Abstract
Chronic kidney disease (CKD) is one of the significant causes of morbidity and mortality worldwide, which could develop and progress to end-stage renal disease. Increased inflammation and reduced antioxidant capacity commonly occur in CKD and hemodialysis patients. Curcumin is a natural bioactive compound with antioxidant and anti-inflammatory properties. This systematic review was undertaken with the main aim of assessing the effects of curcumin/turmeric supplementation on renal diseases based on clinical trials. A comprehensive search was performed in PubMed/MEDLINE, Scopus, ISI Web of Science, and Google Scholar from inception up to April 6, 2020 to identify clinical trials assessing the effects of curcumin or turmeric alone, or in combination with other herbs or nutrients on renal diseases. Twelve studies met the eligibility criteria. These randomized controlled trials (RCTs) comprised 631 patients with either chronic kidney diseases (CKD), hemodialysis, diabetic proteinuria and nephropathy, and lupus nephritis. Curcumin/turmeric supplementation had favorable effects on renal diseases, particularly in terms of inflammation and oxidative stress. However, with the exception for proteinuria, their impact on clinical parameters, such as blood urea nitrogen, creatinine, glomerular filtration rate (GFR), and serum albumin, was weak and not significant. No serious adverse effects were reported following curcumin/turmeric supplementation. Within the limitations of this review, it can be concluded that curcumin/turmeric supplementation might have some beneficial effects on inflammatory and oxidative stress parameters of patients but no considerable positive impact on clinical outcomes of kidney diseases, apart from proteinuria.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
19.1 Introduction
Chronic kidney disease (CKD) is a significant cause of morbidity and mortality worldwide, which imposes a high economic cost to the healthcare systems [1]. CKD has a direct correlation with increased risks of cardiovascular morbidity, premature death, hospitalization, cognitive dysfunction, and decreased quality of life [1,2,3,4]. A recent meta-analysis indicated a high global prevalence of CKD (11–13% with the majority in stage 3) [1]. End-stage renal disease (ESRD) occurs when the glomerular filtration rate (GFR) is substantially decreased and accompanied by signs and symptoms of kidney failure that necessitate replacement therapy [5].
Today, diabetes and hypertension are the primary cause of ESRD [6]. The ever-increasing high prevalence of type 2 diabetes has led to a significant rise in the number of patients with CKD and the number requiring end-stage renal failure management, particularly with the need for dialysis [6].
It has been demonstrated that inflammation plays a central role in the etiology of chronic diseases such as CKD, cardiovascular disease, diabetes, metabolic syndrome, and hypertension [7,8,9,10,11]. Increased inflammation and reduced antioxidant capacity is a common complication among CKD and hemodialysis patients [11, 12]. One of the significant factors involved in the development and progression of nondiabetic or diabetic proteinuric CKD and its related complications is increased oxidative stress among patients with renal disorders [13, 14]. Controlling proteinuria and delaying the progression of CKD in adults are the main targets of the current treatment of kidney diseases [15, 16]. Likewise, although dialysis and transplantation are an effective way of managing end-stage renal failure, these approaches are not optimal therapies for kidney failure since they are associated with several adverse effects [5]. In CKD and hemodialysis patients, it has been shown that high levels of inflammatory and hyperlipidemic factors are associated with the increased risk of cardiovascular disease [17,18,19,20].
Finding new, safe, and practical therapeutic approaches to treat kidney diseases as one of the strategies or complementary therapy, with an emphasis on reducing inflammation, has recently attracted significant attention. In this regard, medicinal plants and herbal bioactive compounds with antidiabetic, antilipidemic, anti-hypertension, and anti-inflammatory properties could be potential candidates to consider as an alternative or complementary medicine in the treatment of renal diseases [21,22,23,24,25].
Curcumin is the main active ingredient in turmeric with several proven health benefits [26,27,28,29,30,31]. It has been shown that this natural bioactive compound has several unique properties such as anti-tumor, anti-inflammatory, antioxidant, antithrombotic, chemosensitizing and chemopreventive, neuroprotective and cardioprotective, lipid-modifying, analgesic, and antirheumatic activities [22, 32,33,34,35,36]. Recently, the favorable effects of curcumin on kidney diseases were shown in preclinical studies [37,38,39]. In addition, the results of these medicinal herbs on kidney diseases have been assessed in clinical trials [40,41,42].
Although the effects of curcumin/turmeric on renal diseases were assessed in some clinical trials, to our knowledge, there has been no review of the main findings of these studies. Thus, the main aim of this systematic review was to assess the effects of curcumin or turmeric supplementation on kidney diseases in clinical trials.
19.2 Methods
This systematic review was designed and reported based on the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [43].
19.2.1 Search Strategy
ISI web of science, Scopus, PubMed, and Google Scholar databases systematically searched up to April sixth, 2020 without any limitation for language and date using the following keywords: (“curcumin” OR “curcuminoids” OR “turmeric”) AND (“hemodialysis” OR “Chronic Kidney Disease” OR “Acute Kidney Disease” OR “nephritis” OR “renal diseases” OR “kidney diseases” OR “nephro” OR “Nephrotic syndrome”) AND (“Intervention Study” OR “Intervention Studies” OR “Controlled trial” OR “Randomized controlled trial” OR “Randomised clinical trial” OR “Non-Randomized Controlled Trials” OR “Clinical Trial” OR “Non-Randomized Controlled Trials” OR “Cross-Over study” OR “Cross-Over trial” OR “Cross Over trial” OR “Cross Over study” OR “Double-Blind Method” OR “Double-Blind” OR “Double-Blind trial” OR “Double-Blind study”). Whenever possible, Medical Subject Headings (MESH) terms were also used. Finally, a direct search was performed of the reference lists of the included original papers and review articles to identify other relevant works for inclusion in the current review.
19.2.2 Study Selection
Two independent authors (MB and GA) reviewed the title and abstract of all papers found in the first stages of the search. Articles were excluded if they did not meet the inclusion criteria using a screen form with a hierarchical approach based on study design, population, exposure, and outcome, and reference lists of relevant review articles were reviewed to explore additional studies. The full texts of eligible citations were considered. Any disagreements were discussed and a final agreement made.
19.2.3 Inclusion and Exclusion Criteria
The main aims of the search were to identify articles investigating the effects of curcumin/turmeric supplementation alone or in combination with other herbs or nutrients on kidney or renal diseases. Original articles were included in the present systematic review which followed these criteria: (1) use of a clinical trial design; (2) curcumin or turmeric supplementation was applied; and (3) the study was conducted on patients with kidney or renal diseases as a primary disease or secondary condition.
Studies were excluded if they (1) were uncontrolled; (2) reported duplicate data; or (3) were reviews, letters, editorial articles, study protocol, or case reports.
19.2.4 Data Extraction
Relevant articles were selected after screening records from the initial search. The following information was extracted from eligible studies and reported in Table 19.1: publication information including the first author’s last name, publication date, study location, details of the clinical trial including sample size, patients, mean age (years), study design, intervention (treatment), control, duration of the study, findings of the studies including main results, side effects, and study quality using Jadad Scores.
19.2.5 Quality Assessment
Two independent reviewers (MB and GA) evaluated the quality of the included studies using the Jadad scale, which contained three domains as follows: (1) randomization (0-2 points); (2) blinding (0-2 points); and (3) dropouts and withdrawals (0-1 points) [44]. According to the mentioned domains, the overall quality of each study was considered as low or high quality if the scores were less than or equal to 2 or greater than or equal to 3, respectively [45].
19.3 Results
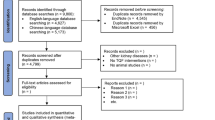
The online search yielded in 679 non-duplicated records, of which, after reading the title and abstracts, 660 irrelevant records were omitted. Nineteen articles were selected for a full assessment, of which 12 records were appropriate (Fig. 19.1). Thus, data extraction was performed on 12 articles [40,41,42, 46,47,48,49,50,51,52,53,54]. The characteristics of each selected paper are shown in Table 19.1. The studies were published between 2011 and 2020 and comprised a total of 631 patients. Eight studies were conducted in Iran [40, 42, 47, 48, 51,52,53,54], two in Mexico [46, 50], one in Brazil [41], and one in the United States of America [49]. Six studies worked on hemodialysis patients [41, 42, 50,51,52,53], three studies were conducted on CKD patients (with or without diabetes) [40, 46, 49], and one of these articles worked on contrast-induced nephropathy patients [40]. One study was conducted on patients with overt type 2 diabetic nephropathy [47], one in patients with type 2 diabetes mellitus with overt albuminuria [54], and one in patients with relapsing or refractory biopsy-proven lupus nephritis [48]. All of the studies were conducted on both male and females, except for two studies which did not mention the gender of the participants [49, 53]. The mean age of the participants varied from 33.6 to 63.8 years. All of the included studies were conducted in the context of randomized controlled trials, all applied randomization, and all had a placebo group. One study used nano-curcumin (120 mg/day) [42], six studies used turmeric (500 mg–2.5 g daily) [41, 47, 48, 51,52,53], three studies used curcumin (320–1500 mg daily) [40, 46, 54], one applied curcumin and Boswellia serrata (each capsule contained 824 mg of purified turmeric extract, 95% curcuminoids, and 516 mg of Boswellia serrata extract; 2 capsules/day) [49], and one study used curcumin (500 mg/daily) in combination with resveratrol (500 mg/day) [50]. The duration of the studies was in the range of 8–16 weeks [41, 42, 46,47,48,49,50,51,52,53,54]. One study used curcumin two days before coronary angiography or angioplasty to three days afterwards in CKD patients [40].
19.3.1 Quality Assessment
Overall , ten studies out of 12 were categorized as high quality [40,41,42, 47,48,49, 51,52,53,54], and two had a low-quality methodological approach [46, 50].
19.3.2 Effects of Curcumin on the Inflammatory and Stress Oxidative Status of Patients
In three studies, curcumin/turmeric consumption significantly reduced high-sensitivity C-reactive protein (hs-CRP) compared with placebo [41, 42, 52]. In one study, hs-CRP levels were significantly reduced after turmeric consumption, although no differences were found compared with the placebo group [53]. In one study, curcumin and Boswellia serrata did not affect CRP levels [49]. Four studies assessed the effects of curcumin/turmeric on serum interleukins (ILs), and one of these found that IL-6 levels were significantly reduced in response to treatment [49]. However, no significant difference was found than in placebo groups. In other studies, curcumin/turmeric had no significant effects on IL-6 [53], IL-8 [47], and IL-1β [41] compared with the placebo group. Likewise, in comparison to the placebo, curcumin/turmeric had no significant effects on TNF-α levels [47, 49, 53]. In one study, turmeric treatment significantly decreased nuclear factor kappa B (NF-κb) mRNA expression. However, mRNA expression of nuclear factor erythroid factor 2-related (Nrf2), Nod-like receptor pyrin domain containing 3 (NLRP3) inflammasome did not significantly change [41]. In one study, plasma malondialdehyde (MDA) was significantly decreased, and red blood cell catalase (CAT) activity was increased in the turmeric group compared with placebo, and glutathione peroxidase (GPX) and glutathione reductase (GR) were increased in both intervention and control groups. However, differences between groups were not significant [51]. One study showed that curcumin and resveratrol did not affect thiobarbituric acid reactive substance (TBARS) levels [50]. Another investigation showed that curcumin attenuated lipid peroxidation in individuals with nondiabetic proteinuric CKD and enhanced the antioxidant capacity in subjects with diabetic proteinuric CKD [46]. In the study of Ortiz et al., ferritin levels were significantly decreased in the curcumin and resveratrol group compared with placebo in patients with CKD and iron overload undergoing hemodialysis [50]. The adhesion molecules, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion protein 1 (VCAM-1) , were significantly decreased after 12 weeks of intervention with nano-curcumin in hemodialysis patients compared with the placebo [42].
19.3.3 Effects of Curcumin on the Clinical and Biochemical Parameters of the Kidney
Blood urea nitrogen (BUN) was assessed in five studies [47,48,49, 52, 54]. In all of these studies, curcumin/turmeric had no significant effect on this parameter compared with placebo. Likewise, creatinine was assessed in six studies, and curcumin/turmeric did not affect this in comparison to the control group [40, 47,48,49, 52, 54]. In the study conducted by Hami et al., serum creatinine was increased after 72-h intervention in both groups, but there was no difference between the groups [40]. In another study, curcumin treatment did not affect proteinuria in nondiabetic or diabetic proteinuric CKD [46]. However, turmeric treatment remarkably reduced proteinuria in relapsing or refractory lupus nephritis and overt type 2 diabetic nephropathy patients compared with placebo [47, 48]. Glomerular filtration rate (GFR) did not improve after curcumin/turmeric supplementation in nondiabetic or diabetic proteinuric CKD [46] and relapsing or refractory lupus nephritis patients [48]. After turmeric supplementation, serum albumin was significantly increased compared with placebo in a study conducted in hemodialysis patients [51]. However, there was no significant effect in five studies conducted on hemodialysis patients [41, 53], CKD patients [49], patients with relapsing or refractory lupus nephritis [48], and diabetic patients with overt albuminuria [54]. In the study of Vanaie et al., curcumin supplementation for 16 weeks resulted in a substantial reduction in albuminuria among diabetic patients with overt albuminuria in comparison to the placebo [54]. Other biochemical factors, such as serum uric acid [50], urea [41], phosphorus [41], potassium [41, 53], and hemoglobin [41, 51], were evaluated in a few studies, which found no significant effects. Moreover, compared with placebo, turmeric treatment had no effects on systolic and diastolic blood pressure, as assessed in two studies in patients with overt type 2 diabetic nephropathy and relapsing or refractory lupus nephritis [47, 48].
19.3.4 Effects of Curcumin on Lipid Profile, Glycemic Indices, and Liver Functional Tests
Curcumin/turmeric supplementation had no significant effects on lipid profile [41, 42, 46, 47, 51, 54], except for one study in which 12 weeks’ treatment with curcumin and resveratrol led to decreased triglycerides, very low density lipoprotein (VLDL), and cholesterol compared with placebo [50]. Fasting blood sugar (FBS) [42, 47, 50, 54] and hemoglobin A1C (HbA1c) [41, 54] levels did not change in response to curcumin/turmeric treatment. Likewise, after curcumin/turmeric supplementation, liver function tests such as measurement of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) did not change in the study groups [49, 53].
19.3.5 Effects of Curcumin/Turmeric on Other Outcomes and Adverse Effects
In their study, Ortiz et al. showed that 12 weeks’ intervention with curcumin and resveratrol significantly increased body mass index (BMI), muscle mass (kg), and bone mass (kg). At the same time, the percentage of fat and subjective global evaluation were significantly decreased (data of the control group were not reported) in patients with CKD and iron overload undergoing hemodialysis [50]. In the study conducted by Alvarenga et al., turmeric did not affect BMI or waist circumference [41]. Overall supplementation with curcumin/turmeric had no adverse side effects in renal diseases, although two studies stated that a few patients reported some adverse effects [49, 54] (Table 19.1).
19.4 Discussion
To the best of our knowledge, this is the first review which systematically assessed the effects of curcumin/turmeric supplementation on renal diseases. The main findings were that curcumin/turmeric supplementation had some favorable effects on renal diseases, particularly in terms of inflammation and stress oxidative, although the effects on clinical parameters such as BUN, creatinine, GFR, and serum albumin were weak and insignificant.
Atherosclerotic cardiovascular disease is the common and important cause of morbidity and mortality among patients with CKD. This appears to be a consequence of abnormalities of lipid metabolism, and a systemic increase in inflammation and oxidative stress in CKD patients [55]. Clinical evidence has documented that consumption of curcumin and turmeric can improve endothelial function and serum lipid profiles among patients with cardiovascular disease by modulating several pathways involved in lipid metabolism, including lipoprotein lipase (LPL), cholesteryl ester transfer protein (CETP), peroxisome proliferator-activated receptor gamma (PPAR-γ), and PPAR-α [56, 57]. In addition, it has well-established anti-inflammatory and anti-oxidative properties [29,30,31,32].
Both preclinical and clinical evidence suggests that curcumin can interfere with multiple signaling pathways linked to tissue injury, including the NF-κB signaling pathway, arachidonic acid (AA) metabolism, and the damaging effects of reactive oxygen species (ROS) [58]. NF-κB is the most critical transcriptional activator protein in the inflammatory cascade. Clinical evidence on hemodialysis patients showed that turmeric supplementation resulted in significant reduction in NF-kB mRNA expression [41]. In vivo and in vitro studies have shown that curcumin down-regulates NF-κB expression through inhibition of the c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinases (p38 MAPK), and extracellular signal-regulated kinase (ERK) pathways in response to pro-inflammatory stimuli and redox status [59,60,61,62]. Also, curcumin appears to inhibit nuclear NF-κB translocation through IκB kinase (IKK) activation and prevent NF-κB binding to the promoter region of inflammation-relevant genes [63].
Some effects of curcumin on inflammation can also be attributed to modulation of leukocyte recruitment into injured tissue, thereby altering AA metabolism and down-regulating NF-κB target genes, such as CXCL1, CXCL2, ICAM-1, and VCAM-1 [64, 65]. In line with this, a nano-formulation of curcumin has been found to reduce the levels of ICAM-1 and VCAM-1 adhesion molecules in hemodialysis patients after 12 weeks’ intervention [42]. AA metabolites, such as leukotrienes, have been implicated in the inflammatory cascade through increasing endothelial membrane permeability and leukocyte recruitment. Curcumin switches AA metabolism through inactivation of phospholipase A2 (PLA2G2A) and arachadonate 5-lipoxygenase (ALOX5) and down-regulation of cyclooxygenase-2 (PTGS2) gene expression via the NF-κB pathway [66]. This is consistent with findings which have shown that curcumin could act as a selective COX-2 inhibitor [67].
Patients with renal diseases have consistently exhibited an imbalance between oxidants and antioxidants status. Elevated free radical levels not only cause damage to DNA and increase lipid peroxidation, but also stimulate signaling pathways that switch on inflammation-relevant gene expression. Curcumin as the main phenolic compound in turmeric directly scavenges free radicals. In addition, increasing evidence supports its roles in enhancing both endogenous enzymatic and non-enzymatic antioxidants, perhaps due to induction of the Nrf2 transcription factor [68,69,70].
One of the major drawbacks of curcumin use is its poor absorption and bioavailability, which has severely limited its applications [71]. To overcome this limitation, one of the studies reviewed here used nano-curcumin [42]. The use of new formulations of curcumin such as curcumin-piperine or phospholipid curcumin with greater bioavailability may lead to better results in treatment outcomes for kidney disease patients [72].
Although this systematic review comprehensively assessed the effects of curcumin and turmeric supplementation on renal diseases based on randomized clinical trials, several limitations should be acknowledged. First, the small number of included studies and corresponding low numbers of participants made it difficult to draw robust evidence-based conclusions on its efficacy. Second, it was difficult to make comparisons across the studies due to their heterogeneous nature, regarding use of different forms of curcumin, different dosages, and treatment durations, as well as dissimilar outcome measures. Finally, more than a half of the studies (eight studies) were conducted in Iran, which restricts the ability to extend the findings to other populations.
19.5 Conclusions
Within the limitations of this review, it can be concluded that curcumin or turmeric supplementation might have some beneficial effects on inflammatory and stress oxidative parameters of patients with renal diseases. However, curcumin/turmeric had no considerable positive impact on clinical outcomes of kidney diseases apart from alleviation of proteinuria. The patients reported no serious adverse effects following curcumin/turmeric supplementation, supporting the case that these natural products are relatively safe. Thus, more randomized controlled trials using larger patient numbers and standardized protocols are needed for more accurate testing of the effects of curcumin supplementation on renal diseases.
References
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS et al (2016) Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS One 11(7):e0158765. https://doi.org/10.1371/journal.pone.0158765
Etgen T, Chonchol M, Forstl H, Sander D (2012) Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol 35(5):474–482
Perlman RL, Finkelstein FO, Liu L, Roys E, Kiser M, Eisele G et al (2005) Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis 45(4):658–666
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF et al (2013) Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382(9889):339–352
Alebiosu C, Ayodele O (2005) The global burden of chronic kidney disease and the way forward. Ethn Dis 15(3):418
Atkins RC (2005) The epidemiology of chronic kidney disease. Kidney Int 67:S14–S18
Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25(1):4–7
Haffner SM (2006) The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol 97(2):3–11
Manabe I (2011) Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J 75(12):2739–2748
Pawelec G, Goldeck D, Derhovanessian E (2014) Inflammation, ageing and chronic disease. Curr Opin Immunol 29:23–28
Akchurin M, Kaskel F (2015) Update on inflammation in chronic kidney disease. Blood Purif 39(1–3):84–92
Yao Q, Axelsson J, Stenvinkel P, Lindholm B (2004) Chronic systemic inflammation in dialysis patients: an update on causes and consequences. ASAIO J 50(6):lii–lvii. https://doi.org/10.1097/01.mat.0000147958.87989.eb
Forbes JM, Coughlan MT, Cooper ME (2008) Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57(6):1446–1454
Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC (2012) Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 17(4):311–321
Chandar J, Abitbol C, Montane B, Zilleruelo G (2007) Angiotensin blockade as sole treatment for proteinuric kidney disease in children. Nephrol Dial Transpl 22(5):1332–1337
Alicic RZ, Tuttle KR (2014) Novel therapies for diabetic kidney disease. Adv Chronic Kidney Dis 21(2):121–133
Pandya V, Rao A, Chaudhary K (2015) Lipid abnormalities in kidney disease and management strategies. World J Nephrol 4(1):83
Keane WF, Tomassini JE, Neff DR (2012) Lipid abnormalities in patients with chronic kidney disease: implications for the pathophysiology of atherosclerosis. J Atheroscler Thromb 20(2):123–133
Wanner C, Zimmermann J, Schwedler S, Metzger T (2002) Inflammation and cardiovascular risk in dialysis patients. Kidney Int 61:S99–S102
Qureshi AR, Alvestrand A, Divino-Filho JC, Gutierrez A, Heimbürger O, Lindholm B et al (2002) Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol 13(Suppl 1):S28–S36
Talebi S, Bagherniya M, Atkin SL, Askari G, Orafai HM, Sahebkar A (2020) The beneficial effects of nutraceuticals and natural products on small dense LDL levels, LDL particle number and LDL particle size: a clinical review. Lipids Health Dis 19(1):66. https://doi.org/10.1186/s12944-020-01250-6
Bagherniya M, Nobili V, Blesso CN, Sahebkar A (2018) Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: a clinical review. Pharmacol Res 130:213–240
Bagherniya M, Johnston TP, Sahebkar A (2020) Regulation of apolipoprotein B by natural products and nutraceuticals: a comprehensive review. Curr Med Chem. https://doi.org/10.2174/0929867327666200427092114. Online ahead of print
Musabayane C (2012) The effects of medicinal plants on renal function and blood pressure in diabetes mellitus. Cardiovasc J Afr 23(8):462–468
Iranshahi M, Sahebkar A, Hosseini ST, Takasaki M, Konoshima T, Tokuda H (2010) Cancer chemopreventive activity of diversin from Ferula diversivittata in vitro and in vivo. Phytomedicine 17(3–4):269–273
Kocaadam B, Şanlier N (2017) Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr 57(13):2889–2895
Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL (2005) A potential role of the curry spice curcumin in Alzheimer’s disease. Curr Alzheimer Res 2(2):131–136
Wongcharoen W, Phrommintikul A (2009) The protective role of curcumin in cardiovascular diseases. Int J Cardiol 133(2):145–151
Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A (2019) Immune modulation by curcumin: the role of interleukin-10. Crit Rev Food Sci Nutr 59(1):89–101
Hasanzadeh S, Read MI, Bland AR, Majeed M, Jamialahmadi T, Sahebkar A (2020) Curcumin: an inflammasome silencer. Pharmacol Res 159:104921. https://doi.org/10.1016/j.phrs.2020.104921. Epub 2020 May 25. PMID: 32464325
Teymouri M, Pirro M, Johnston TP, Sahebkar A (2017) Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: A review of chemistry, cellular, molecular, and preclinical features. BioFactors 43(3):331–346
Mohajeri M, Bianconi V, Ávila-Rodriguez MF, Barreto GE, Jamialahmadi T, Pirro M, Sahebkar A (2020) Curcumin: a phytochemical modulator of estrogens and androgens in tumors of the reproductive system. Pharmacol Res 156:104765. https://doi.org/10.1016/j.phrs.2020.104765. Epub 2020 Mar 23. PMID: 32217147
Sahebkar A (2013) Why it is necessary to translate curcumin into clinical practice for the prevention and treatment of metabolic syndrome? BioFactors 39(2):197–208
Momtazi AA, Derosa G, Maffioli P, Banach M, Sahebkar A (2016) Role of microRNAs in the therapeutic effects of curcumin in non-cancer diseases. Mol Diagn Ther 20(4):335–345
Momtazi AA, Sahebkar A (2016) Difluorinated curcumin: a promising curcumin analogue with improved anti-tumor activity and pharmacokinetic profile. Curr Pharm Des 22(28):4386–4397
Bagheri H, Ghasemi F, Barreto GE, Rafiee R, Sathyapalan T, Sahebkar A (2020) Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors. 46(1):5–20. https://doi.org/10.1002/biof.1566. Epub 2019 Oct 3. PMID: 31580521
Huang SJ, Huang J, Yan YB, Qiu J, Tan RQ, Liu Y et al (2020) The renoprotective effect of curcumin against cisplatin-induced acute kidney injury in mice: involvement of miR-181a/PTEN axis. Ren Fail 42(1):350–357
Damiano S, Andretta E, Longobardi C, Prisco F, Paciello O, Squillacioti C et al (2020) Effects of curcumin on the renal toxicity induced by Ochratoxin A in rats. Antioxidants (Basel) 9(4):E332. https://doi.org/10.3390/antiox9040332
Akomolafe SF, Olasehinde TA, Adewale OO, Ajayi OB (2020) Curcumin improves biomolecules associated with renal function and attenuates oxidative injury and histopathological changes in potassium-induced toxicity in rats. Kidney Biol Trace Elem. https://doi.org/10.1007/s12011-020-02113-y. Online ahead of print
Hami M, Bigdeli A, Rajabi O, Salehi M (2019) The effect of curcumin in prevention of contrast nephropathy following coronary angiography or angioplasty in CKD patients. Iran J Kidney Dis 13(5):304–309
Alvarenga L, Salarolli R, Cardozo LFMF, Santos RS, de Brito JS, Kemp JA et al (2020) Impact of curcumin supplementation on expression of inflammatory transcription factors in hemodialysis patients: a pilot randomized, double-blind, controlled study. Clin Nutr S0261-5614(20):30110–30112. https://doi.org/10.1016/j.clnu.2020.03.007. Online ahead of print
Afshar GV, Rasmi Y, Yagmaye P, Khadem-Ansari MH, Makhdomi K, Rasooli J (2020) The effects of nano-curcumin supplementation on serum level of hs-CRP, adhesion molecules, and lipid profiles in hemodialysis patients, a randomized controlled clinical trial. Iran J Kidney Dis 14(1):52–61
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Moher D, Cook D, Jadad A, Tugwell P, Moher M, Jones A et al (1999) Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses. Health Technol Assess 3(12):i–iv., 1–98
Jiménez-Osorio AS, García-Niño WR, González-Reyes S, Álvarez-Mejía AE, Guerra-León S, Salazar-Segovia J et al (2016) The effect of dietary supplementation with curcumin on redox status and Nrf2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: a pilot study. J Ren Nutr 26(4):237–244
Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH et al (2011) Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol 45(5):365–370
Khajehdehi P, Zanjaninejad B, Aflaki E, Nazarinia M, Azad F, Malekmakan L et al (2012) Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled Study. J Ren Nutr 22(1):50–57
Moreillon JJ, Bowden RG, Deike E, Griggs J, Wilson R, Shelmadine B et al (2013) The use of an anti-inflammatory supplement in patients with chronic kidney disease. J Complement Integr Med 10(1):1–10
Ortiz BOM, Preciado ARF, Emiliano JR, Garza SM, Rodríguez ER, de Alba Macías LA (2019) Recovery of bone and muscle mass in patients with chronic kidney disease and iron overload on hemodialysis and taking combined supplementation with curcumin and resveratrol. Clin Interv Aging 14:2055–2062
Pakfetrat M, Akmali M, Malekmakan L, Dabaghimanesh M, Khorsand M (2015) Role of turmeric in oxidative modulation in end-stage renal disease patients. Hemodial Int 19(1):124–131
Pakfetrat M, Basiri F, Malekmakan L, Roozbeh J (2014) Effects of turmeric on uremic pruritus in end stage renal disease patients: a double-blind randomized clinical trial. J Nephrol 27(2):203–207
Samadian F, Dalili N, Gholi FPR, Fattah M, Malih N, Nafar M et al (2017) Evaluation of Curcumin's effect on inflammation in hemodialysis patients. Clin Nutr ESPEN 22:19–23
Vanaie A, Shahidi S, Iraj B, Siadat ZD, Kabirzade M, Shakiba F et al (2019) Curcumin as a major active component of turmeric attenuates proteinuria in patients with overt diabetic nephropathy. J Res Med Sci 24:77. https://doi.org/10.4103/jrms.JRMS_1055_18
Cachofeiro V, Goicochea M, De Vinuesa SG, Oubiña P, Lahera V, Luño J (2008) Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: New strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int 74:S4–S9
Hallajzadeh J, Milajerdi A, Kolahdooz F, Amirani E, Mirzaei H, Asemi Z (2019) The effects of curcumin supplementation on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Phytother Res 33(11):2989–2995
Qin S, Huang L, Gong J, Shen S, Huang J, Ren H et al (2017) Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials. J Nutr 16(1):68. https://doi.org/10.1186/s12937-017-0293-y
Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14(2):141–153
Camacho-Barquero L, Villegas I, Sánchez-Calvo JM, Talero E, Sánchez-Fidalgo S, Motilva V et al (2007) Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol 7(3):333–342
Cho JW, Lee KS, Kim CW (2007) Curcumin attenuates the expression of IL-1β, IL-6, and TNF-α as well as cyclin E in TNF-α-treated HaCaT cells; NF-κB and MAPKs as potential upstream targets. Int J Mol Med 19(3):469–474
Goel A, Kunnumakkara AB, Aggarwal BB (2008) Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol 75(4):787–809
Ilbey YO, Ozbek E, Cekmen M, Simsek A, Otunctemur A, Somay A (2009) Protective effect of curcumin in cisplatin-induced oxidative injury in rat testis: mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways. Hum Reprod 24(7):1717–1725
Marín YE, Wall BA, Wang S, Namkoong J, Martino JJ, Suh J et al (2007) Curcumin downregulates the constitutive activity of NF-κB and induces apoptosis in novel mouse melanoma cells. Melanoma Res 17(5):274–283
Bachmeier BE, Mohrenz IV, Mirisola V, Schleicher E, Romeo F, Höhneke C et al (2008) Curcumin downregulates the inflammatory cytokines CXCL1 and-2 in breast cancer cells via NFκB. Carcinogenesis 29(4):779–789
Pan Y, Zhang X, Wang Y, Cai L, Ren L, Tang L et al (2013) Targeting JNK by a new curcumin analog to inhibit NF-kB-mediated expression of cell adhesion molecules attenuates renal macrophage infiltration and injury in diabetic mice. PLoS One 8(11):e79084. https://doi.org/10.1371/journal.pone.0079084. eCollection 2013
Hong J, Bose M, Ju J, Ryu J-H, Chen X, Sang S et al (2004) Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: effects on cytosolic phospholipase A 2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 25(9):1671–1679
Jiang H, Deng C-S, Zhang M, Xia J (2006) Curcumin-attenuated trinitrobenzene sulphonic acid induces chronic colitis by inhibiting expression of cyclooxygenase-2. World J Gastroenterol 12(24):3848–3853
Yang C, Zhang X, Fan H, Liu Y (2009) Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res 1282:133–141
Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R et al (2003) Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J 371(3):887–895
Pizzo P, Scapin C, Vitadello M, Florean C, Gorza L (2010) Grp94 acts as a mediator of curcumin-induced antioxidant defence in myogenic cells. J Cell Mol Med 14(4):970–981
Lopresti AL (2018) The problem of curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv Nutr 9(1):41–50
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4(6):807–818
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bagherniya, M., Soleimani, D., Rouhani, M.H., Askari, G., Sathyapalan, T., Sahebkar, A. (2021). The Use of Curcumin for the Treatment of Renal Disorders: A Systematic Review of Randomized Controlled Trials. In: Guest, P.C. (eds) Studies on Biomarkers and New Targets in Aging Research in Iran. Advances in Experimental Medicine and Biology(), vol 1291. Springer, Cham. https://doi.org/10.1007/978-3-030-56153-6_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-56153-6_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-56152-9
Online ISBN: 978-3-030-56153-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)