Abstract
Purpose
This systematic review examined the effects of exercise interventions on depression and anxiety in chronic kidney disease patients.
Methods
Electronic searches were conducted between August 2019 and February 2020 at PubMed, MEDLINE, Web of Science, EBSCO, Scopus, LILACS, EMBASE, Physiotherapy Evidence Database, and Cochrane Library databases. Original clinical trial studies that examined the effects of exercise on depression and anxiety in chronic kidney disease patients, stages 3–5, were included. A total of eight studies were included in the systematic review after applying the eligibility criteria, and six studies used for the meta-analysis procedures.
Results
The meta-analysis demonstrated statistical difference on depression in favour to exercise when compared to active control (SMD = − 0.66 [− 1.00, − 0.33], p < 0.0001) and passive control (MD = − 6.95 [− 8.76, − 5.14], p < 0.00001). Same results on anxiety demonstrated statistical difference between exercise and active control (SMD = − 0.78 [− 1.21, − 0.34], p = 0.0004).
Conclusion
From the current limited number and quality of published studies, exercise seems to be more effective than sedentary control and other active control groups for improving depression and anxiety symptoms in chronic kidney disease patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last 2 decades, the diagnosis of chronic kidney disease (CKD) has been growing worldwide, especially in developing countries [1, 2]. Renal replacement therapies, prescribed in cases of end-stage renal disease, generally affect biopsychosocial health and patient's independence, which may lead to depression, anxiety, and physical function impairment [3, 4]. Previous studies showed that CKD patients undergoing hemodialysis (HD) have a high prevalence of depressive symptoms (DS), which is also confirmed in earlier stages of the disease [5, 6].
Another mood disorder in CKD patients is anxiety, which is also highly prevalent, and both are strong associated [7,8,9]. There is evidence that DS and anxiety are associated with diminished quality of life (QoL) [6]. Besides, a reduction in QoL impairs the metabolism and physical function, which increases disability, morbidities, and mortality [10, 11].
Muscle and physical function are commonly reported as associated factors with hospitalization and independent living in CKD patients [12, 13]. These conditions may contribute to DS and anxiety. Exercise interventions have been effective in improving mood disorders, such as anxiety and DS in this population [14,15,16]. Additionally, exercise plays an important role in maintaining and improving muscle and physical function. Improvements in physical function explain a possible neural mechanism that may mediate the positive effects of exercise on DS and anxiety [17].
Therefore, the need for a systematic review with meta-analysis to assess the effects of exercise on DS and anxiety may reinforce new non-pharmacological perspectives. Thus, this study aimed to conduct a systematic review to examine the effects of exercise intervention programs on DS and anxiety in CKD patients.
Methods
Registration and protocol
This study was registered with the International Prospective Register of Systematic Reviews (CRD42018117242). The systematic review was performed using the PICO method: patients with CKD (Population), physical exercise program (Intervention), active and passive control (Comparison), depressive disorders and anxiety (Results). Also, this work is in line with the recommendations of the PRISMA statement to report systematic reviews.
Search methods to identify the studies
Two independent authors (A.L.A.R and W.R.M) performed an electronic search between August 2019 to February 2020 at PubMed, MEDLINE, Web of Science, EBSCO, Scopus, LILACS, EMBASE, Physiotherapy Evidence Database (PEDro), and Cochrane Library databases and used the following keywords and search strategy: ("renal insufficiency chronic" OR "chronic renal insufficiencies" OR “Renal Insufficiencies, Chronic” OR “Disease, Chronic Renal” OR “Chronic Kidney Disease”) AND (exercises OR “physical activity” OR “activities physical” OR “activity physical” OR “physical activities” OR “exercise physical”).
After that, a full-text reading was done to the potentially eligible studies. Disagreements were resolved by consensus between two reviewers. The reference list of the selected articles was consulted to find possible additional studies. The included articles of similar systematic review but with other outcomes were also consulted to find additional studies. The duplicate items identified after searching the databases were removed.
Selection criteria
Only randomized clinical trials (RCTs) in English were included to investigate the effect of different exercise programs on anxiety and depressive disorder in adults (> 18 years) with CKD.
Physical exercise intervention program was considered as a training with a prescription that is usually regular and done to improve or maintain fitness or health. The exercise considered in the present study was performed only in a supervised manner. Depression was considered as an affective disorder manifested by either a dysphoric mood or loss of interest or pleasure in usual activities. The mood disturbance is prominent and relatively persistent.
To analyze the efficacy of exercise on depression and anxiety in CKD patients, it was selected studies with the active and passive control group as a comparator for analyzes employed by the present study.
The following exclusion criteria were considered: studies in which patients had comorbidities such as neurological and rheumatological impairments. Individuals who performed home-based exercises/without supervision/leisure.
Study selection
Two independent authors screened titles and abstracts of the results identified by the search strategy. A full-text reading was done to the potentially eligible studies. Articles were selected after a sequenced reading of the title, abstract, and full text, always in this order. First, the titles retrieved in the search, then the abstract, which showed relevance with the theme, and finally the methodologic analysis of supervised physical exercise used in the selected studies. Divergence in study selection was resolved by consensus between the reviewers. The reference list of the articles was consulted to find possible additional studies. The included articles of similar systematic review but with another outcome were also consulted to find additional studies. The duplicate items identified after searching the databases were removed.
Data extraction and analysis
The continuous data to perform the meta-analysis were extracted by one reviewer and checked by a second. The values were entered into a database on Excel Software before the use of Review Manager software (Version 5.3.5).
To perform the meta-analysis was used the Standardized Mean Difference ([SMD]: assess the same outcome but measured it in a variety of ways) and 95% of the Confidence Intervals on the following comparisons (1) exercise vs. active control on depression; (2) exercise vs. active control on anxiety. The Mean Difference ([MD]: measures the absolute difference between the mean values in two groups in a clinical trial) and 95% of the Confidence Intervals were considered for comparison between exercise and passive control on depression (i.e., Back Depression Inventory [BDI]). For all analyses were used the data from post-test values (final measurement rather than the changes from baseline), the post-test group standard deviation, and the number of participants (n) in each group [18].
Considering that the included papers had distinct populations, intervention parameters, and settings, a random-effects model (inter-study heterogeneity) was always employed in the meta-analysis. Finally, a sensitivity analysis was planned to identify if a specific study (forest plot inspection for outliers) changes the summary effect, by repeating the meta-analysis with one study omitted at a time. The heterogeneity of the studies was assessed by the I2 statistic and 95% Cl [18]. An I2 statistic: 0–40% might not be important, 30–60% may represent moderate heterogeneity, 50–90% may represent substantial heterogeneity, and 75–100% considerable heterogeneity [18]. Assessment of clinical relevance was made using three categories: small effect (MD < 10% of the scale; SMD < 0.5); medium effect (MD from 10–20% of the scale; SMD from 0.5 to 0.8); large effect (MD > 20% of the scale; SMD > 0.8) [19].
To rate the methodological quality of the included RCTs was scored using the PEDro scale. The PEDro scale consists of 11 criteria (random allocation; concealed allocation; baseline comparability; blind subjects; blind therapists; blind assessor; adequate follow up; intention-to-treat-analysis; between groups comparisons; point estimates and variability), which receives either a “yes”, or “no” rating. As criteria 1 is not used in the calculation, the maximum PEDro score is 10 points. Trials with a PEDro score ≥ 6 points were classified as high-quality, while trials with a PEDro score < 6 points were classified as low quality. The studies were assessed with the Brazilian-Portuguese version of the PEDro scale. Also, to rate the quality of the evidence underlying was used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE). GRADE offers four levels of evidence: high, moderate, low, and very low. Randomized trials begin as higher quality evidence and the quality may be downgraded as a result of limitation in the study design or implementation, imprecision of estimates, variability in results, indirectness of evidence, and publication bias.
Results
Included studies
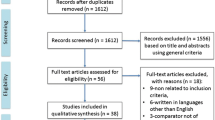
The electronic search retrieved 621 articles, of which 288 were excluded as duplicates, 304 were excluded by reading title and abstract, 21 were excluded by full-text reading. Therefore, eight RCTs [5, 15, 20,21,22,23,24,25] were included in the systematic review after applying the eligibility criteria and six studies used for the meta-analysis procedures [5, 15, 22,23,24,25]. Figure 1 shows the flowchart of the search process and the included studies in the analyses.
Studies not included in the pooled analysis
The studies which not entered in the meta-analysis had inadequate information about the outcomes. A study reported the data only in graphics and another used the instruments (Beck Depression Inventory-II and Beck Anxiety Inventory) only to identify the prevalence of depression and anxiety in participants. Both did not report quantitative data to data extraction. Therefore, the authors of the studies were contacted to provide additional information, however, this procedure was not successful.
General characteristics of the studies
The included studies are randomized controlled trials and measured outcomes before and after exercise training programs. Studies were developed in Greece [22, 23], China [5, 25], India [15], and Iran [24]. Seven studies included maintenance HD patients and only one CKD stages 1–3.
The included studies evaluated 376 patients on maintenance HD and CKD stages 1–3 (289 on meta-analysis) of both genders. The minimum and maximum age ranged between 18 and 70 years, the mean (DP) on control groups among 38.4 (1.8) and 65.2 (3.1), on experimental groups the variations were among 39.1 (2.2) and 64.94 (9.51) years old. The sample size of the included studies considering control and experimental groups was 14–90 participants. The other characteristics of the studies are shown in Table 1. In addition, supplementary data present the results of PEDro scale of the studies included in qualitative and quantitative analyzes.
Interventions
The studies included in the meta-analysis used the following types of exercise interventions: cycling + strengthening + flexibility [23]; warm-up + cycling + strengthening + cool down [22]; audio device-guided breathing training (quiet room) [5]; routine care + pilates exercise (pilates out of hemodialysis) [24]; home-based—aerobic exercise (walking, stationary cycling, and jogging) [25]; intradialytic exercise training [15]. In aerobic exercise training studies, light-to-moderate intensity was used (on average 60% of the patient's total strength capacity). The intervention period ranged from 04 to 48 weeks, 2–3 times a week with a duration of 15–90 min per session.
The control groups were not all passive, some were submitted to education exercise [25] or routine care practice in the hospital [15, 24]. In both cases, treatment was supervised by a physiotherapist, nurse, or exercise physiologist. Therefore, we consider as passive controls only the sedentary [22] or waiting list groups [5, 23]. No study has reported adverse effects (falls, hypertensive spikes, pain, or malaise related to exercise training).
Instruments
The instruments used in the included studies of the meta-analysis were: General Health Dimensions (Anxiety subscale) [24]; Hospital Anxiety and Depression scale (HAD-A = anxiety subscale) [25]; Hospital Anxiety and Depression scale (HAD-D = depression subscale) [25]; Depression, Anxiety and Stress Scale (DASS) [15]; General Health Dimensions (depression subscale) [24]; Beck Depression Inventory (BDI) [5, 22, 23].
Meta-analysis results
The meta-analysis demonstrated statistical difference (1) on depression in favour to exercise when compared to active control (Fig. 2; n = 148 participants; [experimental n = 74; control n = 74 participants], SMD = − 0.66 [− 1.00, − 0.33], p < 0.0001, with very low confidence in effect estimated (GRADE analysis of three studies) and (2) on depression in favour to exercise when compared passive control (Fig. 3; experimental n = 75 vs, control n = 59 participants]; MD = − 6.95 [− 8.76, − 5.14], p < 0.00001, with very low confidence in effect estimated (GRADE analysis of three studies). The clinical relevance was found to be moderate between (1) exercise vs. active control and for (2) exercise vs. passive control (at least of 10% of change in the scales analysed).
The meta-analysis on anxiety demonstrated statistical difference between exercise and active control (Fig. 4; experimental n = 67 vs. control n = 67 participants]; SMD = − 0.78 [− 1.21, − 0.34], p = 0.0004, with very low-quality confidence in effect estimated (GRADE analysis of two studies). In this outcome, it was showed a moderate clinical relevance. All decisions and judgments for GRADE analysis are presented as supplementary material.
Heterogeneity, sensitivity, and risk of bias
There was not important heterogeneity on depression analysis between exercise vs. active control (I2 = 0%; p = 0.98) and between exercise vs. passive control (I2 = 0%; p = 0.38). As on depression, in anxiety also, there was not important heterogeneity between exercise vs active control (I2 = 30%; p = 0.23). The sensitive analysis, which includes checking outliers’ studies by graphic inspection, found no need to remove a specific study.
The risk of bias analysis showed that 100% of studies described the random sequence generation method [5, 15, 22,23,24,25] and only one study adopted a method to conceal randomization [5]. No study included blinding of participants and professionals, and one study blinding of evaluators [5]. In 83.33% of studies, the outcomes were complete [5, 22,23,24,25], and in 50% of the studies, the selective outcome was reported [5, 22, 24]. Half of the studies presented other risks of bias [23,24,25]. The risk of bias was summarized in supplementary data.
Discussion
The results from this systematic review with meta-analyses showed that exercise interventions are effective in attenuating mood disorders, such as DS and anxiety. Also, even when exercise intervention was compared with active control groups it has been observed an improvement in favor of exercise.
Mood disorders, especially DS and anxiety, are important issues that negatively impact CKD treatment. Most of the physicians prescribe medications, which might have numerous emotional and physical undesirable effects [16, 26]. Researchers have been interested in non-pharmacological therapeutics options, such as exercise interventions. Therefore, a recent meta-analysis identified exercise as the best non-pharmacological treatment for reducing DS in ESRD patients [16], which is also confirmed by our findings, where exercise intervention showed better results in comparison to active or control group.
Improvements in muscle and physical function induced by exercise might explain beneficial changes in DS and anxiety. Physical function seems to be associated with a better QoL, moreover, DS has been found as the greatest predictor of QoL [27,28,29]. Mood disorders might be triggered by the reduction in physical function and the dependence of a second person to perform activities of daily living [30, 31]. On the other hand, exercise interventions have been showing positive effects on general health, QoL, and vitality, in addition to reducing the demand for medication intake, disability, episodes of cramps, and fatigue [22, 25, 31, 32].
Exercise as a non-pharmacological therapy to mood disorders has been widely studied in other chronic diseases and healthy population, with many physicians advocating its prescription [33]. In the CKD population, the first evidence is dated from 1997 [34], however, its implementation as routine care in CKD treatment is not completely established [35]. Busch et al. (2016) found that lack of motivation and fatigue were reported as barriers to exercise in depressive people [36]. These barriers are increased in CKD patients, which may impair engagement in exercise programs [37].
Our systematic review and meta-analysis screened only supervised clinical trials, and it is known that supervised exercise prescriptions promote higher adherence, motivation, and benefits [38]. Thus, CKD patients with DS and anxiety may experience better results in mood control when the exercise intervention is supervised by an exercise physiologist, kinesiologist, physical therapist, or similar. Indeed, guidelines for the clinical management of CKD patients should be updated for nephrologists to present the benefits of exercise programs in DS and anxiety [32]. Despite the high amount of evidence, there are still gaps in the scientific literature and further studies are needed to investigate the dose–response of exercise programs on depression and anxiety in CKD patients.
Limitations
The limitations are mainly related to the methodological quality of the selected clinical trials and the absence of information in the description. Regarding the selection criteria, only three presented sample size calculation [5, 24, 25], not all samples were probabilistic, and as the selection criteria were not homogeneous, the mean ages showed great variability. There was a lack of information about the researchers' blinding, about the intention to choose a specific exercise program, which was quite varied, and about the control of patients with CKD who did not perform exercise.
Conclusion
From the current limited number and quality of published studies, exercise seems to be more effective than sedentary control and other active control groups for improving depression and anxiety symptoms in CKD patients.
References
Ng JKC, Li PKT (2018) Chronic kidney disease epidemic: How do we deal with it? Nephrology 23:116–120. https://doi.org/10.1111/nep.13464
Sesso R, Lugon JR (2020) Global dialysis perspective: Brazil. Kidney 360(1):216–219. https://doi.org/10.34067/KID.0000642019
Jesus NM, de Souza GF, Mendes-Rodrigues C et al (2019) Quality of life of individuals with chronic kidney disease on dialysis. Brazilian J Nephrol 41:364–374. https://doi.org/10.1590/2175-8239-jbn-2018-0152
Kutner NG, Zhang R, Huang Y, Johansen KL (2010) Depressed mood, usual activity level, and continued employment after starting dialysis. Clin J Am Soc Nephrol 5:2040–2045. https://doi.org/10.2215/CJN.03980510
Tsai SH, Wang MY, Miao NF et al (2015) The efficacy of a nurse-led breathing training program in reducing depressive symptoms in patients on hemodialysis: a randomized controlled trial. Am J Nurs 115:24–32. https://doi.org/10.1097/01.NAJ.0000463023.48226.16
Lee Y, Kim MS, Cho S, Kim SR (2013) Association of depression and anxiety with reduced quality of life in patients with predialysis chronic kidney disease. Int J Clin Pract. https://doi.org/10.1111/ijcp.12020
Loosman WL, Rottier MA, Honig A, Siegert CEH (2015) Association of depressive and anxiety symptoms with adverse events in Dutch chronic kidney disease patients: a prospective cohort study. BMC Nephrol. https://doi.org/10.1186/s12882-015-0149-7
Hou Y, Li X, Yang L (2014) Factors associated with depression and anxiety in patients with end-stage renal disease receiving maintenance hemodialysis. Int Urol Nephrol. https://doi.org/10.1007/s11255-014-0685-2
dos Valle L S, de Souza VF, Ribeiro AM (2013) Stress and anxiety in chronic renal patients undergoing hemodialysis. Estud Psicol 30:131–138. https://doi.org/10.1590/S0103-166X2013000100014
Barros A, da Costa BE, Mottin CC, D’Avila DO (2016) Depression, quality of life, and body composition in patients with end-stage renal disease: a cohort study. Rev Bras Psiquiatr 38:301–306. https://doi.org/10.1590/1516-4446-2015-1681
Hornik B, Duława J (2019) Frailty, quality of life, anxiety, and other factors affecting adherence to physical activity recommendations by hemodialysis patients. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph16101827
Lee YH, Kim JS, Jung S et al (2020) Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol 21:166. https://doi.org/10.1186/s12882-020-01831-8
Sutcliffe BK, Bennett PN, Fraser SF, Mohebbi M (2018) The deterioration in physical function of hemodialysis patients. Hemodial Int 22:245–253. https://doi.org/10.1111/hdi.12570
Dziubek W, Kowalska J, Kusztal M, Klinger M (2016) The level of anxiety and depression in dialysis patients undertaking regular physical exercise training—a preliminary study. Kidney Blood Press Res. https://doi.org/10.1159/000368548
Santhi AS, Samson R, Srikanth PD (2018) Effectiveness of physical activity on depression, anxiety, stress and quality of life of patients on hemodialysis. Biomed Res 29:1885–1890. https://doi.org/10.4066/biomedicalresearch.29-18-177
Wen X, Wang Y, Zhao Q et al (2019) Nonpharmacological interventions for depressive symptoms in end-stage renal disease: a systematic review. West J Nurs Res. https://doi.org/10.1177/0193945919857540
Gujral S, Aizenstein H, Iii CFR et al (2017) Exercise effects on depression: possible neural mechanisms. Gen Hosp Psychiatry 49:2–10. https://doi.org/10.1016/j.genhosppsych.2017.04.012
Higgins JP, Green S (2008) Cochrane handbook for systematic reviews of interventions. John Wiley & Sons Ltd, Chichester
Furlan AD, Pennick V, Bombardier C, van Tulder M (2009) Updated method guidelines for systematic reviews in the cochrane back review group. Spine (Phila Pa 1976) 34:1929–1941. https://doi.org/10.1097/BRS.0b013e3181b1c99f
Cho JH, Lee JY, Lee S et al (2018) Effect of intradialytic exercise on daily physical activity and sleep quality in maintenance hemodialysis patients. Int Urol Nephrol 50:745–754. https://doi.org/10.1007/s11255-018-1796-y
Frih B, Jaafar H, Mkacher W et al (2017) The effect of interdialytic combined resistance and aerobic exercise training on health related outcomes in chronic hemodialysis patients: the Tunisian randomized controlled study. Front Physiol 8:1–11. https://doi.org/10.3389/fphys.2017.00288
Kouidi E, Karagiannis V, Grekas D et al (2010) Depression, heart rate variability, and exercise training in dialysis patients. Eur J Cardiovasc Prev Rehabil 17:160–167. https://doi.org/10.1097/HJR.0b013e32833188c4
Ouzouni S, Kouidi E, Sioulis A et al (2009) Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil 23:53–63. https://doi.org/10.1177/0269215508096760
Rahimimoghadam Z, Rahemi Z, Mirbagher Ajorpaz N, Sadat Z (2017) Effects of pilates exercise on general health of hemodialysis patients. J Bodyw Mov Ther 21:86–92. https://doi.org/10.1016/j.jbmt.2016.05.012
Tang Q, Yang B, Fan F et al (2017) Effects of individualized exercise program on physical function, psychological dimensions, and health-related quality of life in patients with chronic kidney disease: a randomized controlled trial in China. Int J Nurs Pract 23:1–8. https://doi.org/10.1111/ijn.12519
Zhao C, Ma H, Yang L, Xiao Y (2017) Long-term bicycle riding ameliorates the depression of the patients undergoing hemodialysis by affecting the levels of interleukin-6 and interleukin-18. Neuropsychiatr Dis Treat 13:91–100. https://doi.org/10.2147/NDT.S124630
Mitrou GI, Grigoriou SS, Konstantopoulou E et al (2013) Exercise training and depression in ESRD: a review. Semin Dial 26:604–613. https://doi.org/10.1111/sdi.12112
Barbosa L, Mendonça M, Pacheco M et al (2007) Predictors of quality of life in chronic hemodialysis patients luciana. Brazilian J Nephrol 29:222
Bohlke M, Nunes DL, Marini SS et al (2008) Predictors of quality of life among patients on dialysis in southern Brazil. Sao Paulo Med J 126:252–256. https://doi.org/10.1590/S1516-31802008000500002
Fassbinder TRC, Winkelmann ER, Schneider J et al (2015) Functional capacity and quality of life in patients with chronic kidney disease in pre-dialytic treatment and on hemodialysis—a cross sectional study. Brazilian J Nephrol 37:47–54. https://doi.org/10.5935/0101-2800.20150008
Henrique DMN, de Reboredo M, Chaoubah A, de Paula RB (2010) Aerobic exercise improves physical capacity in patients under chronic hemodialysis. Arq Bras Cardiol 94:823–828. https://doi.org/10.1590/S0066-782X2010005000043
Cheema BS, Chan D, Fahey P, Atlantis E (2014) Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sport Med 44:1125–1138. https://doi.org/10.1007/s40279-014-0176-8
Glowacki K, Arbour-Nicitopoulos K, Burrows M et al (2019) It’s more than just a referral: development of an evidence-informed exercise and depression toolkit. Ment Health Phys Act 17:100297. https://doi.org/10.1016/j.mhpa.2019.100297
Koudi E, Iacovides A, Iordanidis P et al (1997) Exercise renal rehabilitation program: psychosocial effects. Nephron 77:152–158. https://doi.org/10.1159/000190266
Hedayati SS, Yalamanchili V, Finkelstein FO (2012) A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int 81:247–255. https://doi.org/10.1038/ki.2011.358
Busch AM, Ciccolo JT, Puspitasari AJ et al (2016) Preferences for exercise as a treatment for depression. Ment Health Phys Act 10:68–72. https://doi.org/10.1016/j.mhpa.2015.12.004
Clarke AL, Young HML, Hull KL et al (2015) Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant 30:1885–1892. https://doi.org/10.1093/ndt/gfv208
Bogataj Š, Pajek J, Buturovi J et al (2020) Kinesiologist-guided functional exercise in addition to intradialytic cycling program in end-stage kidney disease patients : a randomised controlled trial. Sci Rep. https://doi.org/10.1038/s41598-020-62709-1
Acknowledgements
We thank the Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF) for supporting the study (grant 0193.001.558/2017)
Funding
This study was supported by the Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF) (grant 0193.001.558/2017).
Author information
Authors and Affiliations
Contributions
TLF: study design, data collection, and writing; HSR: study design, writing, and final review; ALAR: data collection and analysis; ACBR: writing and final review; JMSL: data collection and analysis; PAO: data collection and analysis; FRSA: data collection and analysis; APF: study design, writing, and final review; LAVM: study design, supervision, and final review; WRM: data collection and analysis, supervision, and final review.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Availability of data and material
Author may turn data and material available if this is the editor wish.
Consent for publication
Authors consent on the publication of this article to the International and Urology Journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferreira, T.L., Ribeiro, H.S., Ribeiro, A.L.A. et al. Exercise interventions improve depression and anxiety in chronic kidney disease patients: a systematic review and meta-analysis. Int Urol Nephrol 53, 925–933 (2021). https://doi.org/10.1007/s11255-020-02612-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02612-w