Abstract
Background
The effects of coenzyme Q10 (CoQ10) supplementation in chronic kidney disease (CKD) patients remain controversial.
Objective
A systematic review of current evidence was performed to systematically and comprehensively summarize the effects of CoQ10 on cardiovascular outcomes, oxidative stress, inflammation, lipid profiles, and glucose metabolism.
Methods
MEDLINE, EMBASE, and the Cochrane Library database (Cochrane Central Register of Controlled Trials) were searched to identify eligible studies investigating the effects of CoQ10 supplementation on patients with CKD.
Results
Twelve independent studies (including seventeen publications) were included in this systematic review. For CKD patients, six studies reported variable cardiovascular outcomes, which yielded inconsistent results. Regarding oxidative stress and inflammation, pooled analysis showed that CoQ10 supplementation significantly reduced malonaldehyde (WMD: − 1.15 95% CI − 1.48 to − 0.81) and high-sensitivity C reactive protein levels (WMD: − 1.18 95% CI − 2.21 to − 0.15). Regarding glucose metabolism, we found that CoQ10 supplementation resulted in significant improvements in HbA1c (WMD: − 0.80; 95% CI: − 1.35 to − 0.24) and QUICKI (WMD: 0.02; 95% CI: 0.01 to 0.03). The pooled results indicated that CoQ10 supplementation had no effects on total cholesterol, or LDL-cholesterol, or on HDL-cholesterol, and triglycerides.
Conclusions
Our systematic review demonstrated that CoQ10 supplementation might have promising effects on oxidative stress. This work provided some clues that CoQ10 supplementation might have the potential to improve inflammation levels, glucose metabolism, cardiac structure, and cardiac biomarkers. However, the effects of CoQ10 supplementation should be confirmed in larger high-quality studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
CoQ10 is a kind of fat-soluble vitamin-like quinone that transports electrons from complexes 1 or 2 to complex 3 in mitochondria [1]. In addition to its critical role as a component of the electron transport chain, CoQ10 might act as a reactive oxygen species scavenger [2]. It has been demonstrated that CoQ10 can inhibit lipid peroxidation in biological membranes, protect mitochondrial proteins and DNA from oxidative damage, and reduce lipid oxidation [3, 4]. In addition to its antioxidant activity, CoQ10 also attenuates the oxidized low-density lipoprotein (oxLDL)-mediated downregulation of endothelial nitric oxide synthase and the upregulation of inducible nitric oxide synthase [5].
Oxidative stress is characterized by an imbalance between the production of pro-oxidants and antioxidant defense mechanisms [6, 7]. Already existing in the early stage of CKD, oxidative stress deteriorates along with the decline of kidney function, and this state is further exacerbated in hemodialysis patients [2]. The accumulation of uric toxins and hemodialysis itself might contribute to oxidative stress [7]. In CKD and hemodialysis patients, oxidative stress promotes the development of atherosclerosis and is a predictor of all-cause and CVD mortality [7, 8].
In the general population, it has been shown that CoQ10 treatment decreases superoxide production in endothelial cells, improves endothelial function, enhances cardiac capacity, and reduces major adverse cardiovascular events [9,10,11]. In patients with nondialysis CKD and undergoing dialysis, the plasma concentrations of CoQ10 are reduced [12, 13]. Consumption of CoQ10 results in increased electron transport and inefficient active oxygen. CoQ10 can improve mitochondrial function and reduce oxidative stress in hemodialysis patients [13]. In patients with CKD, CoQ10 may have the potential to prevent and treat cardiovascular disease, improve cardiac function, and reduce oxidative stress. Coenzyme Q10 may have benefits for heart function, blood pressure, glucose metabolism, lipids, inflammation, and oxidative stress in patients with nondialysis CKD and those undergoing dialysis, but the results are still controversial. One recent meta-analysis showed that CoQ10 supplementation significantly improved the metabolic profile of CKD patients [14]. However, no published study has systematically and comprehensively summarized the effects of CoQ10 on cardiovascular outcomes, oxidative stress, inflammation, glucose metabolism, and lipid profiles in CKD patients.
The purpose of this systematic review was to systematically assess the impact of CoQ10 supplementation on cardiovascular function, oxidative stress, inflammation, glucose metabolism, lipid profiles and others in patients with CKD.
Methods
Protocol design and eligibility criteria
This systematic review was advanced by using a published protocol [15] and was reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [16]. The protocol of this systematic review was also registered in the International Prospective Register of Systematic Reviews (PROSPERO) and was assigned the registration number CRD42019120201 (website: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD4201912021). The included trials met the following criteria: (1) randomized controlled, quasi-randomized trial, nonrandomized trial or observational study; (2) participants with CKD; (3) the intervention or exposure of interest was CoQ10; and (4) adult patients without intervention or with placebo in trials or without exposure to CoQ10 in the cohort study were comparators.
Prespecified outcomes
The primary outcomes were cardiovascular effects, including (1) cardiac function and structure: left ventricular ejection fraction (determined by echocardiography or contrast or radionuclide angiography); diastolic heart function; cardiac structure (measured by individual trials); (2) biomarkers of cardiac function, such as brain natriuretic peptide and N-terminal pro-b-type natriuretic peptide (NT-pro-BNP); (3) blood pressure and heart rate; (4) symptom improvement (measured by individual trials and/or by exercise capacity), quality of life (measured by individual trials); (5) major cardiovascular events (cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke, and revascularization procedures). Secondary outcomes of interest included effects on oxidative stress, inflammation, glucose metabolism, lipid profiles, all-cause mortality, safety, and tolerability.
Database and search strategy
We performed an electronic search of MEDLINE via Ovid, EMBASE via Ovid and Cochrane Library (Cochrane Central Register of Controlled Trials) in December 2018. We updated our search in August 2019. Relevant text words and medical subject headings were used as follows: kidney diseases, renal replacement therapy, renal insufficiency, dialysis, predialysis, diabetic nephropathies, ubiquinone, ubidecarenone, coenzyme Q10, co-enzyme Q10 and quinone. The clinicaltrials.gov website was searched for relevant studies that have been registered and completed but remain to be published. The reference lists of articles and other reviews retrieved during the search or known to the authors were searched for relevant articles. There were no language restrictions.
Study selection and data extraction
Two independent reviewers assessed the eligibility of the trials with a standardized approach. Discrepancies were resolved by discussion with a third individual. Two authors independently extracted data, including baseline patient characteristics, follow-up duration, intervention, outcome events, and adverse events using a standardized data collection form.
Assessing the risk of bias
Two authors independently assessed the risk of bias of the randomized controlled trials according to the standard criteria. Seven different bias domains, including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and any other potential biases were categorized as low risk of bias, high risk of bias or unclear risk of bias [17]. Observational studies were evaluated with the Newcastle–Ottawa Scale [18].
Statistical analysis
It is possible for baseline imbalances to occur between treatment groups for one or more variables in a randomized controlled trial by chance (especially if a trial’s sample size is small) or through an inadequate randomization strategy [19, 20]. If trials with baseline imbalances are combined in a meta-analysis, then this may result in misleading conclusions [21, 22]. As analysis based on change scores (also called changes from baseline) removes a component of between-person variability, it is more efficient and powerful than the comparison of final values in some circumstances [20]. Given most studies with small sample sizes or (and) poor quality, we conducted a meta-analysis of change scores for continuous variables in our work. The method of imputing standard deviations for changes from baseline outlined in the Cochrane Handbook for Systematic Reviews of Interventions was accepted [20]. The results of dichotomous outcomes were expressed as risk ratios (RRs) with 95% CIs for individual studies. We used first-period data from cross-over trials and combined them with data obtained from parallel studies [23]. There is no reason to assume that the effects being estimated in the different studies will not be identical; therefore, the random-effect model was the most appropriate choice for most meta-analyses [24]. Accordingly, a Dersimonian–Laird random-effect model was used [25]. The heterogeneity of treatment effects between studies was investigated statistically using the test and I2 statistic. I2 values of 25, 50, and 75% correspond to low, medium and high levels of heterogeneity, respectively [26]. If heterogeneity existed and there were a substantial number of studies, subgroup analyses and meta-regression will be undertaken. Funnel plots, Egger’s regression asymmetry test, and Begg’s test were used to evaluate publication bias if appropriate [27, 28]. A two-sided p value < 0.05 was regarded as significant for all analyses. All analyses were calculated using Stata software (V.12.0; StataCorp, College Station, Texas, USA). When there were insufficient clinically homogeneous trials to perform a meta-analysis for some outcomes, we presented a narrative synthesis.
Results
Characteristics of the included studies
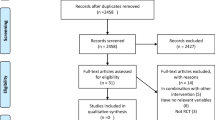
Briefly, we identified 1252 potentially relevant records, of which 207 were removed because they were duplicates. After screening the titles and abstracts and full-text browsing, 12 independent studies (including 17 publications) were included in this systematic review (Fig. 1). No observational studies were included. One trial was presented by two publications [29, 30], and another trial was presented by five publications [31,32,33,34,35]. The basic characteristics of the included studies were shown in Table 1. Nine trials adopted a parallel study design, and three trials had a randomized crossover design. The studies involved participants with non-dialysis-dependent CKD, dialysis-dependent CKD, or both. The study sample size ranged from 21 to 97. The daily dosage of CoQ10 used also differed among studies, and ranged from 30 to 1200 mg/day. The treatment duration of the included studies ranged from 4 weeks to 6 months. The quality assessment for each included trial, based on the authors’ judgments on the risk of bias, was presented in Figure S1 in the Supplementary File. Overall, study quality was varied, and high-quality studies were lacking. We planned to use funnel plots, Egger’s regression asymmetry test and Begg’s test to evaluate publication bias. However, because there were no adequate studies for each outcome, these tests were not performed.
Cardiovascular effects
Six studies evaluated the cardiovascular effects of CoQ10 on patients with CKD, which included cardiac function and structure, blood pressure and heart rate, biomarkers of cardiac function, and symptom improvement (Table 2). In one prospective, double-blind, placebo-controlled, crossover study, the hemodialysis patients received CoQ10 200 mg/d or placebo during the 8 weeks in each phase. Echocardiographic findings showed that intraventricular septum (IVS) thickness and left ventricle mass (LVM) were significantly decreased in the CoQ10 group (p = 0.03 and p = 0.01) compared with the placebo group. However, the results suggested that CoQ10 supplementation did not significantly improve diastolic heart function in hemodialysis patients [36]. A study conducted by Rivara et al. demonstrated a significant reduction in troponin T and NT-pro-BNP concentrations with 1200 mg daily CoQ10 supplementation in a pre-specified per-protocol analysis. However, in the intention-to-treat analysis, the reductions in troponin T (p = 0.09) and NT-pro-BNP (p = 0.10) levels did not reach statistical significance [37]. One abstract of congress reported that after 8 weeks of treatment with CoQ10, ST-segment depression during hemodialysis was significantly improved [1.86 ± 0. 54 to 1.76 ± 0.66 (60 mg CoQ10), and 2.55 ± 0.75 to 2.11 ± 0.70 (90 mg CoQ10)], but no significant change was noted in the placebo group [38]. One study evaluated the effect of CoQ10 supplementation on exercise performance measures. The results showed no effect of CoQ10 on the 6-min walk test (6MWT) and maximal oxygen consumption (VO2max) at 1 min with submaximal exercise in a cycle ergometer in maintenance hemodialysis patients [39]. Three studies [31, 37, 40] investigated the efficacy of CoQ10 supplementation on blood pressure and found no effects on blood pressure. No study reported major cardiovascular events (cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke, and revascularization procedures).
Oxidative stress
Seven studies [29, 33, 37, 39, 41,42,43] reported the effects of CQ10 supplementation on biomarkers of oxidative stress. Markers of oxidative stress were variable in different studies (Table S1 in Supplementary File). Four studies [29, 39, 41,42,43] reported data on the effects of coenzyme Q10 supplementation on MDA levels (Fig. 2). In a pooled analysis of these studies using the random-effect model, CoQ10 significantly decreased the MDA level (WMD: − 1.15 95% CI − 1.48 to − 0.81; Fig. 2) compared with the control group. When excluding the study [41] with imputed change scores, similar results were noted (WMD: − 0.88 95% CI − 1.10 to − 0.67). One study reported the efficacy of CoQ10 supplementation on increases in MDA with exercise [43]. However, there was no significant difference between the placebo and CoQ10 groups in terms of increases in MDA with exercise (data not shown).
Meta-analysis of CoQ10 treatment on changes (95% CI) in MDA. The horizontal lines denote the 95% CIs. The square represents the point estimate of each study. The diamond represents the overall pooled estimate of the treatment effect. CoQ10 coenzyme Q10, CI confidence interval, WMD weighted mean difference
Two studies indicated the effects of CoQ10 on F2-isoprostane and showed that small doses of CoQ10 (100 mg or 600 mg/d) had no effect on F2-isoprostanes [33, 37]. In contrast, large doses (1200 mg/d) have an effect on patients under hemodialysis [37]. Regarding advanced glycation end products (AGEs), one study demonstrated that CoQ10 supplementation for 12 weeks had favorable effects [43]. One recent study reported that CoQ10 can increase the levels of total antioxidant (TAC) and nitric oxide (NO), but did not have any beneficial effects on glutathione (GSH) [29]. There was no significant difference between the placebo and CoQ10 supplementation groups in terms of changes in superoxide dismutase (SOD) activity, glutathione peroxidase (GPx) activity, and serum oxidized low-density lipoprotein cholesterol levels with exercise [39].
Inflammation
Two studies [29] [40] reported information on high sensitivity C-reactive protein (hs-CRP), one of which was a cross-over trial. As stated above, we include only data from the first period of this study. The changes in hs-CRP from baseline were estimated. When combining data from two studies on hs-CRP, we found that CoQ10 supplementation significantly decreased hs-CRP levels (WMD: − 1.18 95% CI − 2.21 to − 0.15; Figure S2 in Supplementary File). Removing the cross-over trial, which also had imputed change scores, did not alter the conclusion (WMD: − 1.65 95% CI − 1.94 to − 1.35). Mori et al. determined the effects of CoQ10 on C-reactive protein (CRP) and found CRP was not different between groups [31]. One study found that CQ10 supplementation in CKD patients significantly improved the gene expression of interleukin-1, and tumor necrosis factor-α [44].
Glucose metabolism
Data on glucose metabolism were obtained from four studies [30, 31, 43, 44]. Two studies [30, 43] investigated the efficacy of CoQ10 supplementation on hemoglobin A1c (HbA1c) and the quantitative insulin sensitivity check index (QUICKI). By a pooled analysis, we found CoQ10 supplementation resulted in significant improvement in HbA1c (WMD: − 0.80; 95% CI: − 1.35 to − 0.24; Fig. 3a) and QUICKI (WMD: 0.02; 95% CI: 0.01 to 0.03; Fig. 3b). Three studies [30, 31, 43] provided intervention effects of CoQ10 on fasting plasma glucose (FPG), insulin, and homeostasis model assessment of insulin resistance (HOMA-IR). However, changes from baseline were imputed in one study [31], in which the investigators enrolled nondiabetic patients. A pooled analysis of these three studies found that CoQ10 supplementation had no effects on FPG (WMD: − 4.23; 95% CI: − 17.88 to 9.42; Fig. 3c), insulin (WMD: − 3.02; 95% CI: − 6.74 to 0.71; Fig. 3d) or HOMA-IR (WMD: − 0.31; 95% CI: − 1.33 to 0.72; Fig. 3e).
Forest plot detailing the WMD and 95% CI for the impact of CoQ10 supplementation on changes in HbA1c (a), QUICKI (b), FPG (c), insulin (d), HOMA-IR (e), and HOMA-IR (f) in patients with chronic kidney disease. The horizontal lines denote the 95% CIs. The square represents the point estimate of each study. The diamond represents the overall pooled estimate of the treatment effect. CoQ10 coenzyme Q10, WMD weighted mean difference, CI confidence interval, HbA1c hemoglobin A1c, QUICKI quantitative insulin sensitivity check index, FPG fasting plasma glucose, HOMA-IR homeostasis model assessment of insulin resistance, HOMA-B homeostatic model assessment for B-cell function
When excluding the study with imputed change scores, the results of FPG (WMD: − 14.52; 95% CI: − 31.87 to 2.82) and HOMA-IR (WMD: − 0.84; 95% CI: − 1.70 to 0.03) did not alter the initial qualitative interpretations. However, the sensitivity analysis showed that CoQ10 supplementation decreased insulin levels compared with the control group (WMD: − 4.97; 95% CI: − 6.97 to − 2.98). One study [43] found that CoQ10 could improve homeostatic model assessment for B-cell function (HOMA-B) (WMD: − 15.80; 95% CI: − 29.52 to − 2.08; Fig. 3f). Heidari explored the effects of CoQ10 on gene expression related to insulin and found that CQ10 supplementation for 12 weeks in patients with diabetic nephropathy significantly improved the gene expression of peroxisome proliferator-activated receptor-γ (PPAR-γ), which is known to be a key regulator of insulin resistance [44].
Lipid metabolism
A total of five studies [30, 31, 43,44,45] reported the effects of CoQ10 supplementation on lipid profiles (Figure S3 in Supplementary File). Four studies [30, 31, 43, 45] reported the effects of CoQ10 supplementation on total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides. Changes from baseline were available from two studies [30, 43]. The remaining two studies [31, 45] provided baseline and final values, so change scores were imputed according to the above-mentioned method. Meta-analysis suggested that CoQ10 supplementation did not significantly affect the levels of total cholesterol (WMD: − 8.56; 95% CI: − 17.81 to 0.69), LDL-cholesterol (WMD: − 4.56; 95% CI: − 12.00 to 2.88), HDL-cholesterol (WMD: 0.15; 95% CI: − 1.56 to 1.86) or triglycerides (WMD: − 10.91; 95% CI: − 25.12 to 3.31). Sensitivity analysis was performed by removing studies in which change scores were imputed. The conclusion remained unchanged (data not shown) regarding total cholesterol, LDL-cholesterol, and HDL-cholesterol. However, regarding triglycerides, when we removed studies with imputed change scores, the result showed that CoQ10 supplementation could decrease the levels of triglycerides (WMD: − 21.05; 95% CI: − 40.68 to − 1.42). Data on VLDL-C were reported from two studies [30, 43]. When combining the two studies, the mean change in VLDL-C concentrations in the treated group compared with the control group was − 3.90 mg/dL (95% CI: − 7.76 to − 0.04). One study provided an intervention effect of CoQ10 on lipoprotein(a) [45]. After 3 months of therapy, serum levels of lipoprotein(a) showed a significant decrease compared with the placebo group (data not shown). One study [44] determining the effects of CoQ10 supplementation on gene expression related to lipids found that CoQ10 supplementation had no effect in this respect.
Safety and tolerability
Only one study reported the safety and tolerability of CoQ10 among the included studies. In Rivara et al.’s study [37], three patients discontinued treatment for abdominal pain and gastrointestinal discomfort or difficulty chewing the study agent; CoQ10 was usually well tolerated.
Discussion
Our work systematically appraised the evidence of CoQ10 supplementation regarding cardiovascular effects, oxidative stress, inflammation, carbohydrate metabolism, and lipid profiles in patients with CKD. The results showed that CoQ10 supplementation might have the potential to improve oxidative stress, glucose metabolism, inflammation levels, cardiac structure, and functional biomarkers.
CoQ10 has important roles in myocardial function. In the general population, there is some evidence that has suggested that decreased CoQ10 concentration in myocardial tissue is associated with impared myocardial function and increasing severity of heart failure [46]. The results of one meta-analysis suggested that CoQ10 supplementation may improve the ejection fraction (EF) in patients with chronic heart failure (CHF) [47]. The benefits tended to be greater in patients with less severe stages of CHF, such as patients with an EF ≥ 30% or those with New York Heart Association (NYHA) class of II or III heart failure. There were some clues that patients with CKD might benefit from CoQ10 treatment. The CoQ10 level was lower and related to coronary flow reserve in hemodialysis patients [48, 49]. Our cross-sectional study showed that the CoQ10 level was independently associated with endothelial dysfunction [50]. CoQ10 treatment might reduce oxidative stress [51, 52], and oxidative stress may constitute a link between CoQ10 levels and endothelial dysfunction in hemodialysis patients [50]. CoQ10 administration might result in a decrease in intraventricular septum thickness, left ventricle mass, and the ratio of velocity early diastolic transmitral blood flow/early diastolic myocardial velocity [36]. In a randomized trial, high doses of CoQ10 might have the potential to reduce biomarkers of cardiac function (troponin T and NT-pro-BNP) in a pre-specified per-protocol analysis [37]. But the results of the intention-to-treat analysis showed that the reductions in troponin T and NT-pro-BNP level did not reach statistical significance (p = 0.09 and 0.10, respectively) [37]. However, not all data support this benefit which might be partly explained by the small sample size. Therefore, large-scale randomized controlled studies are needed to observe the effects of CoQ10 on cardiac function and structure or the long-term cardiovascular prognosis in patients with CKD.
Our meta-analysis showed that CoQ10 supplementation had favorable effects on MDA and other biomarkers of oxidative stress. Previous systematic reviews also demonstrated that CoQ10 supplementation significantly decreased MDA in both the general population and CKD patients [14, 53]. Studies have shown that oxidative stress is associated with kidney disease progression [54, 55]. Several complications of CKD, such as inflammation and cardiovascular disease (CVD), are also linked to an increased level of oxidative stress. Patients with CKD showed increasing concentrations of oxidative stress markers such as mitochondrial superoxide and oxidized LDL [56, 57], homocysteine [58], F2-isoprostanes, MDA and asymmetric dimethylarginine [59, 60]. F2-isoprostanes formation is favored in low oxygen cellular environments, which is aggravated by rarefication of postglomerular capillaries in patients undergoing maintenance dialysis [61]. CoQ10 is an electron carrier and might decrease the oxidative stress status as a reactive oxygen species scavenger [62]. CoQ10 can also improve oxidative stress by reacting with lipids or oxygen radicals through a direct reduction back to tocopherol [63].
Although one previous systematic review [14] found that CRP concentrations did not change following CoQ10 supplementation, our study showed that CoQ10 supplementation could improve hs-CRP levels in hemodialysis patients. The improvement effects of treatment with CoQ10 on inflammation markers were also found in the general population [64]. One recent systematic review [65] assessed the efficacy of CoQ10 supplementation on tumor necrosis factor- α (TNF-α) and interleukin-6 (IL-6) levels in the general population. Overall, nine RCTs were included in the analysis and the results indicated that CoQ10 supplementation resulted in a significant reduction in TNF-α (SMD: − 0.44, 95% CI: [− 0.81 to − 0.07] mg/dl; p = 0.00) and IL-6 levels (SMD: − 0.37, 95% CI: [− 0.65 to − 0.09]; p = 0.01). However, the evidence of the improvement of inflammation markers in CKD patients is relatively nonrobust due to inadequate studies and small sample sizes.
Our review found that CoQ10 supplementation might have the potential to improve glucose metabolism such as HbA1c and QUICKI. In the general population, recent studies also found that coenzyme Q10 may assist glycemic control [66, 67]. There are several mechanisms by which CoQ10 improves glucose metabolism. CoQ10 supplementation may induce the gene expression of PPAR-γ by activating the calcium-mediated AMPK pathway and inhibiting differentiation-induced adipogenesis [68]. PPAR-γ, a nuclear receptor protein, is a ligand-activated transcription factor that regulates the expression of genes involved in insulin [69]. CoQ10 may improve indices of insulin metabolism through modulation of insulin and adiponectin receptors, as well as tyrosine kinase (TK), phosphatidylinositol kinase (PI3K), and glucose transporters. However, not all data supported the benefits. The possible reasons for the discrepancy included differences in study design, study population characteristics, the dosage of CoQ10 used for intervention, and the duration of the intervention.
This meta-analysis suggested that CoQ10 supplementation did not significantly affect the levels of total cholesterol, LDL-cholesterol, HDL-cholesterol, or triglycerides. However, a previous study showed that CoQ10 supplementation significantly improved total cholesterol and LDL-cholesterol [14]. The difference can be partly explained by different inclusion and exclusion criteria. The study conducted by Bakhshayeshkaram et al. included trials combining CoQ10 and other agents [14]. In contrast, we included studies with CoQ10 supplementation alone. Bakhshayeshkaram’s analysis was based on final values [14], and we performed the meta-analysis based on changes from baseline, which seems more appropriate.
This systematic review has several important strengths. First, compared with previous systematic reviews, our review is the first to comprehensively analyze the effects of CoQ10 supplementation on cardiovascular function, oxidative stress, inflammation, glucose metabolism, lipid profiles, and others in patients with CKD. Second, our analysis was based on changes from baseline, which removed a component of between-person variability. Hence, it is more efficient and powerful than the previous study that performed analyses of final values. Third, we only included RCTs using CoQ10 alone, excluding the trials with the combination of CoQ10 with other agents. The pooled results showed the effects of CoQ10 itself, excluding confounders resulting from other agents.
Despite its strengths, this systematic review has several limitations. First, the numbers of trial participants were relatively small, and no studies had sample sizes of more than 100 participants. Second, in some studies, change scores were not available and were imputed. However, sensitivity analyses were performed by excluding the studies with imputed change scores. Third, for each individual outcome, there are relatively few studies. Even though some treatment effects were observed, these were not robust. Due to these limitations, the interpretation of the results should be cautious and further high-quality RCTs are required.
To investigate the cardiovascular effects of CoQ10 supplementation, our pilot study is ongoing, which was previously registered with the Chinese Clinical Trial Registry (ChiCTR) and assigned the registration number ChiCTR1900022258 (available at http://www.chictr.org.cn/edit.aspx?pid=36344&htm=4). This pilot study is focused on the endothelial and cardiac function in hemodialysis patients. We hope that more clues on this issue will be provided.
Conclusion
In conclusion, this systematic review demonstrated that CoQ10 supplementation might have promising effects on oxidative stress. In addition, this work provided some clues that CoQ10 supplementation might have the potential to improve glucose metabolism, inflammation levels, cardiac structure and function biomarkers. However, the effects of CoQ10 supplementation should be confirmed by larger high-quality studies.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The code used in this paper are available from the corresponding author on reasonable request.
Abbreviations
- CKD:
-
Chronic kidney disease
- CoQ10:
-
Coenzyme Q10
- FPG:
-
Fasting plasma glucose
- HbA1c:
-
Hemoglobin A1c
- HDL:
-
High-density lipoprotein
- hs-CRP:
-
High sensitivity C-reactive protein
- HOMA-B:
-
Homeostatic model assessment for B-cell function
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- LDL:
-
Low-density lipoprotein
- MDA:
-
Malonaldehyde
- NT-pro-BNP:
-
N-terminal pro-B-type natriuretic peptide
- PPAR-γ:
-
Peroxisome proliferator-activated receptor-γ
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- QUICKI:
-
Quantitative insulin sensitivity check index
- VLDL:
-
Very low-density lipoprotein
References
Jankowski J, Korzeniowska K, Cieslewicz A, Jablecka A (2016) Coenzyme Q10—a new player in the treatment of heart failure? Pharmacol Rep 68(5):1015–1019. https://doi.org/10.1016/j.pharep.2016.05.012
Liakopoulos V, Roumeliotis S, Gorny X, Eleftheriadis T, Mertens PR (2017) Oxidative stress in patients undergoing peritoneal dialysis: a current review of the literature. Oxid Med Cell Longev 2017:3494867. https://doi.org/10.1155/2017/3494867
Martelli A, Testai L, Colletti A, Cicero AFG (2020) Coenzyme Q(10): clinical applications in cardiovascular diseases. Antioxidants (Basel). https://doi.org/10.3390/antiox9040341
Arenas-Jal M, Suñé-Negre JM, García-Montoya E (2020) Coenzyme Q10 supplementation: efficacy, safety, and formulation challenges. Compr Rev Food Sci Food Saf 19(2):574–594. https://doi.org/10.1111/1541-4337.12539
Tsai KL, Huang YH, Kao CL, Yang DM, Lee HC, Chou HY, Chen YC, Chiou GY, Chen LH, Yang YP, Chiu TH, Tsai CS, Ou HC, Chiou SH (2012) A novel mechanism of coenzyme Q10 protects against human endothelial cells from oxidative stress-induced injury by modulating NO-related pathways. J Nutr Biochem 23(5):458–468. https://doi.org/10.1016/j.jnutbio.2011.01.011
Liakopoulos V, Roumeliotis S, Zarogiannis S, Eleftheriadis T, Mertens PR (2019) Oxidative stress in hemodialysis: causative mechanisms, clinical implications, and possible therapeutic interventions. Semin Dial 32(1):58–71. https://doi.org/10.1111/sdi.12745
Rysz J, Franczyk B, Lawinski J, Gluba-Brzozka A (2020) Oxidative stress in ESRD patients on dialysis and the risk of cardiovascular diseases. Antioxidants (Basel). https://doi.org/10.3390/antiox9111079
Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR (2017) Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev 2017:3081856. https://doi.org/10.1155/2017/3081856
Kawashima C, Matsuzawa Y, Konishi M, Akiyama E, Suzuki H, Sato R, Nakahashi H, Kikuchi S, Kimura Y, Maejima N, Iwahashi N, Hibi K, Kosuge M, Ebina T, Tamura K, Kimura K (2019) Ubiquinol improves endothelial function in patients with heart failure with reduced ejection fraction: a single-center, randomized double-blind placebo-controlled crossover pilot study. Am J Cardiovasc Drugs. https://doi.org/10.1007/s40256-019-00384-y
Fotino AD, Thompson-Paul AM, Bazzano LA (2013) Effect of coenzyme Q(1)(0) supplementation on heart failure: a meta-analysis. Am J Clin Nutr 97(2):268–275. https://doi.org/10.3945/ajcn.112.040741
Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, Steurer G, Littarru GP (2014) The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Failure 2(6):641–649. https://doi.org/10.1016/j.jchf.2014.06.008
Mehmetoglu I, Yerlikaya FH, Kurban S, Erdem SS, Tonbul Z (2012) Oxidative stress markers in hemodialysis and peritoneal dialysis patients, including coenzyme Q10 and ischemia-modified albumin. Int J Artif Organs 35(3):226–232. https://doi.org/10.5301/ijao.5000078
Gazdikova K, Gvozdjakova A, Kucharska J, Spustova V, Braunova Z, Dzurik R (2001) Oxidative stress and plasma concentrations of coenzyme Q10, alpha-tocopherol, and beta-carotene in patients with a mild to moderate decrease of kidney function. Nephron 88(3):285. https://doi.org/10.1159/000046007
Bakhshayeshkaram M, Lankarani KB, Mirhosseini N, Tabrizi R, Akbari M, Dabbaghmanesh MH, Asemi Z (2018) The effects of coenzyme Q10 supplementation on metabolic profiles of patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Curr Pharm Des. https://doi.org/10.2174/1381612824666181112112857
Xu Y, Liu J, Han E, Wang Y, Gao J (2019) Efficacy of coenzyme Q10 in patients with chronic kidney disease: protocol for a systematic review. BMJ Open 9(5):e029053. https://doi.org/10.1136/bmjopen-2019-029053
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P (2010) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa, Canada: Department of Epidemiology and Community Medicine, University of Ottawa. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Riley RD, Kauser I, Bland M, Thijs L, Staessen JA, Wang J, Gueyffier F, Deeks JJ (2013) Meta-analysis of randomised trials with a continuous outcome according to baseline imbalance and availability of individual participant data. Stat Med 32(16):2747–2766. https://doi.org/10.1002/sim.5726
Higgins JPT, Chandler JTJ, Cumpston M, Li T, Page MJ, Welch VA (eds) (2019) Cochrane handbook for systematic reviews of interventions, 3rd edn. John Wiley & Sons, Chichester (UK)
Trowman R, Dumville JC, Torgerson DJ, Cranny G (2007) The impact of trial baseline imbalances should be considered in systematic reviews: a methodological case study. J Clin Epidemiol 60(12):1229–1233. https://doi.org/10.1016/j.jclinepi.2007.03.014
Aiello F, Attanasio M, Tine F (2011) Assessing covariate imbalance in meta-analysis studies. Stat Med 30(22):2671–2682. https://doi.org/10.1002/sim.4311
Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A (2002) Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 31(1):140–149. https://doi.org/10.1093/ije/31.1.140
Borenstein M, Hedges LV, Higgins JP, Rothstein HR (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1(2):97–111. https://doi.org/10.1002/jrsm.12
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Fallah M, Askari G, Soleimani A, Feizi A, Asemi Z (2019) Clinical trial of the effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in diabetic hemodialysis patients. Int J Prev Med 10:12. https://doi.org/10.4103/ijpvm.IJPVM_418_18
Fallah M, Askari G, Soleimani A, Feizi A, Asemi Z (2018) Clinical trial of the effects of coenzyme Q10 supplementation on glycemic control and markers of lipid profiles in diabetic hemodialysis patients. Int Urol Nephrol 50(11):2073–2079. https://doi.org/10.1007/s11255-018-1973-z
Mori TA, Burke V, Puddey I, Irish A, Cowpland CA, Beilin L, Dogra G, Watts GF (2009) The effects of [omega]3 fatty acids and coenzyme Q10 on blood pressure and heart rate in chronic kidney disease: a randomized controlled trial. J Hypertens 27(9):1863–1872
Barden AE, Burke V, Mas E, Beilin LJ, Puddey IB, Watts GF, Irish AB, Mori TA (2015) n-3 fatty acids reduce plasma 20-hydroxyeicosatetraenoic acid and blood pressure in patients with chronic kidney disease. J Hypertens 33(9):1947–1953. https://doi.org/10.1097/HJH.0000000000000621
Barden A, O’Callaghan N, Burke V, Mas E, Beilin LJ, Fenech M, Irish AB, Watts GF, Puddey IB, Huang RC, Mori TA (2016) n-3 fatty acid supplementation and leukocyte telomere length in patients with chronic kidney disease. Nutrients 8(3):175. https://doi.org/10.3390/nu8030175
Barden AE, Shinde S, Burke V, Puddey IB, Beilin LJ, Irish AB, Watts GF, Mori TA (2018) The effect of n-3 fatty acids and coenzyme Q10 supplementation on neutrophil leukotrienes, mediators of inflammation resolution and myeloperoxidase in chronic kidney disease. Prostaglandins Other Lipid Mediat 136:1–8. https://doi.org/10.1016/j.prostaglandins.2018.03.002
Mas E, Barden A, Burke V, Beilin LJ, Watts GF, Huang RC, Puddey IB, Irish AB, Mori TA (2016) A randomized controlled trial of the effects of n-3 fatty acids on resolvins in chronic kidney disease. Clin Nutr 35(2):331–336. https://doi.org/10.1016/j.clnu.2015.04.004
Turk S, Baki A, Solak Y, Kayrak M, Atalay H, Gaipov A, Aribas A, Akilli H, Biyik Z, Okudan N, Gokbel H (2013) Coenzyme Q10 supplementation and diastolic heart functions in hemodialysis patients: a randomized double-blind placebo-controlled trial. Hemodial Int 17(3):374–381. https://doi.org/10.1111/hdi.12022
Rivara MB, Yeung CK, Robinson-Cohen C, Phillips BR, Ruzinski J, Rock D, Linke L, Shen DD, Ikizler TA, Himmelfarb J (2017) Effect of coenzyme Q10 on biomarkers of oxidative stress and cardiac function in hemodialysis patients: the CoQ10 biomarker trial. Am J Kidney Dis 69(3):389–399. https://doi.org/10.1053/j.ajkd.2016.08.041
Kirigaya H (1983) The effect of coenzyme-Q10 on the electrocardiographic ST-segment depression in patients undergoing chronic hemodialysis. Second Asian Pacific Congress of Nephrology, p 45
Gokbel H, Turk S, Okudan N, Atalay H, Belviranli M, Gaipov A, Solak Y (2016) Effects of coenzyme Q10 supplementation on exercise performance and markers of oxidative stress in hemodialysis patients: a double-blind placebo-controlled crossover trial. Am J Ther 23(6):e1736–e1743. https://doi.org/10.1097/MJT.0000000000000166
Zahed NS, Ghassami M, Nikbakht H (2016) Effects of coenzyme Q10 supplementation on C-reactive protein and homocysteine as the inflammatory markers in hemodialysis patients; a randomized clinical trial. J Nephropathol 5(1):38–43. https://doi.org/10.15171/jnp.2016.07
Singh RB, Khanna HK, Niaz MA (2000) Randomized, double-blind placebo-controlled trial of coenzyme Q10 in chronic renal failure: discovery of a new role. J Nutr Environ Med 10(4):281–288. https://doi.org/10.1080/13590840020013266
Singh RB, Kumar A, Niaz MA, Singh RG, Gujrati S, Singh VP, Singh M, Singh UP, Taneja C, Rastogi SS (2003) Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in patients with end-stage renal failure. J Nutr Environ Med 13(1):13–22. https://doi.org/10.1080/1359084031000095002
Gholnari T, Aghadavod E, Soleimani A, Hamidi GA, Sharifi N, Asemi Z (2018) The effects of coenzyme Q10 supplementation on glucose metabolism, lipid profiles, inflammation, and oxidative stress in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. J Am Coll Nutr 37(3):188–193. https://doi.org/10.1080/07315724.2017.1386140
Heidari A, Hamidi G, Soleimani A, Aghadavod E, Asemi Z (2018) Effects of coenzyme Q10 supplementation on gene expressions related to insulin, lipid, and inflammation pathways in patients with diabetic nephropathy. Iran J Kidney Dis 12(1):14–21
Shojaei M, Djalali M, Khatami M, Siassi F, Eshraghian M (2011) Effects of carnitine and coenzyme Q10 on lipid profile and serum levels of lipoprotein(a) in maintenance hemodialysis patients on statin therapy. Iran J Kidney Dis 5(2):114–118
Judy WV, Stogsdill WW, Folkers K (1993) Myocardial preservation by therapy with coenzyme Q10 during heart surgery. Clin Investig 71(8 Suppl):S155-161
DiNicolantonio JJ, Bhutani J, McCarty MF, O’Keefe JH (2015) Coenzyme Q10 for the treatment of heart failure: a review of the literature. Open Heart 2(1):e000326. https://doi.org/10.1136/openhrt-2015-000326
Triolo L, Lippa S, Oradei A, De Sole P, Mori R (1994) Serum coenzyme Q10 in uremic patients on chronic hemodialysis. Nephron 66(2):153–156. https://doi.org/10.1159/000187793
Macunluoglu B, Kaya Y, Atakan A, Ari E, Kaspar C, Demir H, Alp HH, Asicioglu E, Kedrah AE (2013) Serum coenzyme Q10 levels are associated with coronary flow reserve in hemodialysis patients. Hemodial Int 17(3):339–345. https://doi.org/10.1111/hdi.12001
Gao JJ, Xu YX, Jia HP, Zhang L, Cao XY, Zuo XW, Cai GY, Chen XM (2021) Associations of coenzyme Q10 with endothelial function in hemodialysis patients. Nephrology (Carlton) 26(1):54–61. https://doi.org/10.1111/nep.13766
Sakata T, Furuya R, Shimazu T, Odamaki M, Ohkawa S, Kumagai H (2008) Coenzyme Q10 administration suppresses both oxidative and antioxidative markers in hemodialysis patients. Blood Purif 26(4):371–378. https://doi.org/10.1159/000135605
Liakopoulos V, Roumeliotis S, Bozikas A, Eleftheriadis T, Dounousi E (2019) Antioxidant supplementation in renal replacement therapy patients: is there evidence? Oxid Med Cell Longev 2019:9109473. https://doi.org/10.1155/2019/9109473
Jorat MV, Tabrizi R, Kolahdooz F, Akbari M, Salami M, Heydari ST, Asemi Z (2019) The effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in among coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 27(2):233–248. https://doi.org/10.1007/s10787-019-00572-x
Himmelfarb J (2005) Relevance of oxidative pathways in the pathophysiology of chronic kidney disease. Cardiol Clin 23(3):319–330. https://doi.org/10.1016/j.ccl.2005.03.005
Popolo A, Autore G, Pinto A, Marzocco S (2013) Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic Res 47(5):346–356. https://doi.org/10.3109/10715762.2013.779373
Garcia-Bello JA, Gomez-Diaz RA, Contreras-Rodriguez A, Talavera JO, Mondragon-Gonzalez R, Sanchez-Barbosa L, Diaz-Flores M, Valladares-Salgado A, Gallardo JM, Aguilar-Kitsu A, Lagunas-Munoz J, Wacher NH (2014) Carotid intima media thickness, oxidative stress, and inflammation in children with chronic kidney disease. Pediatr Nephrol 29(2):273–281. https://doi.org/10.1007/s00467-013-2626-1
Drozdz D, Kwinta P, Sztefko K, Kordon Z, Drozdz T, Latka M, Miklaszewska M, Zachwieja K, Rudzinski A, Pietrzyk JA (2016) Oxidative stress biomarkers and left ventricular hypertrophy in children with chronic kidney disease. Oxid Med Cell Longev 2016:7520231. https://doi.org/10.1155/2016/7520231
Chien SJ, Lin IC, Hsu CN, Lo MH, Tain YL (2015) Homocysteine and arginine-to-asymmetric dimethylarginine ratio associated with blood pressure abnormalities in children with early chronic kidney disease. Circ J 79(9):2031–2037. https://doi.org/10.1253/circj.CJ-15-0412
Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, Aydin A, Eyileten T, Ozcan O, Acikel C, Tasar M, Genctoy G, Erbil K, Vural A, Zoccali C (2006) The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 47(1):42–50. https://doi.org/10.1053/j.ajkd.2005.09.029
Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC (2005) Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol 289(6):H2649-2656. https://doi.org/10.1152/ajpheart.00548.2005
Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, Willam C (2005) Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl 99:S46-51. https://doi.org/10.1111/j.1523-1755.2005.09909.x
Takashiba S, Van Dyke TE, Shapira L, Amar S (1995) Lipopolysaccharide-inducible and salicylate-sensitive nuclear factor(s) on human tumor necrosis factor alpha promoter. Infect Immun 63(4):1529–1534
Arroyo A, Kagan VE, Tyurin VA, Burgess JR, de Cabo R, Navas P, Villalba JM (2000) NADH and NADPH-dependent reduction of coenzyme Q at the plasma membrane. Antioxid Redox Signal 2(2):251–262. https://doi.org/10.1089/ars.2000.2.2-251
Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH (2017) Effects of coenzyme Q10 supplementation on inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 119:128–136. https://doi.org/10.1016/j.phrs.2017.01.032
Vafa M (2019) Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor-alpha and interleukin-6 levels in chronic inflammatory diseases? A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. https://doi.org/10.1016/j.phrs.2019.104290
Stojanovic M, Radenkovic M (2017) A meta-analysis of randomized and placebo-controlled clinical trials suggests that coenzyme Q10 at low dose improves glucose and HbA1c levels. Nutr Res 38:1–12. https://doi.org/10.1016/j.nutres.2016.12.001
Huang H, Chi H, Liao D, Zou Y (2018) Effects of coenzyme Q10 on cardiovascular and metabolic biomarkers in overweight and obese patients with type 2 diabetes mellitus: a pooled analysis. Diabetes Metab Syndr Obes: Targets Ther 11:875–886. https://doi.org/10.2147/dmso.S184301
Lee SK, Lee JO, Kim JH, Kim N, You GY, Moon JW, Sha J, Kim SJ, Lee YW, Kang HJ, Park SH, Kim HS (2012) Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPARalpha induction in 3T3-L1 preadipocytes. Cell Signal 24(12):2329–2336. https://doi.org/10.1016/j.cellsig.2012.07.022
Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W (2006) From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 45(2):120–159. https://doi.org/10.1016/j.plipres.2005.12.002
Funding
This study was supported in part by a grant (No. 19ZX47) from Chinese PLA Strategic Support Force Characteristic Medical Center (the 306th Hospital of the Chinese PLA).
Author information
Authors and Affiliations
Contributions
YX, JG and JL contributed in conception and design. GY, YX contributed to electronic search. YX and YW contributed in study selection and data collection. GY, YX, and XZ contributed in statistical analysis. YX, GY, EH and HY interpreted data. GY, YX, XZ, JG, and HJ drafted the initial and final manuscript. All authors approved the final version for submission. JG and HJ supervised the study.
Corresponding authors
Ethics declarations
Conflict of interest
Yongxing Xu, Guolei Yang, Xiaowen Zuo, Jianjun Gao, Huaping Jia, Enhong Han, Juan Liu, Yan Wang, and Hong Yan declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, Y., Yang, G., Zuo, X. et al. A systematic review for the efficacy of coenzyme Q10 in patients with chronic kidney disease. Int Urol Nephrol 54, 173–184 (2022). https://doi.org/10.1007/s11255-021-02838-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-02838-2