Abstract
Background and Objective
Skeletal muscle wasting resulting in reduced muscular strength and health-related quality of life (HR-QOL) is common in chronic kidney disease (CKD) and may be reversed with progressive resistance training (PRT). Therefore, we systematically assessed the effect of PRT on measures of skeletal muscle hypertrophy, muscular strength and HR-QOL in this cohort to inform clinical practice and guidelines.
Design
We performed a systematic review and meta-analysis.
Inclusion Criteria
We included randomised controlled trials (RCTs) that investigated the independent effect of PRT (>6 weeks) on measures of skeletal muscle hypertrophy [muscle mass or cross-sectional area (CSA)], muscular strength and/or HR-QOL in adults with CKD.
Data Extraction and Analysis
The standardised mean difference (SMD) from each study was pooled to produce an overall estimate of effect and associated 95 % confidence interval (95 % CI) between treatment and control groups on primary outcomes.
Results
Seven RCTs in 271 patients with Stage 3–5 CKD yielded seven studies on muscular strength (N = 249), six studies on total body muscle mass (N = 200) and six studies on HR-QOL (N = 223). PRT significantly improved standardised muscular strength [SMD 1.15 (95 % CI 0.80–1.49)] and HR-QOL [SMD 0.83 (95 % CI 0.51–1.16)], but not total body muscle mass [SMD 0.29 (95 % CI −0.27 to 0.86)] in our primary analysis. However, secondary analysis of six studies showed that PRT induced significant muscle hypertrophy of the lower extremities (leg mass, or mid-thigh or quadriceps CSA) [SMD 0.43 (95 % CI 0.11–0.76)], a pertinent analysis given that most studies implemented lower-body PRT only.
Conclusions
Robust evidence from RCTs indicates that PRT can induce skeletal muscle hypertrophy and increase muscular strength and HR-QOL outcomes in men and women with CKD. Therefore, clinical practice guidelines should be updated to inform clinicians on the benefits of PRT in this cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to the United States Renal Data System, more than 15 % of the adult population in the USA has chronic kidney disease (CKD) [1], while global estimates reveal a burgeoning epidemic (8–16 % prevalence) [2]. These trends are being driven largely by escalating rates of obesity and type 2 diabetes mellitus [3]. The prevention and treatment of CKD will present a major challenge for healthcare systems in the coming decades [3]. A major part of this challenge will involve providing quality care to patients with advanced CKD, including those with pre-dialysis (Stage 3–4 CKD) and end-stage renal disease (ESRD) [3].
Skeletal muscle wasting is common in advanced CKD [4–6] due to factors such as sedentary behaviour [7], acidosis [8], co-morbid illnesses, corticosteroid usage, aging, oxidative stress, dialysis treatment [9], insulin resistance, chronic inflammation and protein-restricted diet. This wasting contributes to reductions in muscular strength and associated functional impairment [10–12]. Functional impairment, in turn, contributes to impaired health-related quality of life (HR-QOL), particularly the physical dimension of HR-QOL [13]. Many investigations have shown that muscle wasting [14], loss of functional activities [15] and/or low HR-QOL contribute to greater hospitalization and all-cause mortality in patients with CKD [16–18].
Progressive resistance training (PRT) has been shown to induce skeletal muscle hypertrophy and improve functioning and HR-QOL in older adults and those with advanced chronic diseases [19]. Since there is an association of muscle wasting in CKD with high morbidity and mortality, it has been hypothesized that PRT may be important in terms of clinical outcomes in this patient population as well [20–24]. In fact, Exercise and Sport Science Australia has recently recommended PRT as a central component of the exercise prescription for patients with CKD [25]. Since 2001, a number of randomised controlled trials (RCTs) have investigated the independent effect of PRT on measures of skeletal muscle hypertrophy and related health outcomes in patients with CKD [26–32]. However, there is currently no consensus regarding the effectiveness of PRT for counteracting catabolic disease outcomes in this cohort [25]. Accordingly, PRT is not routinely prescribed [33] and recommendations for undertaking this form of exercise remain absent from CKD clinical practice guidelines [34].
Our initial analysis of the published literature indicated an absence of high-quality reviews specifically elucidating the effect of PRT in patients with CKD. We therefore conducted a systematic review of the literature to assess the independent effect of PRT on measures of skeletal muscle hypertrophy, muscular strength and HR-QOL in patients with CKD to inform clinical practice and guidelines.
2 Methods
2.1 Search Strategy
A systematic review of all published literature using the following electronic databases was conducted in June 2013: MEDLINE (OvidSP, Wolters Kluwer), PubMed (NCBI, U.S. National Library of Medicine), ScienceDirect (SciVerse, Elsevier), SPORTDiscus (EBSCOhost, EBSCO), Scopus (SciVerse, Elsevier), Web of Science (Web of Knowledge, Thomson Reuters), the Cochrane Library (John Wiley & Sons), EMBASE (OvidSP, Wolters Kluwer), CINAHL, and Google Scholar. Search syntaxes were developed in consultation with an experienced university librarian, taking into account a broad range of terms and phrases used in definitions related to CKD (e.g. chronic kidney disease, haemodialysis, end-stage renal disease, etc.) and resistance training (e.g. resistance training, resistance exercise, weight training, weight lifting, strength training, etc.). Sample search strategies (PubMed and Scopus) are presented in the Electronic Supplementary Material, Appendix S1. Reference lists of retrieved full-text articles and recent reviews were examined to identify additional articles not found by our search.
2.2 Study Selection
Electronic references were compiled in an Endnote X6© (Thomson Reuters) file and duplicates were identified and deleted. Two authors (BSC and DC) independently reviewed the titles and abstracts of each reference for potential inclusion. Each reviewer then performed a second screening on the full-text version of these articles, and disagreements were resolved by discussion. RCTs that investigated the independent effect of PRT intervention on measures of skeletal muscle hypertrophy [muscle mass or cross-sectional area (CSA)], muscular strength and/or HR-QOL in adults with CKD (Stage 1–5) were eligible. PRT interventions may have included, but were not restricted to, any form of resistive type exercise using body weight (calisthenics), equipment (machine weights, free weights) or apparatus (elastic bands), and had to have been at least 6 weeks in duration. There were no language restrictions for articles.
2.3 Primary Outcomes
The primary outcomes were the mean difference in measures of skeletal muscle hypertrophy (muscle mass or CSA), muscular strength and HR-QOL after intervention (post-treatment) between the treatment and control (e.g. non-treatment, placebo-treatment) group. Where multiple muscular strength outcomes were reported, we prioritised lower-body over upper-body measures, and knee extension over other lower-body measures. Where multiple measures of muscle mass or CSA were reported, we prioritised measures of muscle mass over CSA, and whole-body over regional measures. Where multiple HR-QOL outcomes were reported, we first prioritised subscales then summary measures of the physical component of HR-QOL.
2.4 Data Extraction
Data extraction and quality assessment of included studies were performed and/or verified independently by three reviewers (BSC, DC and PF). Discrepancies were resolved through discussion. Authors of relevant studies were contacted, where possible, for data that could not be extracted from the published articles.
2.5 Quality Assessment
The following data were extracted from included studies using a standard proforma checklist: study design, study population characteristics, PRT intervention [e.g. specific exercises, number of sets per exercise, number of repetitions per set, intensity (load), frequency and duration of training, and loading progression]. Our quality checklist was designed based on established criteria for the assessment of RCTs [35]. Quality items for RCT studies reviewed were (each worth 1.0 numerical point) as follows: (1) evidence of randomisation and concealment of treatment allocation; (2) statistical similarity of groups at baseline; (3) specification of eligibility criteria; (4) blinding of outcomes assessors; (5) reporting of compliance; (6) supervision of exercise sessions; (7) reporting of dropouts; (8) presenting data for primary and secondary outcomes; (9) use of intention-to-treat analysis; and (10) reporting of adverse events. Summed scores ranged from 0 to 10 points with higher scores reflecting better quality.
2.6 Data Synthesis
Three reviewers (DC, BSC and EA) independently collated and/or verified extracted data to present a descriptive synthesis of important study characteristics and a quantitative synthesis of effect estimates.
2.7 Secondary Outcomes
The secondary outcomes were data about adverse events for a descriptive synthesis.
2.8 Statistical Methods
We pooled and weighted studies first using random–effects meta-analysis models, and second using fixed–effects models for verification [36]. The effect was measured as the difference between groups in the improvement in outcome over the treatment period. Where papers did not present the mean and standard deviation of the improvement in outcome, we estimated these from the pre- and post-treatment standard deviations [37]. This estimation requires an estimate of the pre–post correlation, which we obtained from papers which provided pre-, post- and change means and standard deviations [37]. As the estimated correlations were quite consistent across studies (Electronic Supplementary Material, Table S1) we used the average correlation in our calculations.
In examining the effects of PRT on skeletal muscle hypertrophy, muscular strength and HR-QOL outcomes, the standardised mean difference (SMD) from each study was pooled to produce an overall estimate of effect and associated 95 % confidence interval (95 % CI) between treatment and control groups. For each meta-analysis model, the degree of heterogeneity in SMDs was assessed by visual inspection, the I 2 statistic (moderate being <50 %) [38] and the chi-squared (χ 2) test of goodness of fit [39]. Where evidence of heterogeneity was observed, we checked data extracted from individual outlier studies, qualitatively investigated reasons for their different results, and explored the effects of study exclusion in sensitivity analyses.
The subset of studies examining the impact of PRT on lean body mass (in kg) as measured by dual-energy X-ray absorptiometry (DEXA) were pooled to estimate the inverse variance weighted mean difference (WMD), including the DerSimonian and Laird [36] 95 % CI, between cases and controls. This preserved the original measurement units. We also used sensitivity analysis to investigate the robustness of the meta-analyses models. We variously excluded studies that combined PRT with other therapies (including haemodialysis), studies in older patients (>60 years), studies conducted outside the USA, longer duration trials (≥12 weeks), and studies of lower quality (score <6.0). Publication bias, which reflects the tendency for smaller studies to be published in the literature only when findings are positive, was assessed visually using funnel plots [40]. All calculations were performed in Stata® version 12 (StataCorp, College Station, TX, USA) using the ‘metan’ and ‘metafunnel’ commands. A two-tailed P value <0.05 was considered statistically significant throughout the analyses.
3 Results
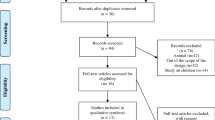
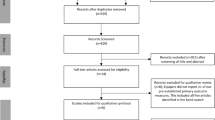
Figure 1 presents a flowchart summarising identification of potentially relevant studies, and those included. Our search strategy identified 187 citations after duplicates were removed. Of these, 164 citations were excluded after the first screening of titles and/or abstracts for inclusion and exclusion criteria. After further assessment of the remaining 23 citations, 16 were excluded (Electronic Supplementary Material, Appendix S2) for reasons listed in Fig. 1, leaving seven for inclusion in the review. Most citations were excluded due to no randomisation or to being redundant citations of the same study.
3.1 Descriptive Data Synthesis
Table 1 presents study characteristics of the seven RCTs included for review, which were published between 2001 and 2013. Four of seven studies were conducted in the USA [26, 28, 30, 31], with others conducted in Australia [27], Brazil [29] and South Korea [32]. The major inclusion criterion was pre-dialysis (Stage 3–4) CKD [30] or ESRD [26–29, 31, 32]. All studies in ESRD involved maintenance haemodialysis patients. In most of these studies it was noted that the patients were adequately dialyzed [haemodialysis treatment adequacy (Kt/V) > 1.2] and receiving dialysis treatment for more than 3 months. Major exclusion criteria primarily emphasised uncontrolled cardiovascular diseases and other conditions that would contraindicate PRT. Analysed sample sizes ranged from 22 to 68, resulting in a total of 271 participants across studies. Mean age of the samples ranged from 43 to 69 years. All studies enrolled both men and women. PRT interventions were prescribed two to three times per week during haemodialysis treatment in four studies with all employing weighted ankle cuffs [26–29]. Only three studies targeted both the upper and lower body musculature with PRT exercises [27, 30, 32], while four targeted the lower body musculature only [26, 28, 29, 31]. Two studies prescribed PRT just prior to each haemodialysis treatment session (3 sessions/week) using machine weights [31] or elastic bands and sandbags [32]. Only one study was conducted in patients not receiving haemodialysis and prescribed PRT using standard machine weights three sessions per week [30]. Three studies compared PRT intervention to usual care (no exercise) [27, 29, 32], one study compared PRT to stretching exercise using light-resistance bands [28], one study compared PRT plus nutritional supplementation with nutritional supplementation only [31], and one study compared PRT plus a protein restricted diet to protein-restricted diet only. Further, a study by Johansen et al. [26] compared PRT + anabolic steroid (i.e. nandrolone decanoate) with anabolic steroid only and PRT + placebo with placebo only. Hence, this study was included as two separate comparisons in relevant meta-analyses. Trial durations ranged from 8 to 24 weeks.
Primary outcomes were muscular strength measures evaluated by knee extension [26–28, 30] and leg press [31, 32], total body muscle mass measures evaluated by total body potassium [30], DEXA [26, 28, 31] and bioelectrical impedance analysis (BIA) [32], mid-thigh muscle CSA evaluated by computed tomography (CT) [27, 30], quadriceps muscle CSA evaluated by magnetic resonance imaging (MRI) [26], lean leg mass evaluated by DEXA [28, 31], and the physical dimension of HR-QOL evaluated by the Medical Outcomes Trust Short Form-36 (SF-36) physical functioning domain [26, 27, 29] and physical component summary scale [28, 32]. Mean quality scores ranged from 5.5 to 9.5, and five studies received a score of 8.0 or higher (Electronic Supplementary Material, Table S2).
3.2 Quantitative Data Synthesis
Figure 2 presents the SMD for muscular strength outcomes after PRT between the treatment and control groups. PRT significantly improved standardised muscular strength outcomes compared with control conditions [SMD 1.15 (95 % CI 0.80–1.49)], and there was only slight evidence of statistical heterogeneity between studies (I 2 = 35.0 %, P = 0.161). The sensitivity analyses presented in Table 2 shows that the pooled SMD was similarly large in the fixed–effect model and after each of the various studies was excluded (SMD 0.82–1.36). In addition, a funnel plot was produced and showed little evidence of publication bias, since the SMD in muscular strength outcomes was consistently medium to large in all studies (Electronic Supplementary Material, Figure S1).
Figure 3 presents the SMD for total body muscle mass outcomes after PRT between the treatment and control groups. Our primary analysis revealed that PRT failed to increase standardised total body muscle mass outcomes compared with control conditions [SMD 0.29 (95 % CI −0.27 to 0.86); I 2 = 73.5 %, P = 0.002]. A funnel plot showed no evidence of publication bias (Electronic Supplementary Material, Figure S2). The sensitivity analyses showed that this null effect was comparable after each of the various studies was excluded (Electronic Supplementary Material, Table S3). Conversely, PRT significantly improved total body muscle mass in the fixed–effect model [SMD 0.34 (95 % CI 0.05–0.63)] but the fixed–effect assumption was violated given the strong evidence of statistical heterogeneity between studies (I 2 = 73.5 %, P = 0.002).
Given that the majority of trials reviewed investigated the effect of lower-body PRT only (Table 1), we pooled studies to investigate the SMD in lower-body muscle mass and CSA outcomes in a secondary analysis (Fig. 4). This analysis of six studies showed that PRT induced significant muscle hypertrophy of the lower extremities (leg mass, or mid-thigh or quadriceps CSA) [SMD 0.43 (95 % CI 0.11–0.76); I 2 = 26.8 %, P = 0.234]. A funnel plot showed little evidence of publication bias (Electronic Supplementary Material, Figure S3). Additionally, we pooled studies to estimate the inverse variance WMD in muscle mass outcomes after PRT between the treatment and control groups. PRT significantly improved quadriceps muscle CSA measured by MRI [pooled WMD for two studies [26] was 3.83 cm2 (95 % CI 1.73–5.94); I 2 = 1.0 %, P = 0.315], but not total body muscle mass measured by DEXA only [pooled WMD for four studies [26, 28, 31] was −0.06 kg (95 % CI −1.94 to 1.83)] or thigh muscle CSA measured by CT [pooled WMD for two studies [27, 30] was 3.03 cm2 (95 % CI −0.15 to 6.21)].
Figure 5 presents the SMD for HR-QOL outcomes after PRT between the treatment and control groups. PRT significantly improved standardised HR-QOL outcomes compared with control conditions [SMD 0.83 (95 % CI 0.51–1.16)], and there was little evidence of statistical heterogeneity between studies (I 2 = 27.8 %, P = 0.226). The sensitivity analyses presented in Table 3 shows that the pooled SMD was similarly large in the fixed–effect model and after each of the various studies was excluded (SMD 0.70–0.94). In addition, a funnel plot was produced and showed little evidence of publication bias, since the SMD in HR-QOL outcomes was consistently medium to large in all studies (Electronic Supplementary Material, Figure S4).
3.3 Adverse Events
Four studies reported that no adverse events occurred as a consequence of PRT [27, 28, 30, 32]. One study that prescribed intradialytic PRT reported no statistically significant differences between the experimental and control group in the number of dialysis-related complaints (i.e. headache, hypotension, cramping and fistula cannulation difficulties), falls, acute illnesses and number of visits to healthcare professionals [27]. However, one adverse event was documented in this study: a 73-year-old woman in the PRT group sustained a partial tear of the right supraspinatus. The injury was documented [41] and managed conservatively; the patient resumed lower-body PRT for the remainder of the trial [27]. One study reported on adverse events related to anabolic steroid use, but not in relation to PRT [26]. Two studies did not report on adverse events [29, 31].
4 Discussion
4.1 Summary of the Evidence
Based on RCT evidence in patients with CKD, our results were consistent and indicate that PRT significantly improves measures of muscular strength [SMD 1.15 (95 % CI 0.80–1.49)] and HR-QOL [SMD = 0.83 (95 % CI 0.51–1.16)]. There was an absence of evidence showing that PRT significantly increases total body muscle mass [SMD 0.29 (95 % CI −0.27 to 0.86)]. However, secondary analysis of lower body muscle mass and CSA outcomes (i.e. leg mass, or mid-thigh or quadriceps CSA) revealed a significant effect for PRT versus control conditions [SMD 0.43 (95 % CI 0.11–0.76)], a pertinent analysis given that the majority of trials (4/7) were limited to lower-body training [26, 28, 29, 31]. Overall, this robust evidence from RCTs indicates that PRT can induce skeletal muscle hypertrophy and increase muscular strength and HR-QOL with no risk of serious adverse events in men and women with CKD.
The size of the effect of PRT on these key outcomes is moderate to large, and clinically relevant. For instance, studies have consistently shown that skeletal muscle wasting is a strong predictor of mortality in patients with ESRD [14, 42, 43], and a recent observational study noted that the loss of muscle is particularly rapid in pre-dialysis CKD [10]. Carrero et al. [43] have shown that incident and prevalent haemodialysis patients (dialysis vintage 8–78 months) with mild to moderate/severe muscle wasting (SMD 0.38–0.69) suffer a greater risk of systemic inflammation [odds ratio (OR) 2.81 (95 % CI 1.33–5.91)], cardiovascular disease [OR 3.08 (95 % CI 1.43–6.65)] and all-cause mortality (hazard ratio 1.29–3.04) than CKD patients with no evidence of muscle wasting. Similarly, studies have shown that the loss of muscular strength (SMD 0.66) is associated with significantly greater risk of renal endpoint (i.e. pre-dialysis mortality or reaching ESRD) in CKD [44] while impairments in the physical component of HR-QOL (SMD 0.60) have been shown to predict mortality [45]. Therefore, the results of our study suggest that the size of the effect of PRT on skeletal muscle hypertrophy [SMD 0.43 (95 % CI 0.11–0.76)], muscular strength [SMD 1.15 (95 % CI 0.80–1.49)] and HR-QOL [SMD 0.83 (95 % CI 0.51–1.16)], which could be expected in practice, could theoretically protect against disease-related complications and reduce the mortality burden in patients with CKD. Hence, our findings are clinically relevant.
Notably, the effect of PRT on muscle strength and HR-QOL outcomes remained robust in fixed–effect models and after exclusion of studies that combined PRT with other therapies (including haemodialysis), studies in older patients, studies conducted outside the US, longer duration trials, and studies of lower quality. In summary, our results indicate that PRT should be considered for inducing muscle hypertrophy and increasing muscular strength and HR-QOL outcomes in men and women with CKD.
4.2 Limitations
Several limitations require careful consideration. Since only a small number of studies were included, the findings of this review may not be relevant to other countries and key groups within the CKD population. In particular, most of the RCTs reviewed were conducted in patients with ESRD undergoing haemodialysis treatment, while only one trial enrolled patients with pre-dialysis CKD. We found no RCTs that tested the efficacy of PRT in patients undergoing peritoneal dialysis or kidney transplant and hence research on these unique CKD populations is required. Second, there was heterogeneity with respect to the exercise prescriptions (Table 1). Several studies did not prescribe full-body PRT, while others prescribed low-intensity [26, 29] or few exercises [26, 29, 31], factors that can potentially reduce the effectiveness of the training regimen. It has been shown that patients with CKD can safely tolerate higher-intensity and more comprehensive PRT regimens (i.e. involving a greater number of exercises) [27, 30]. Such programmes, involving longer training durations, are likely to be most effective in terms of adapting outcome measures. However, we did not investigate any dose–response effects in the present review and, accordingly, the optimal dosages of PRT to adapt the specific outcomes in this cohort remain unknown and require further research. Finally, combined across all studies, the total number of participants is relatively modest (N = 200–249).
5 Conclusions
We believe that our meta-analytic results are sufficiently reliable to recommend that clinicians consider prescribing PRT for inducing skeletal muscle hypertrophy and increasing muscular strength and HR-QOL outcomes in patients with CKD. Future high-quality research is needed to clarify the long-term clinical benefits and risks of PRT in this cohort.
References
USRDS: U.S. Renal Data System, USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–72.
El Nahas AM, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365(9456):331–40.
Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–8.
Kopple J. Pathophysiology of protein-energy wasting in chronic renal failure. J Nutr. 1999;129(1S Suppl):247S–51S.
Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91(4):1128S–32S.
Johansen KL, Chertow GM, Ng AV, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57:2564–70.
Caso G, Garlick P. Control of muscle protein kinetics by acid-base balance. Curr Opin Clin Nutr Metab Care. 2005;8(1):73–6.
Raj D, Zager P, Shah V, et al. Protein turnover and amino acid transport kinetics in end-stage renal disease. Am J Physiol Endocrinol Metab. 2004;286:E136–43.
John SG, Sigrist MK, Taal MW, et al. Natural history of skeletal muscle mass changes in chronic kidney disease stage 4 and 5 patients: an observational study. PLoS One. 2013;8(5):e65372.
Cheema B, Abas H, Smith B, et al. Investigation of skeletal muscle quantity and quality in end-stage renal disease. Nephrology (Carlton). 2010;15:454–63.
Johansen KL, Shubert T, Doyle J, et al. Muscular atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63:291–7.
McClellan WM, Anson C, Birkeli K, et al. Functional status and quality of life: predictors of early mortality among patients entering treatment for end stage renal disease. J Clin Epidemiol. 1991;44(1):83–9.
Desmeules S, Levesque R, Jaussent I, et al. Creatine index and lean body mass are excellent predictors of long-term survival in haemodiafiltration patients. Nephrol Dial Transplant. 2004;19(5):1182–9.
Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24(5):822–30.
Knight EL, Ofsthun N, Teng M, et al. The association between mental health, physical function, and hemodialysis mortality. Kidney Int. 2003;63(5):1843–51.
DeOreo P. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30(2):204–12.
Lowrie EG, Curtin RB, LePain N, et al. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41(6):1286–92.
Fiatarone Singh M. Exercise comes of age: rationale and recommendations for a geriatric exercise prescription. J Gerontol A Biol Sci Med Sci. 2002;57A:M262–82.
Cheema B, Fiatarone Singh M. Exercise training in patients receiving maintenance hemodialysis: a systematic review of clinical trials. Am J Nephrol. 2005;25:352–64.
Cheema B, Smith B, Fiatarone Singh M. A rationale for intradialytic exercise training as standard clinical practice in end stage renal disease. Am J Kidney Dis. 2005;45:912–6.
Chan M, Cheema B, Fiatarone Singh M. Progressive resistance training and nutrition in renal failure. J Ren Nutr. 2007;17(1):84–7.
Cheema B, Chan D. Resistance training in chronic kidney disease. In: Ciccolo J, Kraemer W, editors. Resistance training for the prevention and treatment of chronic disease. Boca Raton: Taylor & Francis; 2014.
Smart N, Steele M. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology (Carlton). 2011;16:626–32.
Smart NA, Williams AD, Levinger I, et al. Exercise and Sports Science Australia (ESSA) position statement on exercise and chronic kidney disease. J Sci Med Sport. 2013;21(13):005.
Johansen K, Painter P, Sakkas G, et al. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized controlled trial. J Am Soc Nephrol. 2006;17:2307–14.
Cheema B, Abas H, Smith B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18(5):1594–601.
Chen JL, Godfrey S, Ng TT, et al. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant. 2010;25(6):1936–43.
de Lima MC, Cicotoste Cde L, Cardoso KDa S, et al. Effect of exercise performed during hemodialysis: strength versus aerobic. Ren Fail. 2013;35(5):697–704.
Castaneda C, Gordon P, Uhlin K, et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann Intern Med. 2001;135:965–76.
Dong J, Sundell MB, Pupim LB, et al. The effect of resistance exercise to augment long-term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. J Ren Nutr. 2011;21(2):149–59.
Song WJ, Sohng KY. Effects of progressive resistance training on body composition, physical fitness and quality of life of patients on hemodialysis. J Korean Acad Nurs. 2012;42(7):947–56.
Cheema B. Tackling the survival issue in end stage renal disease: Time to get physical on haemodialysis. Nephrology (Carlton). 2008;3(7):560–9.
KDOQI clinical practice guideline for diabetes and CKD. 2012 update. Am J Kidney Dis. 2012;60(5):850–86.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org
Higgins J, Thompson S, Deeks J, et al. Measuring inconsistency in meta-analysis. BMJ. 2003;327(7414):557–60.
Higgins J, Thompson S. Quantifying heterogeneity in meta-analysis. Stat Med. 2002;21(11):1539–58.
Eggers M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–63.
Cheema B, Lassere M, Shnier R, et al. Rotator cuff tear in an elderly woman performing progressive resistance training: case report from a randomized controlled trial. J Phys Act Health. 2007;4(1):1–8.
Honda H, Qureshi A, Axelsson J, et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007;86(3):633–8.
Carrero JJ, Chmielewski M, Axelsson J, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr. 2008;27(4):557–64.
Chang YT, Wu HL, Guo HR, et al. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol Dial Transplant. 2011;26(11):3588–95.
Kalantar-Zadeh K, Kopple JD, Block G, et al. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol. 2001;12(12):2797–806.
Acknowledgments
BSC is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. BSC, DC and EA conceived and designed the review, identified articles for inclusion and exclusion, extracted and interpreted the data and drafted the article. PF analysed and interpreted the data and revised the article. All authors have approved and read the final article. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors declare they have no competing interests. We sincerely thank Ms Katrina Chaudhary for her work in developing the database searches.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheema, B.S., Chan, D., Fahey, P. et al. Effect of Progressive Resistance Training on Measures of Skeletal Muscle Hypertrophy, Muscular Strength and Health-Related Quality of Life in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Sports Med 44, 1125–1138 (2014). https://doi.org/10.1007/s40279-014-0176-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-014-0176-8