, crossover and placebo-controlled trial. Food Funct 11:2617–2625. https://doi.org/10.1039/c9fo02939g
Stockler-Pinto MB, Soulage CO, Borges NA, Cardozo LFMF, Dolenga CJ, Nakao LS, Pecoits-Filho R, Fouque D, Mafra D (2018) From bench to the hemodialysis clinic: protein-bound uremic toxins modulate NF-κB/Nrf2 expression. Int Urol Nephrol 50:347–354. https://doi.org/10.1007/s11255-017-1748-y
Alvarenga LA, Leal VO, Borges NA, Aguiar AS, Faxen-Irving G, Stenvinkel P et al (2018) Curcumin—a promising nutritional strategy for chronic kidney disease patients. J Funct Foods 40:715
Moreillon JJ, Bowden RG, Deike E, Griggs J, Wilson R, Shelmadine B, Cooke M, Beaujean A (2013) The use of an anti-inflammatory supplement in patients with chronic kidney disease. J Complement Integr Med. https://doi.org/10.1515/jcim-2012-0011
Samadian F, Dalili N, Poor-Reza Gholi F, Fattah M, Malih N, Nafar M, Firoozan A, Ahmadpoor P, Samavat S, Ziaie S (2017) Evaluation of Curcumin’s effect on inflammation in hemodialysis patients. Clin Nutr ESPEN 22:19–23. https://doi.org/10.1016/j.clnesp.2017.09.006
Ali BH, Al-Salam S, Al Suleimani Y, Al Kalbani J, Al Bahlani S, Ashique M, Manoj P, Al Dhahli B, Al Abri N, Naser HT, Yasin J, Nemmar A, Al Za’abi M, Hartmann C, Schupp N (2018) Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin Pharmacol Toxicol 122:65–73. https://doi.org/10.1111/bcpt.12817
Ghosh SS, Massey HD, Krieg R, Fazelbhoy ZA, Ghosh S, Sica DA, Fakhry I, Gehr TW (2009) Curcumin ameliorates renal failure in 5/6 nephrectomized rats: role of inflammation. Am J Physiol Renal Physiol 296:F1146–F1157. https://doi.org/10.1152/ajprenal.90732.2008
Ghosh SS, Gehr TW, Ghosh S (2014) Curcumin and chronic kidney disease (CKD): major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 19:20139–20156. https://doi.org/10.3390/molecules191220139
Lourenço ES, Alves GG, de Lima BR, Spiegel CN, de Mello-Machado RC, Al-Maawi S, Ghanaati S, de Almeida Barros Mourão CF (2021) Effects of rotor angle and time after centrifugation on the biological in vitro properties of platelet rich fibrin membranes. J Biomed Mater Res B Appl Biomater 109(1):60–68. https://doi.org/10.1002/jbm
Mondal K, Chakraborty P, Kabir SN (2018) Hyperhomocysteinemia and hyperandrogenemia share PCSK9-LDLR pathway to disrupt lipid homeostasis in PCOS. Biochem Biophys Res Commun 503:8–13. https://doi.org/10.1016/j.bbrc.2018.04.078
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U et al (2004) Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem 279:51163–51171
Zhong W, Qian K, Xiong J, Ma K, Wang A, Zou Y (2016) Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed Pharmacother 83:302–313. https://doi.org/10.1016/j.biopha.2016.06.036
Matsuguchi T, Musikacharoen T, Ogawa T, Yoshikai Y (2000) Gene expressions of Toll-like receptor 2, but not Toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J Immunol 165:5767–5772
Aggarwal BB, Gupta SC, Sung B (2013) Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol 169:1672–1692. https://doi.org/10.1111/bph.12131
Brennan P, O’Neill LA (1998) Inhibition of nuclear factor κB by direct modification in whole cells–mechanism of action of nordihydroguaiaretic acid, curcumin and thiol modifiers. Biochem Pharmacol 55:965–973. https://doi.org/10.1016/S0006-2952(97)00535-2
Kong R, Kang OH, Seo YS, Zhou T, Kim SA, Shin DW, Kwon DY (2018) MAPKs and NF-κB pathway inhibitory effect of bisdemethoxycurcumin on phorbol 12-myristate13-acetate and A23187-induced inflammation in human mast cells. Mol Med Rep 17:630–635. https://doi.org/10.3892/mmr.2017.7852
Soetikno V et al (2011) Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab 8:35. https://doi.org/10.1186/1743-7075-8-35
Xu Y, Liu L (2017) Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-kappaB signaling pathway. Influenza Other Respir Viruses 11:457–463. https://doi.org/10.1111/irv.12459
Lee KH et al (2012) BDMC33, a curcumin derivative suppresses inflammatory responses in macrophage-like cellular system: role of inhibition in NF-kappaB and MAPK signaling pathways. Int J Mol Sci 13:2985–3008. https://doi.org/10.3390/ijms13032985
Kahkhaie KR, Mirhosseini A, Aliabadi A, Mohammadi A, Mousavi MJ, Haftcheshmeh SM, Sathyapalan Sahebkar T (2019) A. Curcumin: a modulator of inflammatory signaling pathways in the immune system. Inflammopharmacology 27:885–900. https://doi.org/10.1007/s10787-019-00607-3.Erratum.In:Inflammopharmacology.2019
Gupta SC, Prasad S, Kim JH et al (2011) Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep 28:1937–1955. https://doi.org/10.1039/c1np00051a
Vafadar Afshar G, Rasmi Y, Yaghmaei P, Khadem-Ansari MH, Makhdomii K, Rasooli J (2020) The effects of nano-curcumin supplementation on serum level of hs-CRP, adhesion molecules, and lipid profiles in hemodialysis patients, a randomized controlled clinical trial. Iran J Kidney Dis 14:52–61. Erratum in: Iran J Kidney Dis. 2020, 14:165
Vafadar-Afshar G, Rasmi Y, Yaghmaei P, Khadem-Ansari MH, Makhdoomi K, Rasouli J (2021) The effects of nano curcumin supplementation on inflammation in hemodialysis patients: a randomized controlled trial. Hemodial Int 25:232–239. https://doi.org/10.1111/hdi.12911
Rodrigues HCN, Martins TFP, Santana NCFES, Braga CC, Silva MAC, Cunha LCD, Sugizaki CSA, Freitas ATVS, Costa NA, Peixoto MDRG (2021) Antioxidant and anti-inflammatory response to curcumin supplementation in hemodialysis patients: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr ESPEN 44:136–142. https://doi.org/10.1016/j.clnesp.2021.06.006
Bagherniya M, Soleimani D, Rouhani MH, Askari G, Sathyapalan T, Sahebkar A (2021) The use of curcumin for the treatment of renal disorders: a systematic review of randomized controlled trials. Adv Exp Med Biol 1291:327–343. https://doi.org/10.1007/978-3-030-56153-6_19
Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, Mambet C, Anton G, Tanase C (2018) Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res 2018:2180373. https://doi.org/10.1155/2018/2180373
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–818. https://doi.org/10.1021/mp700113r
Capozzi F (2017) NMR-based metabolomics: the foodome and the assessment of dietary exposure as a key step to evaluate the effect of diet on health. In: Webb G (ed) Modern magnetic resonance. Springer, Cham
Acknowledgements
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) support Denise Mafra research. CAPES-COFECUB (Comité français d'évaluation de la coopération universitaire et scientifique avec le Brésil) support Denis Fouque and Denise Mafra.
Author information
Authors and Affiliations
Contributions
LA contributed to the data collection and sample analysis; performed statistical analyses; produced the tables and figures; and preparation of the manuscript. BOdaC and BRP actively contributed to samples analysis of the present study. LC participated of all analysis; restructured and revised the manuscript. DF contributed to the interpretation, restructured and revised the manuscript. DM coordinated all the steps of research, restructured and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alvarenga, L., Cardozo, L.F.M.F., Da Cruz, B.O. et al. Curcumin supplementation improves oxidative stress and inflammation biomarkers in patients undergoing hemodialysis: a secondary analysis of a randomized controlled trial. Int Urol Nephrol 54, 2645–2652 (2022). https://doi.org/10.1007/s11255-022-03182-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03182-9