Abstract
Background
Endothelial dysfunction is reportedly associated with worse outcomes in patients with chronic heart failure. Ubiquinol is a reduced form of coenzyme Q10 (CoQ10) that may improve endothelial function.
Objective
We assessed the hypothesis that ubiquinol improves peripheral endothelial function in patients with heart failure with reduced ejection fraction (HFrEF).
Methods
In this randomized, double-blind, placebo-controlled, crossover pilot study, 14 patients with stable HFrEF were randomly and blindly allocated to ubiquinol 400 mg/day or placebo for 3 months. After a 1-month washout period, patients were crossed over to the alternative treatment. Before and after each treatment, we assessed peripheral endothelial function using the reactive hyperemia index (RHI) and analyzed it using the natural logarithm of RHI (LnRHI).
Results
Peripheral endothelial function as assessed by LnRHI tended to improve with ubiquinol 400 mg/day for 3 months (p = 0.076). Original RHI values were also compared, and RHI significantly improved with ubiquinol treatment (pre-RHI 1.57 [interquartile range (IQR) 1.39–1.80], post-RHI 1.74 [IQR 1.63–2.02], p = 0.026), but not with placebo (pre-RHI 1.67 [IQR 1.53–1.85], post-RHI 1.51 [IQR 1.39–2.11], p = 0.198).
Conclusions
Ubiquinol 400 mg/day for 3 months led to significant improvement in peripheral endothelial function in patients with HFrEF. Ubiquinol may be a therapeutic option for individuals with HFrEF. Large-scale randomized controlled trials of CoQ10 supplementation in patients with HFrEF are needed.
Clinical Trial Registration
Japanese University Hospital Medical Information Network (UMIN-ICDR). Clinical Trial identifier number UMIN000012604.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Heart failure is a condition wherein the heart cannot maintain adequate cardiac output or can maintain cardiac output only with an elevated left ventricular filling pressure. Regardless of heart failure etiology, dysfunction of the bioenergetics of cardiac myocytes leading to energy starvation is an important contributing factor in the failing heart. In recent decades, numerous improvements have been made in the therapeutic management of heart failure; however, the outcomes for patients with heart failure with reduced ejection fraction (HFrEF) remain poor [1]. Most of the current therapies for heart failure act by modulating maladaptive neurohormonal pathways, such as the renin-angiotensin-aldosterone system and sympathetic nervous system. Although drugs that have a positive influence on bioenergetics can play a role in the treatment and management of heart failure, they have not yet been put into clinical use.
Coenzyme Q10 (CoQ10) is a lipophilic molecule that plays a crucial role in energy production in mitochondria. It has been reported that CoQ10 improves nitric oxide (NO) bioavailability and electron transport chain function, prevents left ventricular hypertrophy, and decreases left ventricular fibrosis [2]. The physiological role of CoQ10 facilitates the production of adenosine triphosphate (ATP) and acts as an antioxidant, which improves endothelial function. In patients with heart failure, the level of CoQ10 in myocardial tissue is, on average, 33% lower than in control patients [3, 4], and the level correlates with the severity of symptoms and the degree of left ventricular dysfunction [5]. Furthermore, a recent randomized controlled trial (Q-SYMBIO) reported a significant benefit from CoQ10 in improving outcomes in patients with heart failure irrespective of left ventricular EF [6].
Peripheral endothelial function has been assessed using brachial artery flow-mediated vasodilation (FMD) in clinical research. Several studies have demonstrated that CoQ10 improved brachial artery FMD in patients with coronary artery disease [7], diabetes mellitus [8], and heart failure [9]. All these studies used the oxidized form of CoQ10 (ubiquinone). Ubiquinol, the reduced form of CoQ10, is already in its active form of antioxidant [10] and has received more attention because recent studies have shown that it is more bioavailable [11] and has demonstrated greater efficacy at lower doses than oxidized CoQ10 [12,13,14]. It has also been reported that ubiquinol has the potential to modulate the overexpression of inflammatory and thrombotic risk markers in antiphospholipid syndrome [15].
Measurement of peripheral vasodilator response with fingertip peripheral arterial tonometry (PAT) technology (EndoPAT; Itamar Medical Inc., Caesarea, Israel) is a noninvasive method of assessing vascular endothelial function [16, 17]. It has been demonstrated that endothelial dysfunction assessed by EndoPAT is a predictor of future cardiovascular events in patients with heart failure independent of conventional risk factor assessment such as age, diabetes, New York Heart Association (NYHA) classification, and left ventricular EF [18, 19]. Importantly, the Framingham Heart Study found no significant relationship between EndoPAT and FMD after adjustment for risk factors and underlying cardiovascular diseases, indicating that these two methods reflect distinct aspects of vascular function [20]. Both methods are based on the same principle of reactive hyperemia phenomenon. EndoPAT measures the finger pulse amplitude of microvessels and uses a control arm as its internal control to correct for systemic changes during testing. FMD measures vasodilation in a conduit artery and assesses the endothelial response to shear stress. NO plays a substantial role in both methods. Vasodilatory responses result from complex interactions between vascular smooth muscle and vasoactive substances such as prostaglandin, adenosine, and hydrogen peroxide and could occur differently in conduit arteries and microvessels. Importantly, studies, including a meta-analysis [21], have indicated that both FMD and EndoPAT have significant predictive value for future cardiovascular events. One advantage of the EndoPAT technique is that it requires less operator input than the FMD technique. Several studies have indicated that CoQ10 improved FMD in patients with heart disease [7, 9]. The mechanisms by which CoQ10 exerts a beneficial effect on endothelial function are thought to mainly be via antioxidant activity, anti-inflammatory activity, and enhancing endothelial NO bioavailability [22, 23]. As mentioned, both EndoPAT and FMD are tests for endothelial function but might reflect different aspects of vascular conditions. Furthermore, EndoPAT is less operator dependent and thus may be compared among hospitals and internationally. The effect of CoQ10 on endothelial function as assessed by EndoPAT has not yet been reported in patients with heart failure.
The optimal dose of CoQ10 supplementation remains unclear. Ubiquinol 400 mg/day was selected to attain a high enough plasma level of CoQ10 [24]. In a previous study, patients with advanced chronic heart failure were treated with the oxidized form of CoQ10 at 300 mg/day, and patients with plasma CoQ10 levels > 2.4 μg/mL after supplementation showed six times greater improvement in endothelium-dependent relaxation of the brachial artery compared with those with plasma CoQ10 levels < 2.4 μg/mL.
The aim of this study (VERTIS SITA2) was to compare the safety and efficacy of the addition of ertugliflozin (5 mg and 15 mg QD) to that of placebo in patients with T2DM and inadequate glycemic control on a combination of metformin and sitagliptin.
We performed a randomized, placebo-controlled, double-blind, crossover pilot study to assess whether the addition of ubiquinol 400 mg daily to standard heart failure therapy would improve peripheral endothelial function, as assessed by EndoPAT, compared with placebo in patients with HFrEF.
2 Methods
2.1 Study Design and Patients
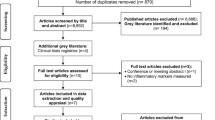
This was a single-center, randomized, double-blind, placebo-controlled, crossover study performed at the Yokohama City University Medical Center in Japan between July 2013 and October 2014. The study design is shown in Fig. 1. Study participants were recruited from the outpatient clinic of the division of cardiology at the Yokohama City University Medical Center. The inclusion criteria were ambulatory patients aged 20–89 years with chronic heart failure and EF ≤ 40% as documented using the modified Simpson method via echocardiogram, and who had received standard heart failure treatment for at least 1 month. Exclusion criteria were (1) malignant disease, (2) severe infectious disease, (3) patients with trauma, (4) patients undergoing hemodialysis, and (5) the decision of the attending doctor. We excluded patients who were receiving other supplements. Participants were randomized to receive either oral CoQ10 supplementation (ubiquinol 200 mg twice daily (400 mg/day); Kaneka, Japan) or matched placebo for 3 months. After a 1-month washout period, patients were crossed over to the alternative treatment for 3 months. Patients took the medication or placebo after breakfast and dinner. The study protocol was approved by the institutional review board and was implemented in accordance with the guidelines of the ethics committee of our institution and the provisions of the Declaration of Helsinki. Written informed consent was obtained from each patient before participation.
Study flow chart. Participants were randomized to receive either oral coenzyme Q10 supplementation or matched placebo for 3 months. After a 1-month washout period, patients were crossed over to the alternative treatment for 3 months. Six of the 20 patients were excluded from the analysis (five withdrawals and one lack of data), and 14 patients completed the final follow-up
2.2 Blood Tests and Clinical Assessment
At the start of the first treatment period (baseline), body height and weight, B-type natriuretic peptide (BNP) levels, and blood pressure were measured. Cardiovascular risk factors and etiology of heart failure were documented. At baseline and after 3 months of treatment, all patients underwent blood tests to measure creatinine, cystatin C as a marker of renal function, lipid profile, BNP as a marker of severity of heart failure, C-reactive protein (CRP) measured with the latex immunoturbidimetric method, and high-sensitivity troponin I to assess myocardial damage. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) was measured to assess oxidative stress. The total plasma concentration of CoQ10, both the reduced form (ubiquinol) and the oxidized form (ubiquinone), was analyzed using high-performance liquid chromatography methods as previously described [25].
2.3 Endothelial Function
Endothelial function was evaluated via reactive hyperemia PAT (RH-PAT) (Endo-PAT2000; Itamar Medical, Caesarea, Israel). This technique measures digital pulse amplitude and blood volume changes noninvasively after occluding the unilateral brachial artery. Previous studies have demonstrated good reproducibility (intraclass correlation coefficient 0.61–0.78) of the EndoPAT data recorded by this procedure [26, 27]. We assessed peripheral endothelial function with reactive hyperemia index (RHI) at baseline and after each treatment. The natural logarithm of RHI (LnRHI) was calculated and used for analyses because RHI values have a skewed distribution.
2.4 Statistical Analysis
Statistical analyses were performed using JMP version 12.2.0 (SAS Institute, Inc.; Cary, NC, USA). Continuous variables are presented as means ± standard deviations or median (interquartile range [IQR]) when appropriate. Categorical variables are shown as numbers with percentages. In this study, as the number of included patients was very small, we did not examine the distribution. We expressed median values with IQRs for CRP, BNP, and troponin I, because these are generally known to have non-normal distributions. We used LnRHI because RHI also has a non-normal distribution. We used Fisher’s exact test for categorical variables. Differences in variables, including RHI before and after treatment, were evaluated using the t-test or the Wilcoxon test as appropriate (difference in RHI with the Wilcoxon test and LnRHI with the t-test). The correlation between changes in LnRHI and in total plasma CoQ10 levels was analyzed with Spearman’s rank correlation. A two-sided p value < 0.05 was considered statistically significant.
3 Results
In total, 20 patients with HFrEF were enrolled, 14 of whom completed the final follow-up. Six patients were excluded from the data analysis (five because of a break in contact; one because of lack of data) (Fig. 1). The baseline characteristics of the study population are shown in Table 1. In total, 12 (85.7%) participants were male, and the mean age was 70 years. Seven participants (50.0%) had ischemic heart disease, nine (64.3%) had hypertension, five (35.7%) had diabetes mellitus, and four (28.6%) had dyslipidemia. The etiology of heart failure was ischemic heart disease in seven (50%) participants; valvular heart disease in two; dilated cardiomyopathy, sarcoidosis, and noncompaction cardiomyopathy in one participant each; and unknown in two. β-Blockers were prescribed in 85.7% of the participants, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in all participants, and statins in 71.4%. In the placebo group, 50% each were NYHA class I and class II at baseline; this did not change after the intervention. In the ubiquinol group, 42.9% were NYHA class I and 57.1% were class II at baseline; after the intervention, 50% each were NYHA class I and II. No significant changes were found in any clinical data, including body weight, blood pressure, NYHA classification, creatinine, cystatin C, lipid profiles, BNP, high-sensitivity troponin I, and urinary 8-OHdG (Table 2).
3.1 Plasma Coenzyme Q10 (CoQ10) Levels
Table 3 shows plasma CoQ10 levels in total, in reduced form, and in oxidized form. Baseline plasma CoQ10 levels were around 0.8–0.9 µg/mL. Ubiquinol treatment led to a large increase in plasma CoQ10 levels (total CoQ10 levels in plasma after ubiquinol treatment was 7.338 ± 5.597 µg/mL), whereas placebo had no significant effect on plasma CoQ10 levels. CoQ10 mostly exists as the reduced form (ubiquinol) rather than the oxidized form (ubiquinone). Table 3 shows the ratio of the reduced form to the oxidized form of coenzyme Q10. There was a significant decrease in the ratio before and after the intervention with placebo (p = 0.0008) and no difference in the ubiquinol group (p = 0.90). We also examined correlations between the concentration of the reduced form of CoQ10, reduced form to oxidized form of CoQ10, and biomarkers such as 8-OHdG/Cr, troponin I, BNP, and CRP before and after the intervention. As a result, reduced form to oxidized form of CoQ10 and BNP have negative correlations both before (Spearman’s ρ = − 0.3822, p = 0.04) and after (Spearman’s ρ = − 0.4051, p = 0.04) the intervention.
3.2 Effect of Supplementation of CoQ10 on Endothelial Function
Figure 2 shows samples of RH-PAT signals before and after the intervention. Peripheral endothelial function as assessed by LnRHI using the t-test tended to be improved by treatment with ubiquinol 400 mg/day for 3 months (p = 0.076) (Fig. 3a). We also compared the original RHI values without logarithmic transformation using a nonparametric Wilcoxon test and found that RHI was significantly improved with ubiquinol (pre-RHI 1.57 [IQR 1.39–1.80], post-RHI 1.74 [IQR 1.63–2.02], p = 0.026) but not with placebo (pre-RHI 1.67 [IQR 1.53–1.85], post-RHI 1.51 [IQR 1.39–2.11], p = 0.198) (Fig. 3b). LnRHI improved after the intervention for 11 (78.6%) patients in the ubiquinol group and six (42.9%) in the placebo group (between-group difference, p = 0.12). There was no significant correlation between change in LnRHI and change in total CoQ10 levels (spearman’s ρ = 0.21, p = 0.31). We analyzed the relationship between the level of reduced CoQ10, reduced form to oxidized form of CoQ10, and LnRHI before and after the intervention. The reduced CoQ10 and the reduced form to oxidized form of CoQ10 did not relate to LnRHI at baseline. Although it did not reach significance, there was a positive relation between the reduced form to oxidized form of CoQ10 and LnRHI after the intervention (Spearman’s ρ = 0.3593, p = 0.07). We analyzed the association between the etiology of heart failure (ischemic or nonischemic) with LnRHI and the levels of the reduced form of CoQ10 at baseline. Although there was no significant difference in LnRHI (0.44 ± 0.07 vs. 0.50 ± 0.13; p = 0.30) and the levels of the reduced form of CoQ10 (0.80 ± 0.34 vs. 0.94 ± 0.27; p = 0.40) between ischemic and nonischemic heart failure, LnRHI and the level of the reduced form of CoQ10 tended to be lower in patients with ischemic heart failure.
Changes in endothelial function in each group. Peripheral endothelial function as assessed by natural logarithm of reactive hyperemia index (LnRHI) tended to be improved in the ubiquinol group (a). Original RHI values was significantly improved by the ubiquinol treatment, but placebo did not lead to improvement (b)
3.3 Safety and Tolerability
Six patients were lost to follow-up over the whole period because of dropout and lack of data, but no adverse events were reported.
4 Discussion
We investigated the effects on peripheral endothelial function in patients with HFrEF of adding oral ubiquinol, a reduced form of CoQ10, at a dosage of 400 mg/day for 3 months to standard therapies for heart failure. Adding ubiquinol had no effect on any clinical variables, including renal function, lipid profile, fasting glucose, BNP, and oxidative stress compared with placebo. However, our results highlighted the potential of ubiquinol to improve peripheral endothelial function. This is the first study to demonstrate that CoQ10 significantly improved endothelial function assessed by RHI. LnRHI increased from 0.45 to 0.55 as a result of CoQ10 therapy, and this increment tended to be significant. According to a recent meta-analysis of RHI, an increase in LnRHI of 0.1 corresponds to a 21% risk reduction for a cardiovascular event [21].
There was no significant difference in basal LnRHI levels between the two groups (0.51 vs. 0.45; p = 0.29). Although the number of patients was too small to analyze with multivariate models, we conducted covariance analysis to adjust for basal LnRHI. After adjustment for the basal LnRHI levels, the effect of ubiquinol in improving endothelial function was not significant compared with the placebo group (p = 0.18). We cannot exclude the influence of basal LnRHI levels on our results. After the washout period of the first therapies, there was no difference in LnRHI between the two groups (LnRHI 0.64 ± 0.27 vs. 0.52 ± 0.28; p = 0.44). We also analyzed the change in LnRHI during the washout period between the first and the second interventions. In patients who received placebo first, LnRHI was 0.51 before placebo, 0.47 after placebo, 0.39 before ubiquinol, and 0.65 after ubiquinol; in patients who received ubiquinol first, LnRHI was 0.52 before ubiquinol, 0.60 after ubiquinol, 0.64 before placebo, and 0.58 after placebo. In patients who received ubiquinol first, the LnRHI level remained elevated after the washout period, which might be due to the shortness of that period. Although this is a small sample study and there was no significant difference between the groups, it is possible that the washout period was too short.
Heart failure, a terminal stage of all kinds of heart diseases, is a major challenge to the health system because it causes significant increases in healthcare costs and the degree of patient disability. Endothelial function-guided therapy for heart failure could be useful in reducing mortality and preventing the worsening of heart failure and complications. Associations between endothelial dysfunction and increased cardiovascular risk have been reported by many studies and in various populations [28]. The endothelium releases several vasoactive factors, such as NO, and plays a crucial role in maintaining vascular tone and inhibiting platelet aggregation, smooth muscle cell proliferation, and inflammation. Thus, dysfunctional endothelium is associated with increased inflammation and thrombogenicity and, in accordance with this pathological feature, leads to atherothrombotic vascular complications. Moreover, endothelial dysfunction has an important involvement in myocardial ischemia and can increase vascular stiffness and resistance, resulting in progression of heart failure and adverse outcomes. We have previously reported the significant predictive value of RHI in patients with HFrEF and heart failure with preserved EF (HFpEF) [18, 19]. Furthermore, deterioration of endothelial function as assessed by RHI in patients with heart failure after implantation of a continuous-flow left ventricular assist device was associated with subsequent adverse cardiovascular events [29]. Importantly, endothelial function can serve not only as a diagnostic and prognostic marker but also as a therapeutic target even in conditions of heart failure. In patients with ischemic heart failure, improvement in peripheral endothelial function was associated with a significant reduction in cardiovascular events [30]. Thus, endothelial function is predictive for adverse events and reversible through the course of heart diseases, including heart failure.
Although the mechanisms of CoQ10 in improving endothelial function remain obscure, it is suggested that CoQ10 can improve energy metabolism and prevent oxidative stress in myocardial cells. CoQ10 is an electron carrier in mitochondria and plays the role of an endothelial NO synthase (eNOS) cofactor by maintaining its “coupled” structure and NO, and it may improve endothelial dysfunction by “recoupling” eNOS and mitochondrial oxidative phosphorylation. CoQ10 could also act synergistically with anti-atherogenic agents, such as statins, to improve endothelial dysfunction [31]. Thus, we hypothesized that reduced inflammation and oxidative stress could be the important mechanism through which CoQ10 improves endothelial function. However, inconsistent with the previous data [32, 33], inflammatory markers and 8-OHdG, a marker of oxidative stress, did not change with CoQ10 therapy in the present study. Insufficient statistical power is one possible reason for this; another is that CoQ10 improved endothelial function assessed by RHI by mediating other mechanisms. In this study population, CRP values were < 3 mg/dL, and patients were not in the proinflammatory state. As such, these participants may not have been the appropriate population in which to observe anti-inflammatory effects from this intervention.
Several studies have reported the effects of CoQ10 on endothelial function [34]. A randomized controlled study of patients with heart failure [9] reported that CoQ10 supplementation (ubiquinone) improved FMD, and this improvement was associated with reductions in the plasma lactate/pyruvate ratio, suggesting that endothelial function was improved via improvement in mitochondrial function after CoQ10 supplementation. Consistent with this, our study also demonstrated the significant improvement in RHI by CoQ10 therapy. Dai et al. [9] also reported a positive relationship between improvement in FMD and an increase in plasma CoQ10 levels. However, our study found no significant relationship between changes in RHI and plasma CoQ10 levels. One possible reason for this discrepancy is the small number of participants in our study. Another potential cause is based on the difference between FMD and RHI. As mentioned in Sect. 1, FMD and RHI may reflect different vascular aspects as these methods use the same principle of reactive hyperemia after ischemia but the target vasculatures differ (brachial artery diameter in FMD and fingertip pulse amplitude in RHI). Vasodilatory responses after ischemia are mediated by a complex interaction between a large number of vasoactive substances and vascular smooth muscle and differ between conduit arteries and microvessels. Belardinelli et al. [24] reported that plasma CoQ10 levels in patients with advanced chronic heart failure treated with the oxidized form of CoQ10 300 mg/day for 4 weeks were 3.25 ± 1.52 μg/mL. In addition, Mortensen et al. [6] observed that serum CoQ10 levels in patients with moderate or severe heart failure after treatment with the oxidized form of CoQ10 300 mg/day for 16 weeks were 3.01 ± 0.17 μg/mL. In our study, patients received the reduced form of CoQ10 for 3 months, and we found a serum CoQ10 level of 7.34 ± 5.60 μg/mL. The CoQ10 level in this study was obviously higher than in the previous study with the oxidized form of CoQ10. The result indicates that the reduced form of CoQ10 has better bioavailability than the oxidized form. Although a dose-dependent relationship with CoQ10 and endothelial function is possible, we did not investigate this, and further studies are required.
4.1 Study Limitations
Our study includes some limitations. First, this was a pilot study, and the small number of patients resulted in limited statistical power, so the study might have been underpowered to detect any relationship between biomarkers and CoQ intervention. Second, although we confirmed that the post-intervention concentration levels of CoQ10 were elevated in all patients, we did not evaluate compliance. Third, although patients underwent an echocardiogram before and after the first intervention and after the second intervention, it was not routinely performed after the washout period (just before the second intervention). Therefore, we could not analyze EF after the intervention. Fourth, we did not assess circulating markers of endothelial function and oxidative stress such as intercellular adhesion molecule, soluble vascular adhesion molecule, E-selectin, von Willebrand factor, NO, total antioxidant capacity, etc. Further study is needed because these important markers could complement and reinforce the results obtained from the RHI analysis. Fifth, we did not include a healthy control group. Finally, we could not rule out that basal LnRHI affected the response.
5 Conclusion
Treatment with ubiquinol 400 mg/day for 3 months resulted in significant and clinically meaningful improvements in peripheral endothelial function in patients with HFrEF. Ubiquinol, the reduced form of CoQ10, and ubiquinone may be therapeutic options for the treatment of individuals with HFrEF. Large-scale randomized controlled trials of CoQ10 supplementation in patients with HFrEF are needed before any firm conclusions can be made.
References
Meta-analysis Global Group in Chronic Heart Failure. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–7.
Sharma A, Fonarow GC, Butler J, Ezekowitz JA, Felker GM. Coenzyme Q10 and heart failure: a state-of-the-art review. Circ Heart Fail. 2016;9:e002639.
Mortensen SA, Vadhanavikit S, Muratsu K, Folkers K. Coenzyme Q10: clinical benefits with biochemical correlates suggesting a scientific breakthrough in the management of chronic heart failure. Int J Tissue React. 1990;12:155–62.
Mortensen SA. Perspectives on therapy of cardiovascular diseases with coenzyme Q10 (ubiquinone). Clin Investig. 1993;71:S116–23.
Folkers K, Vadhanavikit S, Mortensen SA. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc Natl Acad Sci USA. 1985;82:901–4.
Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2:641–9.
Tiano L, Belardinelli R, Carnevali P, Principi F, Seddaiu G, Littarru GP. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double-blind, randomized controlled study. Eur Heart J. 2007;28:2249–55.
Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32:810–2.
Dai YL, Luk TH, Yiu KH, Wang M, Yip PM, Lee SW, et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: a randomized controlled trial. Atherosclerosis. 2011;216:395–401.
Frei B, Kim MC, Ames BN. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc Natl Acad Sci USA. 1990;87:4879–83.
Langsjoen PH, Langsjoen AM. Comparison study of plasma coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin Pharmacol Drug Dev. 2014;3:13–7.
Garcia-Corzo L, Luna-Sanchez M, Doerrier C, Ortiz F, Escames G, Acuna-Castroviejo D, et al. Ubiquinol-10 ameliorates mitochondrial encephalopathy associated with CoQ deficiency. Biochim Biophys Acta. 2014;1842:893–901.
Yoritaka A, Kawajiri S, Yamamoto Y, Nakahara T, Ando M, Hashimoto K, et al. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:911–6.
Yen CH, Chu YJ, Lee BJ, Lin YC, Lin PT. Effect of liquid ubiquinol supplementation on glucose, lipids and antioxidant capacity in type 2 diabetes patients: a double-blind, randomised, placebo-controlled trial. Br J Nutr. 2018;120:57–63.
Perez-Sanchez C, Aguirre MA, Ruiz-Limon P, Abalos-Aguilera MC, Jimenez-Gomez Y, Arias-de la Rosa I, et al. Ubiquinol effects on antiphospholipid syndrome prothrombotic profile: a randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2017;37:1923–32.
Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. J Am Heart Assoc. 2013;2:e000426.
Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–96.
Fujisue K, Sugiyama S, Matsuzawa Y, Akiyama E, Sugamura K, Matsubara J, et al. Prognostic significance of peripheral microvascular endothelial dysfunction in heart failure with reduced left ventricular ejection fraction. Circ J. 2015;79:2623–31.
Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, et al. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1778–86.
Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57:390–6.
Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015. https://doi.org/10.1161/JAHA.115.002270.
Kumar A, Kaur H, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol Ther. 2009;124:259–68.
Pepe S, Marasco SF, Haas SJ, Sheeran FL, Krum H, Rosenfeldt FL. Coenzyme Q10 in cardiovascular disease. Mitochondrion. 2007;7(Suppl):S154–67.
Belardinelli R, Mucaj A, Lacalaprice F, Solenghi M, Seddaiu G, Principi F, et al. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J. 2006;27:2675–81.
Yamashita S, Yamamoto Y. Simultaneous detection of ubiquinol and ubiquinone in human plasma as a marker of oxidative stress. Anal Biochem. 1997;250:66–73.
Brant LC, Barreto SM, Passos VM, Ribeiro AL. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens. 2013;31:1984–90.
McCrea CE, Skulas-Ray AC, Chow M, West SG. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med. 2012;17:29–36.
Mortensen SA. Overview on coenzyme Q10 as adjunctive therapy in chronic heart failure. Rationale, design and end-points of “Q-symbio”—a multinational trial. Biofactors. 2003;18:79–89.
Hasin T, Matsuzawa Y, Guddeti RR, Aoki T, Kwon TG, Schettle S, et al. Attenuation in peripheral endothelial function after continuous flow left ventricular assist device therapy is associated with cardiovascular adverse events. Circ J. 2015;79:770–7.
Takishima I, Nakamura T, Hirano M, Kitta Y, Kobayashi T, Fujioka D, et al. Predictive value of serial assessment of endothelial function in chronic heart failure. Int J Cardiol. 2012;158:417–22.
Chew GT, Watts GF. Coenzyme Q10 and diabetic endotheliopathy: oxidative stress and the ‘recoupling hypothesis’. QJM. 2004;97:537–48.
Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH. Effects of coenzyme Q10 supplementation on inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2017;119:128–36.
Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–99.
Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221:311–6.
Acknowledgements
Kaneka, Japan, supplied the ubiquinol and matching placebo capsules used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of interest
C.K., Y.M., M. Konishi, E.A., H.S., R.S., H.N., S.K., Y.K., N.M., N.I., K.H., M. Kosuge, T.E., K.T., and K.K. have no conflicts of interest that are directly relevant to the content of this study.
Rights and permissions
About this article
Cite this article
Kawashima, C., Matsuzawa, Y., Konishi, M. et al. Ubiquinol Improves Endothelial Function in Patients with Heart Failure with Reduced Ejection Fraction: A Single-Center, Randomized Double-Blind Placebo-Controlled Crossover Pilot Study. Am J Cardiovasc Drugs 20, 363–372 (2020). https://doi.org/10.1007/s40256-019-00384-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-019-00384-y