Abstract
Purpose
Insulin resistance, dyslipidemia and increased systemic inflammation are important risk factors for chronic kidney disease (CKD). Hence, vitamin D administration might be an appropriate approach to decrease the complications of CKD. Randomized controlled trials assessing the effects of vitamin D supplementation or treatment on glycemic control, lipid profiles, and C-reactive protein (CRP) among patients with CKD were included.
Methods
Two independent authors systematically searched online databases including EMBASE, Scopus, PubMed, Cochrane Library, and Web of Science in November 2018 with no time restriction. Cochrane Collaboration risk of bias tool was applied to assess the methodological quality of included trials. Between-study heterogeneity was estimated using the Cochran’s Q test and I-square (I2) statistic. Data were pooled using a random-effects model and weighted mean difference (WMD) was considered as the overall effect size.
Results
Of the 1358 citations identified from searches, 17 full-text articles were reviewed. Pooling findings from five studies revealed a significant reduction in fasting glucose (WMD: − 18.87; 95% CI: − 23.16, − 14.58) and in homeostatic model assessment of insulin resistance (HOMA-IR) through three studies (WMD: − 2.30; 95% CI: − 2.88, − 1.72) following the administration of vitamin D. In addition, pooled analysis revealed a significant reduction in triglycerides (WMD: − 32.52; 95% CI: − 57.57, − 7.47) through six studies and in cholesterol concentrations (WMD: − 7.93; 95% CI: − 13.03, − 2.83) through five studies, following vitamin D supplementation or treatment, while there was no effect on insulin, HbA1c, LDL and HDL cholesterol, and CRP levels.
Conclusions
This meta-analysis demonstrated the beneficial effects of vitamin D supplementation or treatment on improving fasting glucose, HOMA-IR, triglycerides and cholesterol levels among patients with CKD, though it did not influence insulin, HbA1c, LDL and HDL cholesterol, and CRP levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
People with chronic kidney disease (CKD), including patients undergoing hemodialysis (HD), have a high incidence of cardiovascular diseases (CVDs) due to enhanced atherosclerosis [15]. Although the physiopathology of atherosclerosis is not yet fully recognized, there is a close relationship between inflammation and atherosclerosis [50]. The inflammatory cascade can be initiated by metabolic disorders and risk factors of atherosclerosis such as atherogenic dyslipidemia, insulin resistance and elevated blood glucose concentration [25]. Impaired glucose metabolism and increased insulin resistance are well-known complications at all stages of CKD [8, 12].
Vitamin D is a fat-soluble secosteroid that is well recognized due to its function in calcium metabolism and bone mineralization [24]. Prior investigations have demonstrated that hypovitaminosis D is common in people with CKD [7, 8]. Reduced vitamin D levels are related with elevated systemic inflammatory factors and insulin resistance in this condition [35]. Based on a previous meta-analysis, each 10 ng/ml decrement in vitamin D value elevates the all-cause mortality risk by 14% in people with CKD [36]. Another meta-analysis documented a 37% reduction in CVDs mortality with vitamin D supplementation in CKD patients [10]. Our recent meta-analyses also showed the favorable impacts of vitamin D intake on C-reactive protein (CRP) levels among patients with diabetes mellitus and polycystic ovarian syndrome [2, 26]. In a meta-analysis of 17 randomized controlled trials (RCTs), Jafari et al. [18] revealed that vitamin D administration ameliorated total, LDL cholesterol, and triglyceride concentrations in patients with type 2 diabetes mellitus (T2DM). In another study, vitamin D supplementation significantly reduced insulin resistance in patients with T2DM [22]. However, administration of vitamin D did not affect glycemic control and lipid profiles in people with non-alcoholic fatty liver disease [39]. Some RCTs have evaluated the effect of vitamin D on glucose homeostasis, lipid profiles, and CRP in people with CKD [20, 21, 40, 46]. Therefore, the results of those clinical trials are inclusive, with studies that favored vitamin D effects, while other studies did not show any beneficial effects of this micronutrient. Using different dosages of vitamin D, as well as differences in study design and characteristics of participants are the probable reasons that may explain the conflicting results about glycemic control, lipid profile and CRP in patients CKD.
To the best of our knowledge, no earlier study has systematically reviewed findings from RCTs on the effect of vitamin D supplementation or treatment or metabolic status in patients with CKD. Therefore, the present study was done to systematically summarize the present evidence of RCTs on the influence of vitamin D supplementation or treatment on glycemic control, lipid profiles, and CRP in these patients and to run a meta-analysis, if possible.
Methods
Search and study selection strategies
The scientific international databases, including Cochrane Library, EMBASE, PubMed, and Web of Science were searched for relevant studies published up to November 2018 with no time restriction. A search strategy was developed using the following MeSH and text keywords: patients [“CKD” OR “HD” OR “chronic renal failure” OR “ESRD” OR “disorders related to kidney], AND intervention (“vitamin D” OR “colecalciferol” OR “ergocalciferol” OR “calcitriol” AND “supplementation” OR “intake” OR “administration” OR “treatment”), and outcomes (“fasting glucose” OR “fasting plasma glucose (FPG)” OR “insulin” OR “homeostasis model assessment of insulin resistance (HOMA-IR)” OR “HbA1c” AND “total cholesterol (TC)” “triglycerides (TG)” OR “low-density lipoprotein (LDL cholesterol)” OR “LDL-C” OR “high-density lipoprotein (HDL cholesterol)” OR “HDL-C” OR “CRP”). We also checked the reference list of published reviews to avoid missing any potentially eligible studies. A professional librarian educated in research strategies help us to perform the literature review.
Inclusion and exclusion criteria
All CTs with either parallel or cross-over design that investigated the effect of any dosages of vitamin D supplementation or treatment on glycemic control and used placebo as the control group among patients with CKD were included. Furthermore, we included calcitriol due to fewer side effects. Animal experiments, in vitro studies, case reports, observational studies, studies that did not have control group, and those studies that did not achieve the least quality score were excluded. In addition, studies in which vitamin D analogs other than calcitriol were used as the intervention and those without placebo group were also excluded.
Data extraction and quality assessment
Two independent authors (SA and VO) screened retrieved articles for the eligibility. In the first step, the title and abstract of studies were reviewed. Then, the full text of relevant studies was retrieved and assessed to ascertain the suitability of a study for inclusion into the meta-analysis. Any disagreement was discussed and resolved by the third author (ZA).
Following data were then extracted and entered into an Excel database: first authors name, year of publication, study location, age, study design, sample size, dosage of intervention, duration of study, type of disease, the mean and standard deviation (SD) for fasting glucose, insulin, HOMA-IR, HbA1c, lipid profiles and CRP in each intervention group. The quality of studies included was assessed by the same independent authors using the Cochrane Collaboration risk of bias tool based on the following criteria: “randomization generation, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, and selective outcome reporting, and other sources of bias” [17].
Data synthesis and statistical analysis
We fitted fixed effects (FE) models and used forest plots to display the weighted mean differences (WMDs) with 95% CI for fasting plasma glucose, insulin, HOMA-IR, HbA1c, triglycerides, total cholesterol, LDL cholesterol, HDL cholesterol and CRP which were calculated from mean changes in these variables throughout the vitamin D supplementation or treatment comparing to placebo. If a study had no sufficient data, we tried to get them through contact with the authors.
Between-study heterogeneity across included studies was evaluated using Cochran’s Q test (with significant P value < 0.1) and I-square test. I2 greater than 50% was considered as significant heterogeneity [17]. If between-study heterogeneity was high, we used a random-effects model to calculate the pooled estimates. Baseline and final values of the mentioned outcomes for both intervention and placebo groups were extracted from the included studies to calculate mean changes with SD for each variable. In addition, we used a subgroup analysis to detect probable sources of heterogeneity using a FE model. Visual inspection of the funnel plot as well as Egger’s regression test was used to explore the publication bias. Both STATA 11.0 (Stata Corp., College Station, TX) and Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) were used for data analysis.
Results
Characteristics of included studies
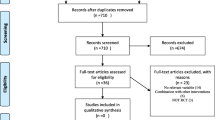
Overall, 17 studies were included in this systematic review and meta-analysis. A PRISMA flow diagram was used to provide a detailed description of the review process including the number of trials that were finally included (Fig. 1). Included studies were published from 1992 to 2018. They enrolled 1781 patients with CKD (888 intervention and 893 control patients). Characteristics of the included studies are shown in Table 1. The mean age of patients was 60.78 years. Among them, 1395 patients (through 11 studies) were under HD. Five studies were done in Iran, three studies in Denmark, two studies in USA, and the others were done in Turkey, India, China, Brazil, Germany, Spain, and UK. Calcidiol, calcitriol, and ergocalciferol were used as different types of vitamin D in the included studies. Supplements were used in daily, weekly, single, and double doses. The duration of intervention varied between 3 and 52 weeks. The measured outcomes in these studies were concentrations of fasting glucose, insulin, HOMA-IR, HbA1c, triglycerides, total, LDL, HDL cholesterol, and CRP.
Findings for the effect of vitamin D supplementation or treatment on glycemic control
Combining five studies, we found 18.87 mg/day reduction in fasting blood glucose following vitamin D supplementation or treatment compared with the placebo (WMD: − 18.87; 95% CI: − 23.16, − 14.58) (Fig. 2a). Subgroup analysis by the participants’ age (< 50 years vs. ≥ 50 years) did not change the overall findings (Table 2). When we combined effect sizes from four studies on the effect of vitamin D supplementation or treatment on serum insulin levels and from another set of four studies on HbA1C, no significant changes were seen [for insulin: (WMD: − 2.25; 95% CI: − 7.18, 2.67), for HbA1C: (WMD: − 0.69; 95% CI: − 1.71, 0.33)] (Fig. 2b, c). Combining findings from three studies, vitamin D supplementation or treatment resulted in significant reduction in HOMA-IR (WMD: − 2.30; 95% CI: − 2.88, − 1.72) (Fig. 2d).
Findings for the effect of vitamin D supplementation or treatment on lipid profiles
Compared with the placebo, vitamin D supplementation or treatment resulted in 32.52 mg/day reduction in serum concentrations of triglycerides through six studies (WMD: − 32.52; 95% CI: − 57.57, − 7.47) (Fig. 3a). To find an explanation for the between-study heterogeneity, we performed subgroup analyses by the participants’ age (< 50 years vs. ≥ 50 years), administration method (single vs. double dose/continuous), and the duration of study (< 12 weeks v. ≥ 12 weeks) (Table 2); none of these subgroup analyses changed our overall findings. In addition, we repeated our analysis after excluding one study that was conducted on hemodialysis patients, and findings remained unchanged (WMD: − 43.41; 95% CI: − 71.68, − 15.15) (data not shown). Combining findings from five studies showed a significant reduction in cholesterol concentrations following the administration of vitamin D (WMD: − 7.93; 95% CI: − 13.03, − 2.83) (Fig. 3b). These findings remained unchanged after excluding a study that was done on HD patients (WMD: − 9.41; 95% CI: − 15.71, − 3.11) (data not shown). However, no significant effect of vitamin D supplementation or treatment was seen on LDL (WMD: − 22.66; 95% CI: − 47.58, 2.25) and HDL cholesterol levels (WMD: 0.93; 95% CI: − 0.85, 2.72), through four included studies (Fig. 3c, d).
Findings for the effect of vitamin D on CRP levels
Pooling findings from 11 studies showed no significant effect of vitamin D supplementation or treatment on CRP concentrations (WMD: − 0.54; 95% CI: − 1.79, 0.71) (Fig. 3e). Due to a high between-study heterogeneity (I2 = 88.2%), we performed subgroup analyses based on participants’ age (< 50 years vs. ≥ 50 years), study design (RCT vs. open-label CT), administration method (daily vs. single or double dose/continuous), study duration (< 6 months vs. ≥ 6 months), control type (placebo vs. non-placebo), and disease stage (HD vs. non-HD) (Table 2). Vitamin D supplementation or treatment resulted in a significant reduction in circulating CRP among studies with participants ≥ 50 years of age (WMD: − 1.64; 95% CI: − 2.42, − 0.86), RCTs (WMD: − 0.48; 95% CI: − 0.87, − 0.09), when vitamin D was administered continuously (WMD: − 0.44; 95% CI: − 0.81, − 0.07), with an intervention duration of < 6 months (WMD: − 0.68; 95% CI: − 1.09, − 0.27), or used placebo among controls (WMD: − 0.48; 95% CI: − 0.87, − 0.09), and among patients with HD (WMD: − 0.51; 95% CI: − 0.93, − 0.10). The effect of vitamin D supplementation or treatment on CRP concentrations was not significant in all other subgroups. Furthermore, meta-regression did not provide a significant dose–response association between dosage and serum levels of CRP (P = 0.42).
Sensitivity analysis and publication bias
We had sufficient data to explore sensitivity analysis and publication bias only for the effect of vitamin D supplementation or treatment on CRP levels. Visual inspection of the funnel plot and Egger regression test (P = 0.88) provided no evidence for the presence of publication bias (Fig. 4). In addition, sensitivity analysis showed that no individual study had a great influence on the final results.
Discussion
To the extent of our knowledge, this is the first meta-analysis to assess the effect of vitamin D supplementation or treatment on glycemic control, lipid profiles and CRP levels in CKD patients. Based on our results, vitamin D supplementation or treatment significantly improved fasting glucose, HOMA-IR, triglycerides, and cholesterol levels among patients with CKD, though it did not influence insulin, HbA1c, LDL and HDL cholesterol, and CRP levels.
Effects of vitamin D supplementation or treatment on glycemic control
It was reported that 415 million adults (20 to 79 years old) had diabetes mellitus in 2015 [49]. Patients with diabetes have a greater risk of death and cardiovascular disease than the general population [37]. This meta-analysis showed that vitamin D had a therapeutic impact on glycemic control (fasting glucose and HOMA-IR) of CKD patients, but did not affect insulin and HbA1c levels. There are many studies examining the effect of vitamin D supplementation or treatment on glycemic control, but results are conflicting. In a meta-analysis conducted by Mirhosseini et al. [29], vitamin D supplementation significantly decreased fasting glucose, HOMA-IR and HbA1c levels in individuals with pre-diabetes. Moreover, vitamin D supplementation led to a significant reduction in HOMA-IR in patients with T2DM, though no significant improvement in fasting glucose, HbA1C and insulin was observed. However, in subgroup analysis, vitamin D supplementation of > 2000 IU/day resulted in a significant reduction in fasting glucose [22]. Another review has evaluated the effects of vitamin D supplementation on glycemic control among patients with non-alcoholic fatty liver disease [39]. They observed that taking vitamin D supplements had no significant effect on fasting glucose, insulin, HOMA-IR and HbA1c [39]. However, they included five trials while a high dosage of vitamin D (50,000 IU/week) was administered only in one trial in which reduction in HOMA-IR and fasting glucose was observed [13]. Also, Akbari et al. conducted a meta-analysis to examine the effects of vitamin D supplementation on gestational diabetes mellitus (GDM) patients and found a significant reduction in HOMA-IR, but vitamin D supplementation had no effect on fasting glucose, insulin, and HbA1c [1]. Although the exact mechanisms through which vitamin D supplementation or treatment might influence glucose homeostasis, limited possible mechanisms are suggested. It has been observed that vitamin D can modify function of β-cells in the pancreas. In addition, vitamin D can affect insulin receptors and subsequently improve insulin sensitivity. Another proposed mechanism is mediated through suppressing inflammatory markers, which in turn improves insulin sensitivity [3].
Effects of vitamin D supplementation or treatment on lipid profiles
This meta-analysis showed that vitamin D supplementation or treatment in patients with CKD could decrease triglycerides and total cholesterol levels, while it did not influence LDL and HDL cholesterol levels. Tabrizi et al. [39] observed that vitamin D supplementation had no significant effect on blood lipids in patients with non-alcoholic fatty liver disease. In another meta-analysis, vitamin D administration resulted in a significant rise in LDL cholesterol levels, but did not have any effect on triglycerides, total and HDL cholesterol levels [44]. Another meta-analysis showed that vitamin D supplements could reduce triglycerides, total and HDL cholesterol, whereas it did not improve HDL cholesterol levels [18]. Supplementation with vitamin D in patients with GDM resulted in a significant reduction in serum LDL cholesterol concentrations, while it did not influence serum levels of triglycerides, total and HDL cholesterol [1]. These differences in findings might be partially due to the fact that the effect of vitamin D supplementation or treatment on serum lipids might be influenced by some other factors including dosage, duration of intervention, and baseline levels of vitamin D, which can be also altered by geographical latitudes. Therefore, bigger studies considering baseline levels of vitamin D are required to shed light on this issue. The probable role of vitamin D on lipid metabolism could be explained by the PPAR-α pathway. Vitamin D can increase the expression of PPAR-α, which has an essential role in lipid metabolism in hepatocytes [34]. In the current meta-analysis, insulin and HbA1c levels were not affected by vitamin D which was against our hypothesis. This may have few reasons. The study duration may be one possible explanation for such discrepancy. Most included RCTs were conducted ≤ 12 weeks, which is far shorter than those observational studies. It is known that HbA1c represents an integrated measure of glycemic control over a period of > 12 weeks. The use of such measurements in studies with a short duration of vitamin D administration may underestimate any effects of vitamin D on glycemic control. A longer duration of RCTs is required to obtain a more reliable conclusion. In addition, dosage of vitamin D may affect insulin and HbA1c levels. Therefore, higher doses of vitamin D (4000 IU/day) appear to be required to achieve a significant increase in insulin and HbA1c levels. Also, pro-inflammatory markers in patients with T2DM may influence glucose levels and HOMA-IR, but this may not affect insulin and HbA1c levels.
Effects of vitamin D supplementation or treatment on CRP
We observed that vitamin D supplementation or treatment in patients with CKD did not affect CRP levels. It is a well-known fact that vitamin D has anti-inflammatory properties [6, 42]. Mansournia et al. [26] conducted a meta-analysis consisting of 16 studies and found that vitamin D intervention significantly decreased CRP levels in patients with diabetes. In another meta-analysis, vitamin D supplementation in patients with diabetes resulted in a 0.45 μg/mL reduction in serum CRP concentrations [47]. In another meta-analysis, Akbari et al. [2] showed a significant reduction in serum concentrations of CRP following supplementation with vitamin D. One pathway through which vitamin D can affect CRP concentrations is the inhibition of inflammatory markers synthesis as well as inhibition of inflammatory markers production by influencing on the immune cells like T helper, lymphocytes, and monocytes. One of these inflammatory markers is interleukin-6, which stimulates CRP production in the liver [23]. There is growing evidence that vitamin D exerts regulatory influence on immune system cells [33]. It has been shown that serum levels of cholecalciferol are pivotal for the optimum anti-inflammatory response of monocytes in humans [48]. The conversion of cholecalciferol to its active form, calcitriol, occurs locally in cells of immune system [5]. Calcitriol has an anti-inflammatory impact on the inflammatory profile of monocytes, decreasing production of various pro-inflammatory markers such as TNF-α, IL-1β, IL-6, and IL-8 [14]. Furthermore, immediate anti-inflammatory impact of vitamin D occurs in cells that have the vitamin D receptor (VDR) [43]. Interaction of cholecalciferol and VDR leads to anti-inflammatory influences via inhibition of NF-κB and STAT1/5 signaling pathways. This causes reduced transcription of pro-inflammatory cytokines [5].
In conclusion, this meta-analysis demonstrated the beneficial effects of vitamin D supplementation or treatment on improving fasting glucose, HOMA-IR, triglycerides and cholesterol levels among patients with CKD, though it did not influence insulin, HbA1c, LDL and HDL cholesterol, and CRP levels. These findings indicated that vitamin D supplementation or treatment might have some other beneficial effects for metabolic control in patients with CKD, who are mostly suffering from diabetes, rather than bone health protection. Due to the heterogeneity between studies, as a result of variations in the dosage, frequency, or duration of vitamin D supplementation or treatment, the results of this meta-analysis should be interpreted with caution.
Data availability
The primary data for this study are available from the authors on direct request.
References
Akbari M, Moosazaheh M, Lankarani KB, Tabrizi R, Samimi M, Karamali M, Jamilian M, Kolahdooz F, Asemi Z (2017) The effects of vitamin D supplementation on glucose metabolism and lipid profiles in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 49(9):647–653
Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, Heydari ST, Kavari SH, Mirhosseini N, Mafi A, Dastorani M, Asemi Z (2018) The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 50(4):271–279
Al-Shoumer KA, Al-Essa TM (2015) Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? World J Diabetes 6(8):1057–1064
Bonakdaran S, Ayatollahi H, Mojahedi MJ, Sharifipoor F, Shakeri M (2008) Impact of treatment with oral calcitriol on glucose intolerance and dyslipidemia(s) in hemodialysis patients. Saudi J Kidney Dis Transpl 19(6):942–947
Calton EK, Keane KN, Newsholme P, Soares MJ (2015) The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One 10(11):e0141770. https://doi.org/10.1371/journal.pone.0141770
Crescioli C (2014) Vitamin D receptor agonists: suitable candidates as novel therapeutic options in autoimmune inflammatory myopathy. Biomed Res Int 2014:949730
Cupisti A, Vigo V, Baronti ME, D’Alessandro C, Ghiadoni L, Egidi MF (2015) Vitamin D status and cholecalciferol supplementation in chronic kidney disease patients: an Italian cohort report. Int J Nephrol Renov Dis 8:151–157
de Boer IH (2008) Vitamin D and glucose metabolism in chronic kidney disease. Curr Opin Nephrol Hypertens 17(6):566–572
Dreyer G, Tucker AT, Harwood SM, Pearse RM, Raftery MJ, Yaqoob M (2014) Ergocalciferol and microcirculatory function in chronic kidney disease and concomitant vitamin D deficiency: an exploratory, double blind, randomised controlled trial. PLoS One 9(7):e99461
Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daures JP, Argiles A (2013) Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol 37(3):239–248
Esfandiari A, Gargari BP, Noshad H, Sarbakhsh P, Mobasseri M, Barzegari M, Arzhang P (2019) The effects of vitamin D3 supplementation on some metabolic and inflammatory markers in diabetic nephropathy patients with marginal status of vitamin D: a randomized double blind placebo controlled clinical trial. Diabetes Metab Syndr 13(1):278–283
Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, Ritz E (1998) Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int 53(5):1343–1347
Foroughi M, Maghsoudi Z, Askari G (2016) The effect of vitamin D supplementation on blood sugar and different indices of insulin resistance in patients with non-alcoholic fatty liver disease (NAFLD). Iran J Nurs Midwifery Res 21(1):100–104
Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C (2007) Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract 77(1):47–57
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351(13):1296–1305
Gravesen E, Hofman-Bang J, Lewin E, Olgaard K (2013) Ergocalciferol treatment and aspects of mineral homeostasis in patients with chronic kidney disease stage 4–5. Scand J Clin Lab Invest 73(2):107–116
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Jafari T, Fallah AA, Barani A (2016) Effects of vitamin D on serum lipid profile in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Clin Nutr 35(6):1259–1268
Khajehdehi P (2000) Effect of vitamins on the lipid profile of patients on regular hemodialysis. Scand J Urol Nephrol 34(1):62–66
Khajehdehi P, Taheri S (2003) Effect of oral calcitriol pulse therapy on the lipid, calcium, and glucose homeostasis of hemodialysis-patients: its safety in a combination with oral calcium carbonate. J Ren Nutr 13(2):78–83
Kumar V, Yadav AK, Lal A, Kumar V, Singhal M, Billot L, Gupta KL, Banerjee D, Jha V (2017) A randomized trial of vitamin D supplementation on vascular function in CKD. J Am Soc Nephrol 28(10):3100–3108
Li X, Liu Y, Zheng Y, Wang P, Zhang Y (2018) The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu10030375
Liefaard MC, Ligthart S, Vitezova A, Hofman A, Uitterlinden AG, Kiefte-de Jong JC, Franco OH, Zillikens MC, Dehghan A (2015) Vitamin D and C-reactive protein: a mendelian randomization study. PLoS One 10(7):e0131740. https://doi.org/10.1371/journal.pone.0131740
Lim S, Kim MJ, Choi SH, Shin CS, Park KS, Jang HC, Billings LK, Meigs JB (2013) Association of vitamin D deficiency with incidence of type 2 diabetes in high-risk Asian subjects. Am J Clin Nutr 97(3):524–530
Mannarino E, Pirro M (2008) Molecular biology of atherosclerosis. Clin Cases Miner Bone Metab 5(1):57–62
Mansournia MA, Ostadmohammadi V, Doosti-Irani A, Ghayour-Mobarhan M, Ferns G, Akbari H, Ghaderi A, Talari HR, Asemi Z (2018) The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 50(6):429–440
Marckmann P, Agerskov H, Thineshkumar S, Bladbjerg E-M, Sidelmann JJ, Jespersen J, Nybo M, Rasmussen LM, Hansen D, Scholze A (2012) Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant 27(9):3523–3531
Meireles MS, Kamimura MA, Dalboni MA, de Carvalho JTG, Aoike DT, Cuppari L (2016) Effect of cholecalciferol on vitamin D-regulatory proteins in monocytes and on inflammatory markers in dialysis patients: a randomized controlled trial. Clin Nutr 35(6):1251–1258
Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM (2018) Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis. J Endocr Soc 2(7):687–709
Miskulin DC, Majchrzak K, Tighiouart H, Muther RS, Kapoian T, Johnson DS, Weiner DE (2016) Ergocalciferol supplementation in hemodialysis patients with vitamin D deficiency: a randomized clinical trial. J Am Soc Nephrol 27(6):1801–1810
Molina P, Górriz JL, Molina MD, Peris A, Beltrán S, Kanter J, Escudero V, Romero R, Pallardó LM (2014) The effect of cholecalciferol for lowering albuminuria in chronic kidney disease: a prospective controlled study. Nephrol Dial Transplant 29(1):97–109
Mose FH, Vase H, Larsen T, Kancir AS, Kosierkiewic R, Jonczy B, Hansen AB, Oczachowska-Kulik AE, Thomsen IM, Bech J (2014) Cardiovascular effects of cholecalciferol treatment in dialysis patients: a randomized controlled trial. BMC Nephrol 15(1):50
Neve A, Corrado A, Cantatore FP (2014) Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin Exp Med 14(3):275–283
Ning C, Liu L, Lv G, Yang Y, Zhang Y, Yu R, Wang Y, Zhu J (2015) Lipid metabolism and inflammation modulated by vitamin D in liver of diabetic rats. Lipids Health Dis 14:31. https://doi.org/10.1186/s12944-015-0030-5
Petchey WG, Hickman IJ, Duncan E, Prins JB, Hawley CM, Johnson DW, Barraclough K, Isbel NM (2009) The role of 25-hydroxyvitamin D deficiency in promoting insulin resistance and inflammation in patients with chronic kidney disease: a randomised controlled trial. BMC Nephrol 10:41. https://doi.org/10.1186/1471-2369-10-41
Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S (2011) Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis 58(3):374–382
Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjornsdottir S (2018) Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 379(7):633–644
Seibert E, Heine GH, Ulrich C, Seiler S, Köhler H, Girndt M (2013) Influence of cholecalciferol supplementation in hemodialysis patients on monocyte subsets: a randomized, double-blind, placebo-controlled clinical trial. Nephron Clin Pract 123(3–4):209–219
Tabrizi R, Moosazadeh M, Lankarani KB, Akbari M, Heydari ST, Kolahdooz F, Samimi M, Asemi Z (2017) The effects of vitamin D supplementation on metabolic profiles and liver function in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr 11(Suppl 2):S975–S982
Tamadon MR, Soleimani A, Keneshlou F, Mojarrad MZ, Bahmani F, Naseri A, Kashani HH, Hosseini ES, Asemi Z (2018) Clinical trial on the effects of vitamin D supplementation on metabolic profiles in diabetic hemodialysis. Horm Metab Res 50(1):50–55
Türk S, Yeksan M, Tamer N, Gürbilek M, Erdoğan Y, Erkul I (1992) Effect of 1, 25 (OH) 2D3 treatment on glucose intolerance in uraemia. Nephrol Dial Transplant 7(12):1207–1212
Vojinovic J (2014) Vitamin D receptor agonists’ anti-inflammatory properties. Ann N Y Acad Sci 1317:47–56
Vuolo L, Di Somma C, Faggiano A, Colao A (2012) Vitamin D and cancer. Front Endocrinol (Lausanne) 3:58. https://doi.org/10.3389/fendo.2012.00058
Wang H, Xia N, Yang Y, Peng DQ (2012) Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis 11:42. https://doi.org/10.1186/1476-511x-11-42
Wang Y, Liu Y, Lian Y, Li N, Liu H, Li G (2016) Efficacy of high-dose supplementation with oral vitamin D3 on depressive symptoms in dialysis patients with vitamin D3 insufficiency: a prospective, randomized, double-blind study. J Clin Psychopharmacol 36(3):229–235
Wasse H, Huang R, Long Q, Singapuri S, Raggi P, Tangpricha V (2012) Efficacy and safety of a short course of very-high-dose cholecalciferol in hemodialysis. Am J Clin Nutr 95(2):522–528
Yu Y, Tian L, Xiao Y, Huang G, Zhang M (2018) Effect of vitamin D supplementation on some inflammatory biomarkers in type 2 diabetes mellitus subjects: a systematic review and meta-analysis of randomized controlled trials. Ann Nutr Metab 73(1):62–73
Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E (2012) Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 188(5):2127–2135
Zheng Y, Ley SH, Hu FB (2017) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14:88. https://doi.org/10.1038/nrendo.2017.151
Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, Tang D, Chen R (2018) Research progress on the relationship between atherosclerosis and inflammation. Biomolecules. https://doi.org/10.3390/biom8030080
Funding
This study was funded by the research grant provided by Research Deputy of Kashan University of Medical Sciences (KAUMS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Milajerdi, A., Ostadmohammadi, V., Amirjani, S. et al. The effects of vitamin D treatment on glycemic control, serum lipid profiles, and C-reactive protein in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Int Urol Nephrol 51, 1567–1580 (2019). https://doi.org/10.1007/s11255-019-02236-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02236-9