Abstract
Ginsenoside Rb1 (GS-Rb1) is a well-known antioxidant derived from traditionally used herbal medicine ginseng. It has been suggested that reactive oxygen species (ROS) is involved in chronic kidney disease (CKD) in which GS-Rb1 may play a protective role. The aim of this study was to evaluate prospectively the effects of GS-Rb1 in patients with early chronic kidney disease. 197 patients who have been diagnosed with early CKD (stage 2 or 3) were recruited and randomly assigned to receive GS-Rb1 (500 mg daily oral administration, n = 103) or placebo (n = 94) for consecutive 6 months. Analytical procedures performed at baseline, the end of the treatments, and 6 months after the treatments included renal function evaluation (creatinine and urea clearance), oxidative stress measurement, inflammation assessment, and lipid profile. Of 177 patients completing the study, the GS-Rb1 group (n = 91) showed a positive response in significantly alleviating renal function impairments compared to the placebo group (n = 86). In addition, GS-Rb1 treatment was effective in reducing the extent of oxidative stress and inflammation in CKD patients, whereas continued deterioration was observed in the placebo group. Thus, extended treatment of patients using GS-Rb1 may present an antioxidant-based approach to slow the progression of CKD at the early stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) affects millions of people worldwide and the incidence continues to increase at all ages. Specifically, patients with CKD are at high risk of cardiovascular complications including atherosclerosis, coronary calcification, and stroke that are characterized by a substantial mortality and cause enormous burden to medical costs (Coresh et al. 2007; Go et al. 2004; Vanholder et al. 2005). With rising numbers of elderly people and global epidemic increase of metabolic disorders, significant numbers of CKD patients are expected to progress to chronic kidney failure or end-stage renal disease (Meguid El Nahas and Bello 2005; Nugent et al. 2011). Currently, the routine laboratory assessments of albuminuria and glomerular filtration rate estimated from the serum creatinine clearance, along with other measurements for risk factors, have been used for diagnosing and staging of CKD (Levey et al. 2005).
A significant reduction in renal function is already noticeable at early stages of CKD in which kidney damage progresses due to vascular dysfunction, immune cell activation, and podocyte injury, etc. As a common manifestation and mediator of organ injury, oxidative stress is known to be involved in the pathogenesis of CKD including glomerulosclersosis (Small et al. 2012; Vaziri 2004). The therapeutic potential of antioxidant in treating CKD patients has, thus, been suggested by both animal models and clinical studies (Chen and Siriki 2015; Jun et al. 2012; Nangaku 2006; Sahni et al. 2012; Zachara 2015). Ginsenosides, the active ingredients of widely used herbal medicine ginseng, are identified as efficient antioxidant compounds in vitro and in vivo. The containing sugar moieties possess the antioxidant function of ginsenosides, and hydroxyl radical scavenging capacity of ginsenosides has been demonstrated (Liu et al. 2003). These properties, together with their reported functions in preventing endothelial dysfunction (Zhang et al. 1999) and ischemia/reperfusion kidney injury (Yokozawa et al. 1998), suggest that ginsenosides could potentially reduce the oxidative stress-associated inflammatory damage in kidney, thus limiting the progression of CKD. The present study was designed to test this hypothesis by prospectively assessing whether GS-Rb1, a ginsenoside isolated from root extract of Panax ginseng, can ameliorate kidney function impairments in individuals with CKD at early stages of the disease.
Methods and materials
Patients
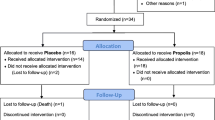
Between 2012 and 2015, 232 CKD patients who have been received treatments at Wuxi No. 2 People’s Hospital Affiliated to Nanjing Medical University were assessed for eligibility of the study. Participants were recruited through advertisement. Recruitment was assisted by the faculty members in Wuxi No. 2 People’s Hospital, and the study took place at the Department of Nephrology at Wuxi No. 2 People’s Hospital. The sampling frame encompassed urban and rural areas, with various education and social class diversity. Inclusion criteria included subjects who were aged 18–90 years old and had an estimated glomerular filtration rate (eGFR) in the range of 30–90 mL/min/1.73 m2 (based on the modification of (Levey et al. 2006)). The establishment of chronicity of kidney dysfunction was implied by previously (3 months before) elevated serum creatinine. All recruited patients were early CKD at the stage of 2 (mild reduction in GFR at 60–89 mL/min/1.73 m2 with kidney damage) or stage 3 (moderate reduction in GFR at 30–59 mL/min/1.73 m2). People who have inflammatory diseases (hepatitis, pneumonia, etc.), cancer, infection, liver failure (ALT and AST >40 U/L), or prior use of antioxidant vitamin (supplemental intake >1000 IU) were excluded. All participant patients have signed consent forms, and the study was approved by the ethics committee of human subject study of Wuxi No. 2 People’s Hospital Affiliated to Nanjing Medical University, and was conducted according to the Declaration of Helsinki and Good Clinical Practices.
Study design
Consenting patients were diagnosed and then randomly assigned to two groups with either ginsenoside Rb1 (GS-Rb1) treatment group (oral administration of 500 mg once daily for consecutive 6 months) or placebo group. The trial is a parallel study in which participants were randomized to receive placebo or GS-Rb1, based on a computer-generated block randomization scheme with respect to age and gender. The computer-generated sequential numbers were allocated to individual patient by the assistant. The assigned drugs were prepared at the center of Wuxi No. 2 People’s Hospital and kept in numbered envelopes with patient identification. During the study, patients were blind to the treatment assignment with concealed labels. Baseline physical examination was performed and medical history was recorded. Next, patients were randomly assigned to receive the custom-made capsules. Each capsule contains placebo or 500 mg GS-Rb1, which is recommended for oral administration once daily with the evening meal. The amounts of capsules sufficiently covered the use of 6-month period with an additional 10%. Patients were encouraged to contact the physicians if they felt any adverse effects, and serious side effects were assessed by investigators who have been compliance with the criteria for good clinical practice (GCP) training. For standard treatment for early stages of chronic kidney disease, patients in the both groups received usual medicine during the whole study. No patients experienced adverse events, such as severe renal dysfunction, hemorrhage, or any allergic reactions. Patients who had associated inflammation diseases or developed late stage of CKD during the treatment have been excluded from the study.
Whole blood was collected for fasting patient at baseline, end of the treatments, and 6 months after the treatments. 24-h urine was also collected for the measurements of the endogenous creatinine clearance and urea clearance. Serum or urine levels of creatinine, albumin, and blood lipid profile were determined using standard laboratory methods in clinic. The peripheral blood mononuclear cells (PBMC) were harvested from patient blood by Ficoll–Paque method, and the isolated cell pellets were transferred to a new sterile cryotube with DMEM cell culture medium and stored at −80 °C. All assays were performed by independent investigators who were not aware of the intervention.
Oxidative stress assessment
The levels of derivative reactive oxygenmetabolites (dROMs), the d-ROMs test as an oxidative stress marker, were determined by measuring the amount of organic hydroperoxide (ROOH) converted into radicals that oxidize N,N-diethyl-p-phenylenediamine (Nagatomo et al. 2012). The levels of dROMs were expressed in relative levels to baseline of placebo group (%). The GPx (Glutathione peroxidase) activity was determined through assay kits (Cayman Chemical, no. 703102) which measures GPx activity indirectly by a coupled reaction with glutathione reductase. The plasma level of 8-isoprostane, a product of lipid peroxidation, was measured by a competitive enzyme immunoassay kit (Cayman Chemical, no. 516351). The DNA base-modified product, 8-hydroxy-2′-deoxyguanosine (8-OHdG), a commonly used marker for oxidative DNA damage, was also measured by a competitive enzyme immunoassay kit (Cayman Chemical, no. 589320).
Inflammation markers
Pro-inflammatory cytokines, interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), were quantified with ELISA kits (R&D systems, DuoSet kits DY 206 and DY 210).
Statistical analysis
Data were expressed as the mean ± SD as indicated. Statistical comparison was analyzed by one-way (different time points within the same group) or two-way ANOVA analysis (different time points between two treatment groups) followed by a Tukey’s post hoc test. p value less than 0.05 was considered significant.
Results
197 patients with chronic kidney disease at the stage 2 or 3 were randomly assigned to receive either GS-Rb1 or placebo treatments as described (Fig. 1). No obviously adverse effects of treatment were observed in patients of both groups. 8 patients in placebo group and 12 in GS1-Rb group were removed from the study, either because of associated inflammation diseases (such as infection) or developed late stage of CKD during the study. Nevertheless, there is no correlation of such exclusion with the different treatment conditions. For patients who have successfully completed the study (86 placebo and 91 treated), the results of baseline characterization are shown in Table 1. The general data including ages, genders, metabolic status, and the extent of kidney dysfunction are almost identical between the two groups. Of note, more than 70% of recruited patients were male in both groups, which is consistent with previously reported high prevalence of CKD in men compared to women (Meguid El Nahas and Bello 2005; Okada et al. 2014). The similar association was found in patients with diabetes (Fox et al. 2012). Baseline tests were performed once on the subjects prior to the trial. After participants started treatments, follow-up visits were carried out at the end of the treatment (6 months) and 6 months after the end of treatments (12 months). The analysis included renal function, oxidative stress and inflammation assessments.
Renal function
Effects of placebo on renal function were minimal. There were no differences in the levels of serum creatinine (Fig. 2a) and endogenous creatinine clearance (Fig. 2c) between all time points of the placebo patient group (p > 0.05). In contrast, 500 mg daily oral administration of GS-Rb1 for consecutive 6 months significantly enhanced the clearance of creatinine and reduced the serum levels of creatinine (Fig. 2a, c, p < 0.01). Importantly, these effects of GS-Rb1 can persist for months, even after the patients stop taking the drug, compared to the baselines (p < 0.05). In addition, continuing deterioration in the placebo group was seen by the trends of progressive increase in serum urea and decrease in urea clearance (Fig. 2b, d). In these patients, urea markers at 12 months became significantly worse than those values before the treatment (p < 0.05). Similar to creatinine data, the effects of GS-Rb1 in urea tests were also greatly improved at both 6 months (p < 0.01) and 12 months (p < 0.05), compared with the baselines.
Effects of GS-Rb1 treatment on renal functions in CKD patients. Renal functions were characterized by serum creatinine (a), serum urea (b), endogenous creatinine clearance (c) and endogenous urea clearance (d). These parameters were collected and analyzed at three time points: before treatment, at 6 months after treatment, at 12 months after treatment. Data were given as mean ± SD. *p < 0.05 and **p < 0.01
Oxidative stress and inflammation markers
Several oxidative stress markers were measured in the study as potential denominators associated with the extent of kidney injury. In the placebo group, the levels of increased dROMs (derivative reactive oxygenmetabolites), decreased GPx activity (Glutathione peroxidase), and elevated 8-OHdG (8-hydroxy-2′-deoxyguanosine) were found in patients (Fig. 3a, b, d), all reaching significance when analyzed at 12 months (Fig. 3, p < 0.05). No significant change in 8-isoprostane was observed after placebo (Fig. 3c, p > 0.05). Together with Fig. 2, these data indicated a positive association of enhanced oxidative stress and disease progression in early stages of CKD patients. As a comparison, the majority of patients receiving GS-Rb1 showed the signs of oxidative stress alleviation. At the end of treatment (6 months), these findings included the reduced levels of dROMs and 8-isoprostane (Fig. 3a, c, p < 0.01), greatly enhanced GPx activity (Fig. 3b, 50% increase, p < 0.01) and diminished DNA damage (Fig. 3d, 50% decrease in 8-OHdG, p < 0.01). Remarkably, the effects were still evident when patients were analyzed 6 months after stopping the medication, compared to baselines (p < 0.05, Fig. 3).
GS-Rb1 treatment alleviated oxidative stress in CKD patients. Oxidative stress was reflected by levels of dROMs (a), GPx (b), 8-isoprostane (c) and 8-OHdG (d). Data were collected and analyzed at three time points: before treatment, at 6 months after treatment, at 12 months after treatment. Data were given as mean ± SD. *p < 0.05 and **p < 0.01
Figure 4 shows the effect of treatments on markers for systemic inflammation including cytokines TNF-α (The tumor necrosis factor alpha) and IL-6 (Interleukin 6). The measured TNF-α level was unchanged in the placebo patients at all time points, whereas GS-Rb1 administration has reduced TNF-α level at the end of the treatment (6 months, Fig. 4a, p < 0.05). Similar finding was also observed in IL-6 production in which patients who received GS-Rb1 administration (6 months, p < 0.01) had significantly less IL-6 than their baselines (Fig. 4b). In contrast, there was no statistical evidence of IL-6 alteration in the control patients at 6 months (p > 0.05). By 12 months, both inflammation markers rose again in GS-Rb1 group after the cessation of drug treatment. In placebo control patients, TNF-α remained the same while IL-6 levels were significantly higher than before the treatment (p < 0.05). The results of lipid profile at 6 months are also summarized in Table 2. No significant changes were observed in both groups for total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TG) levels when compared to the baselines.
GS-Rb1 treatment reduced inflammation in CDK patients, including TNF-α (a) and IL-6 (b) production at 6 months after treatment. TNF-α and IL-6 production was measured at three time points: before treatment, at 6 months after treatment, at 12 months after treatment. Data were given as mean ± SD. *p < 0.05 and **p < 0.01
Discussion
It is generally agreed that uncontrolled oxidative stress and inflammation are associated with the progression of chronic kidney disease. Thus, antioxidant agents might provide therapeutic success for CKD patients. This randomized, prospective, placebo-controlled clinical trial shows that 500 mg of daily administration of GS-Rb1, a member of the class of natural antioxidant known as ginsenosides, was effective in improving renal function, alleviating oxidative stress, and attenuating systemic inflammation in early CKD patients. Moreover, a lasting effect on limiting the regression of kidney disease was also observed up to 6 months after patients stopped GS-Rb1 administration. These impacts were significantly superior to the placebo treatment in which renal function continued to deteriorate in patients with elevated markers for oxidative stress and inflammation. As the study had examined the comparisons between patients randomly assigned based on similar conditions including genders, ages, diabetes, etc., our data indicate that the GS-Rb1 administration could benefit the patients in a typical setting of chronic kidney disease. It will be important to evaluate the effects of GS-Rb1 in a larger and more diverse cohort of CKD patients.
The presence of albuminuria is frequently used as a sign of kidney damage or decreased kidney function in delineating the progression of chronic kidney disease. In addition, serum creatinine and urea are also accepted as surrogate markers for the indication of GFR (glomerular filtration rate) decline: normal creatinine clearance is 88–128 and 97–137 mL/min for women and men, respectively; the normal BUN (blood urea nitrogen) level for adults is 7–20 mg/dL (Go et al. 2004; Levey et al. 2005; Meguid El Nahas and Bello 2005). Because the direct clinical measurement of GFR is often difficult, we used serum creatinine and urea in estimating the progression of renal dysfunction in our patients. The comparisons were made in patients receiving same treatment at specific points during the trial, compared to their pre-treatment levels. The responses to treatment in these two groups of patients were then analyzed, and distinct effects were noticed. Nevertheless, these measurements could lead to discrepancy in prediction of kidney injury. For instance, patients with the same value of serum creatinine may have a wide range of renal insufficiency (Levey et al. 1999). With various parameters that affect the correlation between these markers and GFR, more accurate analysis of patient response will be needed in future studies by the inclusion of additional data such as serum albumin concentrations or GFR measurement.
Ginsenosides, with the established antioxidant and anti-inflammatory properties, have been used as herbal remedies in several countries including China. For instances, ginsenoside compounds are known for efficiently protecting against liver injury (Lee et al. 2005), neurodegenerative damage (Bao et al. 2005; Radad et al. 2006), and myocardial ischemia/reperfusion injury (Wu et al. 2011; Xia et al. 2011). Thus, its potential in therapeutic use has been examined in many clinical scenarios and ongoing trials (Vogler et al. 1999). In a randomized, double-blind trial, Liu et al. (2012) identified an amelioration of the disability score in acute ischemic stroke patients who received ginsenoside-Rd via intravenous infusion. Within a small cohort of well-controlled type 2 diabetes patient, Vuksan et al. (2008) found that a 12-week oral supplementation of Korean red ginseng was able to improve the subjects’ plasma glucose and insulin sensitivity. It was also hinted by a systematic review and meta-analysis that ginseng extracts may have favorable effects in systolic blood pressure in specific groups of patients (Komishon et al. 2016). These beneficial effects of GS-Rb1 are believed to be attributed to the modulation of vascular inflammation, prevention of oxidative cell damage and promotion of cell survival, all of which may help the management of chronic kidney disease (Fogo 2007). Up to now, no study has been reported for the role of ginsenosides in CKD patients. Our randomized controlled study is the first time showing the positive influence of ginsenosides on CKD. The optimal dose and administration route of ginsenosides is still unclear. In addition, there is no consensus on the appropriate duration of the treatment. Oral ingestion may be a key component of ginsenoside intake, since it has been demonstrated that ginsenosides can be modified by intestinal bacteria in order to become active molecules (Hasegawa 2004). Because they are isolated from naturally occurring compound, ginsenosides are well tolerated and minimally toxic. A safe dose at 1000–2000 mg/day intake of American ginseng has been documented in a pilot study of cancer patients (Barton et al. 2010). In the present study, our data suggested that oral administration of 500 mg GS-Rb1 daily in patients for consecutive 6 months have significant impacts in alleviating chronic kidney disease, comparing to the placebo. Interestingly, the improvement can sustain for up to 6 months after the treatment. Since little information is known with respect to the extended effects of ginsenoside administration, these data may suggest novel characteristic of ginsenoside for the possible prevention of disease progression. Further study in the future is necessary for dissecting the underlying mechanism.
Other important factors implicated in the progression of CKD include metabolic parameters that can also be improved by ginsenosides (Yin et al. 2008). It is well known that accelerated nephropathy is tightly associated with both type 1 and type 2 diabetes (Fox et al. 2012). A link between hyperlipidemia and kidney diseases has been shown as well (Kwan et al. 2007). In our study, no effect was found at GS-Rb1-treated groups on lipid profile when compared to either pre-treatment or placebo levels (Table 2). Two possible explanations may underlie the negative results. First, in the selected patients at early stages of CKD, although majority was accompanied by diabetes, the average lipid levels were well controlled by their medications. Second, the dose or duration of GS-Rb1 treatment in the study might not be sufficient to modulate blood lipids. In any case, our finding suggests a possible dissociation between the effects of anti-oxidative stress and actions of ginsenoside on lipid profile in inflammation-associated disorders such as CKD. Further work is needed to test this notion in CKD patients with more severe forms of metabolic abnormalities.
Conclusion
Whether reducing oxidative stress could mitigate the impaired renal function in chronic kidney disease remains undetermined. In the current study, we found that in CKD patients an extended administration of ginsenoside GS-Rb1, an antioxidant, can significantly alleviate the disease progression. The serum creatinine and urea clearance can be improved by GS-Rb1, along with the reductions in markers for both oxidative stress and systemic inflammation. There were no positive effects found in the placebo-treated patients in which renal function continued to deteriorate. These findings are consistent with a beneficial role of ginsenosides as antioxidants in ameliorating kidney damages. Further investigation is warranted to study the effects of ginsenosides on CKD in the form of a blinded study with more patient numbers.
References
Bao HY et al (2005) Memory enhancing and neuroprotective effects of selected ginsenosides. Arch Pharm Res 28:335–342
Barton DL et al (2010) Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: nCCTG trial N03CA. Support Care Cancer 18:179–187. doi:10.1007/s00520-009-0642-2
Chen J, Siriki R (2015) Antioxidants therapy for patients with chronic kidney disease: a question of balance. Am J Nephrol 42:318–319. doi:10.1159/000441628
Coresh J et al (2007) Prevalence of chronic kidney disease in the United States. JAMA 298:2038–2047. doi:10.1001/jama.298.17.2038
Fogo AB (2007) Mechanisms of progression of chronic kidney disease. Pediatr Nephrol 22:2011–2022. doi:10.1007/s00467-007-0524-0
Fox CS et al (2012) Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 380:1662–1673. doi:10.1016/S0140-6736(12)61350-6
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351:1296–1305. doi:10.1056/NEJMoa041031
Hasegawa H (2004) Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci 95:153–157
Jun M et al (2012) Antioxidants for chronic kidney disease. Cochrane Database Syst Rev 10:CD008176. doi:10.1002/14651858.CD008176.pub2
Komishon AM et al (2016) The effect of ginseng (genus Panax) on blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. J Hum Hypertens. doi:10.1038/jhh.2016.18
Kwan BC, Kronenberg F, Beddhu S, Cheung AK (2007) Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol 18:1246–1261. doi:10.1681/ASN.2006091006
Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH (2005) Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int 25:1069–1073. doi:10.1111/j.1478-3231.2005.01068.x
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130:461–470
Levey AS et al (2005) Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 67:2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
Levey AS et al (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254
Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC, Sun YX (2003) In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. J Agric Food Chem 51:2555–2558. doi:10.1021/jf026228i
Liu X et al (2012) Ginsenoside-Rd improves outcome of acute ischaemic stroke—a randomized, double-blind, placebo-controlled, multicenter trial. Eur J Neurol 19:855–863. doi:10.1111/j.1468-1331.2011.03634.x
Meguid El Nahas A, Bello AK (2005) Chronic kidney disease: the global challenge. Lancet 365:331–340. doi:10.1016/S0140-6736(05)17789-7
Nagatomo F, Fujino H, Kondo H, Ishihara A (2012) Oxygen concentration-dependent oxidative stress levels in rats. Oxid Med Cell Longev 2012:381763. doi:10.1155/2012/381763
Nangaku M (2006) Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17:17–25. doi:10.1681/ASN.2005070757
Nugent RA, Fathima SF, Feigl AB, Chyung D (2011) The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract 118:c269–277. doi:10.1159/000321382
Okada K, Yanai M, Takeuchi K, Matsuyama K, Nitta K, Hayashi K, Takahashi S (2014) Sex differences in the prevalence, progression, and improvement of chronic kidney disease. Kidney Blood Press Res 39:279–288. doi:10.1159/000355805
Radad K, Gille G, Liu L, Rausch WD (2006) Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci 100:175–186
Sahni N, Gupta KL, Rana SV, Prasad R, Bhalla AK (2012) Intake of antioxidants and their status in chronic kidney disease patients. J Ren Nutr 22:389–399. doi:10.1053/j.jrn.2011.09.002
Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC (2012) Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 17:311–321. doi:10.1111/j.1440-1797.2012.01572.x
Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N, European Uremic Toxin Work G (2005) Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 20:1048–1056. doi:10.1093/ndt/gfh813
Vaziri ND (2004) Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens 13:93–99
Vogler BK, Pittler MH, Ernst E (1999) The efficacy of ginseng. A systematic review of randomised clinical trials. Eur J Clin Pharmacol 55:567–575
Vuksan V et al (2008) Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis 18:46–56. doi:10.1016/j.numecd.2006.04.003
Wu Y et al (2011) Protective effect of ginsenoside Rb1 against myocardial ischemia/reperfusion injury in streptozotocin-induced diabetic rats. Mol Biol Rep 38:4327–4335. doi:10.1007/s11033-010-0558-4
Xia R, Zhao B, Wu Y, Hou JB, Zhang L, Xu JJ, Xia ZY (2011) Ginsenoside Rb1 preconditioning enhances eNOS expression and attenuates myocardial ischemia/reperfusion injury in diabetic rats. J Biomed Biotechnol 2011:767930. doi:10.1155/2011/767930
Yin J, Zhang H, Ye J (2008) Traditional chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets 8:99–111
Yokozawa T, Liu ZW, Dong E (1998) A study of ginsenoside-Rd in a renal ischemia-reperfusion model. Nephron 78:201–206
Zachara BA (2015) Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv Clin Chem 68:131–151. doi:10.1016/bs.acc.2014.11.006
Zhang JM, Matsuura Y, Sueda T, Orihashi K (1999) Beneficial effects of ginsenosides of stems and leaves on cardiac and coronary vascular functions after 12-hour rat heart preservation. Transplant Proc 31:2175–2178
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
This work was funded by Developing Medicine Funds.
Rights and permissions
About this article
Cite this article
Xu, X., Lu, Q., Wu, J. et al. Impact of extended ginsenoside Rb1 on early chronic kidney disease: a randomized, placebo-controlled study. Inflammopharmacol 25, 33–40 (2017). https://doi.org/10.1007/s10787-016-0296-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-016-0296-x