Abstract
Purpose
Physical inactivity and sleep disturbance are frequently observed and relate to poor clinical outcomes in maintenance hemodialysis patients. We aimed to investigate the effect of intradialytic exercise on daily physical activity and sleep quality, measured by an accelerometer, in maintenance hemodialysis patients.
Methods
This study randomly assigned ambulatory maintenance hemodialysis patients aged ≥ 20 years on dialysis ≥ 6 months, without a hospitalization history for the previous 3 months to 4 groups: aerobic exercise (AE), resistance exercise (RE), combination exercise (CE), and control. A stationary bike was used for AE and a TheraBand®/theraball for RE. A 12-week intradialytic exercise program (3 times/week) was completed in the AE (n = 11), RE (n = 10), and CE (n = 12) groups. The control group (n = 13) received only warm-up stretching. At baseline and 12-week follow-up, daily physical activity and sleep quality were measured with a triaxial accelerometer (wActiSleep-BT; ActiGraph, Pensacola, FL) during a continuous 7-day wear period.
Results
We observed a significant increase in metabolic equivalent (MET; kcal/h/kg) in the AE (1.02 ± 0.03 vs 1.04 ± 0.04, P = 0.04) and CE (1.06 ± 0.05 vs 1.09 ± 0.08, P = 0.01) groups at 12 weeks compared with baseline. When comparing between-group changes in MET, there was a significant increase in METs in the CE group (0.03 ± 0.03 vs − 0.01 ± 0.04, P = 0.02) compared with the control group. The total number of sedentary bouts (per week) decreased significantly in the AE (200 ± 37 vs 174 ± 36, P = 0.01), RE (180 ± 31 vs 130 ± 49, P = 0.03), and CE groups (180 ± 45 vs 152 ± 46, P = 0.04) at 12 weeks compared with baseline. The average sleep fragmentation index, indicating poor sleep quality, decreased significantly at 12 weeks compared with baseline in the AE (51.4 ± 8.0 vs 44.5 ± 9.6, P = 0.03) and RE groups (52.3 ± 7.3 vs 40.0 ± 15.4, P = 0.01).
Conclusions
Intradialytic exercise appears to be clinically beneficial in improving daily physical activity and sleep quality in maintenance hemodialysis patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well established that maintenance hemodialysis (MHD) patients have very low levels of daily physical activity (DPA), even when compared with sedentary individuals without renal failure [1, 2]. In MHD patients, three main factors contribute to low levels of DPA: renal failure itself (with its associated malaise), side effects of hemodialysis (HD) therapy, and worsening comorbidities [3]. Physical inactivity is therefore regarded as a major factor leading to impaired physical condition, reduced exercise capacity, and ultimately muscle wasting [4]. Previous studies reported that lack of DPA is associated with an increased risk of mortality and poor quality of life (QOL) among MHD patients [5,6,7]. Although strategies to increase DPA in this population began to receive attention recently, they remain a neglected aspect of dialysis care.
Poor sleep quality (SQ) is also common among MHD patients [8]. The high prevalence of potential sleep disorders such as sleep apnea and restless legs syndrome (RLS)/periodic limb movement disorder (PLMD) in MHD population is one major factor which contributes to the poor SQ of these patients [9]. The main determinants of sleep disorders in MHD patients are not well understood, and previous studies demonstrated several potential intrinsic and environmental causes [10,11,12,13,14]. These sleep problems directly contribute to poor QOL in MHD patients, which further deteriorates their health status [15].
It has been shown that exercise training improves physical function, physical performance, nutritional status, cardiovascular function, HD efficiency, QOL, and SQ in MHD patients [16,17,18,19]. Intradialytic exercise (IDE), performed only during the HD treatment, is one of the promising choices for MHD patients, as it can be conducted in a setting with close monitoring, does not involve additional time or travel, and may alleviate a patient’s fear of musculoskeletal injury, which may improve adherence and supervision relative to exercise on non-dialysis days [17, 20]. In general, exercise can be classified into the following categories: (1) aerobic exercise (AE), or exercise that is rhythmic, continuous, uses large muscle groups, and is generally prescribed to improve endurance; (2) resistance exercise (RE), or strength training, known to increase muscle size and strength; and (3) combination exercise (CE), a combination of AE and RE [16]. Each training program has specific fitness and health goals. It is well known that AE is the best option in terms of improving cardiopulmonary fitness (muscle mass of the legs can improve as well), whereas RE is superior compared to the other forms of exercise in terms of improving muscle mass, strength, and power. Therefore, additional positive effects are expected by combining AE and RE. Although exercise participation could lead to greater benefits among MHD patients than the general population, it is possible that dialysis patients may also incur greater risk because of underlying heart or musculoskeletal disease. Despite numerous published studies, primarily uncontrolled and/or with small patient samples, the “best” exercise program for MHD patients has not been determined [17, 21, 22].
Thus, the objective of our study was to examine the effect of IDE, using a commercially available, objective accelerometer, in MHD patients. The primary aim was to compare the differences between each of three intervention arms (AE, RE, and CE) and the control arm in changes from baseline to 12 weeks in DPA and SQ parameters (between-group differences). A secondary aim was to investigate the changes within each arm (within-group changes).
Methods
Participants
Fifty-seven MHD patients (mean age 57 years; 26 men) who met the inclusion criteria between December 2014 and March 2015 were recruited (Fig. 1). Inclusion criteria for participants were as follows: (1) age ≥ 20 years; (2) MHD vintage ≥ 6 months; (3) MHD treatment, thrice weekly; (4) no hospitalizations during the previous 3 months, except for vascular access repair; (5) no amputations or prostheses in upper and lower extremities; (6) cognitive capacity sufficient for communication; (7) able to ambulate and wear the physical activity monitor for 7 days; and (8) good compliance with the study protocol. Exclusion criteria included the following: (1) any acute infectious or other inflammatory illnesses; (2) current malignancy except basal cell carcinoma; (3) acute myocardial infarction or unstable angina within the past 12 months; (4) current heart or lung failure or severe liver disease; (5) severe uncontrolled diabetes; (6) severe retinal diseases, such as proliferative diabetic retinopathy and vitreous hemorrhage; and (7) orthopedic disorders exacerbated by activity. This study was approved by the institutional review board, and all participants provided written informed consent.
Study design
This 12-week study was a randomized parallel design investigating the effects of different exercise regimes on changes in DPA and SQ. Participants were randomized to four different groups as they were recruited by the researcher (using a block randomization scheme and sealed envelopes). The AE group performed 30 min of intradialytic AE thrice a week, which consisted of stationary cycling; the RE group completed the programmed session of intradialytic RE thrice a week using elastic resistive bands and soft weights; the CE group performed both intradialytic AE and RE thrice a week; and the control group performed no IDE but received warm-up stretches only. The warm-up stretches included the following: (1) neck stretch; (2) arm/hand stretch; (3) shoulder shrug and rotation; (4) chest and upper back stretch; (5) side stretch; (6) single knee pull; (7) leg stretch; and (8) calf stretch. Allocation was concealed at the time of participant consent; however, participants and the researcher were made aware of their group at the commencement of the intervention. Due to the nature of the intervention, blinding of intervention groups to the researcher and dialysis patients was not possible.
At baseline, participant demographic characteristics, height, and weight were collected and body mass index was calculated. Each participant’s comorbid status was analyzed on the basis of the Charlson comorbidity index, using the original scoring system [23]. A 7-point scale subjective global assessment scoring method was used to assess nutritional status among participants [24]. The Beck Depression Inventory-II (BDI-II) and Beck Anxiety Inventory (BAI) were also used to identify the prevalence of depression (defined as a BDI-II score ≥ 14) and anxiety (defined as a BAI score ≥ 8) in each participant [25, 26].
All participants underwent blood and serum measurements, as described in Table 1. Blood was obtained immediately before a routine midweek HD. Blood tests results were values obtained monthly and averaged over the last 3 months.
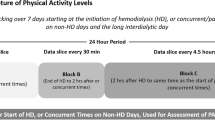
The wActiSleep-BT accelerometer (ActiGraph, Pensacola, FL) was used as an objective method for monitoring physical activity and sleep status. At baseline and again at 12 weeks post-intervention, participants were instructed to wear the accelerometer on a belt in the mid-axillary line above their non-dominant hip for 7 complete days (3 HD and 4 non-HD days), including while sleeping, except during water activities (e.g., bathing, swimming, showering).
Exercise interventions
The exercise program consisted of a 5-min warm-up, AE for maximum of 30 min or programmed RE, and a 5-min cool down period within the first 2 h of each HD session over 12 weeks. AE participants performed recumbent stationary cycling (SP-2100R; SUNGDO MC, South Korea) at an intensity of 11–13 out of 20 at the rate of perceived exertion (RPE) on Borg’s 15-point scale, so that the intensity involved 60–70% of an individual’s maximal capacity, a level at which cardiovascular health can be increased [27]. The speed, load, and duration were gradually increased with change in mode (automatic and passive). According to patients’ performance, training loads were adjusted. The RE program consisted of seven exercises, involving muscles of both the lower (quadriceps femoris, vastus lateralis, adductor magnus, and biceps femoris) and upper body (biceps brachialis, triceps brachialis, and deltoid muscles). All the exercises were performed in a supine or a sitting position, using TheraBand® colored elastic resistive bands and soft weights (The Hygenic Corporation, Akron, OH). When participants could perform three sets of 10–15 repetitions (RPE 13–15) for each exercise, the exercises were made progressively harder using different color-graded elastic bands and soft weights. Participants were encouraged to perform each exercise to optimize movement speed and muscle power. The CE group performed both the intradialytic AE and RE mentioned above. The intensity of both the AE and RE programs remained constant during the 12 weeks of the intervention.
Accelerometer data processing
The small (46 × 33 × 15 mm) lightweight (19 g) triaxial accelerometer measures accelerations in three individual axes (vertical, horizontal and perpendicular), has a dynamic range of ± 8 units of gravity, and determines posture (sitting or lying, standing and stepping) based on acceleration information. The accelerometer was set to record at a frequency of 30 Hz (i.e., collect acceleration data 30 times per second in each axis). ActiLife desktop analysis software version 6.13.2 was used to download these data from the accelerometer, and to convert acceleration data into 3 axes (counts per minute, cpm) and vector magnitude (VM) activity counts (square root of the sum of squares of cpm from all 3 axes) at epoch lengths of 60 s with the low-frequency extension setting. Wear time (the time that the accelerometer was worn) was validated using the NHANES wear time algorithm prior to scoring; segments calculated as non-wear segments were removed [28].
Data were scored via the ActiLife software using the default algorithms to derive the following DPA parameters: metabolic equivalent of task (MET, kcal/h/kg; as an index of the intensity of activities), the number of occurrences of a bout (counts per week), the total time spent in the bouts (min per week), sedentary bouts (counts per week), daily average of sedentary bouts (min), and the percentage of time spent in moderate to vigorous physical activity (MVPA). The bouts were defined as 10 or more consecutive minutes above the relevant threshold of 1952 cpm on the vertical axis, with allowance for interruptions of 1–2 min below threshold. Sedentary bouts were defined as a minimum of 10 consecutive minutes below the cut point of 100 cpm (the vertical axis) without tolerance. The time spent in MVPA was determined by VM cpm ≥ 2690 [29]. Physical activity energy expenditure (PAEE, kcal/day) was also estimated using the prediction algorithm labeled the “Freedson Combination (1998)” equation in the ActiLife software.
Accelerometer sleep recordings were analyzed with the ActiLife using the Cole–Kripke sleep scoring algorithm [30]. The following SQ parameters were examined in this study: total sleep time (TST), defined as the number of minutes scored as sleep between lights off and lights on; wake after sleep onset (WASO), the number of minutes scored as wake after sleep onset; sleep efficiency (SE, %), 100 times the ratio between TST and total time spent in bed; and sleep fragmentation, an index of restlessness during the sleep period expressed as a percentage. The higher the index, the more sleep is disrupted. The ActiLife calculated three values for sleep fragmentation: movement index (MI), the percentage of epochs with vertical axis counts greater than zero in the sleep period; fragmentation index (FI), the percentage of 1-min periods of sleep versus all periods of sleep during the sleep period; and sleep fragmentation index (SFI), the sum of the MI and the FI.
Statistical analyses
The results are presented as mean ± SD or number (percentage). Statistical significance was estimated using a Pearson’s Chi-square test for categorical variables and a one-way analysis of variance (ANOVA) test for continuous variables. The ANOVA tests were used to compare groups on change scores (follow-up score minus baseline score) of DPA and SQ parameters. If the ANOVA test (as an overall test) indicated significance, the Bonferroni test was used as a post hoc testing. Paired t-tests compared within-group changes from baseline to 12 weeks. Statistical significance was defined as P < 0.05. Statistical analyses were performed using IBM SPSS Statistics, version 23 (IBM Corp., Armonk, NY). A post hoc power analysis was conducted using G*Power 3.1.9.2 software (Universität Kiel, Germany) to see whether the number of patients was sufficient to detect differences between groups [31].
Results
Fifty-seven participants were randomized into the AE (n = 15), RE (n = 14), CE (n = 15), and control (n = 13) groups. Finally, 46 participants (AE, n = 11; RE, n = 10; CE, n = 12; control, n = 13) completed the 12-week study, resulting in a 19% attrition rate (Fig. 1). There were no reported adverse events, such as musculoskeletal injuries, hypoglycemic episodes, cardiovascular events, or hospitalizations, as result of the intervention. The 46 participants were 55 ± 12 years of age, on MHD for 64 ± 72 months, 50% female, and 44% diabetic. The baseline characteristics of participants in the four intervention groups are shown in Table 1. These groups had similar characteristics except for dialysis vintage.
Tables 2 and 3 show between- and within-group comparisons in both DPA and sleep-related parameters after the 12-week intervention.
In the primary analysis (between-group differences), when comparing between-group changes to MET (kcal/h/kg), there was a significant increase in the CE group (0.03 ± 0.03 vs − 0.01 ± 0.04, P = 0.02) compared to the control group. Compared with control group, the 12-week IDE group interventions (AE, RE, or CE) showed no significant effect on DPA and SQ values, except for METs. The post hoc power analysis revealed that at least 152 subjects per groups (a total of 610) would have been needed to detect much more significant differences between intervention groups.
In the secondary analysis (within-group changes), we observed a significant MET increase in the AE (1.02 ± 0.03 vs 1.04 ± 0.04, P = 0.04) and CE groups (1.06 ± 0.05 vs 1.09 ± 0.08, P = 0.01) at 12 weeks compared with baseline. The total number of bouts (per week) increased significantly in the CE group (2 ± 3 vs 3 ± 5, P = 0.04) at 12 weeks compared with baseline. The total number of sedentary bouts (per week) decreased significantly in the AE (200 ± 37 vs 174 ± 36, P = 0.01), RE (180 ± 31 vs 130 ± 49, P = 0.03), and CE groups (180 ± 45 vs 152 ± 46, P = 0.04) at 12 weeks compared with baseline. The daily average of sedentary bouts (min) also decreased significantly in the AE (694 ± 159 vs 575 ± 158, P = 0.003), RE (617 ± 118 vs 434 ± 139, P = 0.009), and CE groups (623 ± 112 vs 512 ± 123, P = 0.003) at 12 weeks compared with baseline. The percentage of time spent in MVPA increased significantly in the CE group (2.3 ± 1.7 vs 3.5 ± 2.9, P = 0.03) at 12 weeks compared with baseline. Sleep data analysis showed the average MI (%) decreased significantly in the AE (39.5 ± 5.9 vs 31.3 ± 9.4, P = 0.006), RE (39.3 ± 6.4 vs 28.2 ± 10.2, P = 0.001), and CE groups (38.8 ± 9.0 vs 29.6 ± 9.1, P = 0.02) at 12 weeks compared with baseline. The average FI (%) increased significantly in the control group (8.5 ± 5.0 vs 12.8 ± 2.9, P = 0.01) at 12 weeks compared with baseline. The average SFI (%), indicating poor SQ, decreased significantly at 12 weeks compared with baseline in the AE (51.4 ± 8.0 vs 44.5 ± 9.6, P = 0.03) and RE groups (52.3 ± 7.3 vs 40.0 ± 15.4, P = 0.01). We identified no significant changes after the 12-week intervention in PAEE, total time in bouts, TST, WASO, and SE.
The frequencies of depressive and anxiety disorders did not change significantly in any group during the 12-week study period, and there were no significant differences between exercise interventions at 12 weeks (data not shown).
Discussion
The primary aim of this study was to investigate whether 12 weeks of intradialytic training with AE, RE, or CE would induce improvements to DPA or SQ in MHD patients compared with a no IDE group. To the best of our knowledge, this is the first report to investigate the effect of IDE on DPA and SQ in MHD patients using an accelerometer. A previous randomized pilot study of a 6-month exercise training program in 70 MHD patients indicated that self-reported physical activity increased in the IDE group compared with the usual care group [32]. In this study, however, we did not observe any significant changes in DPA (except for METs) or SQ between the control and IDE groups at 12 weeks. Our primary outcome data also indicated there was no evidence of superiority of AE over RE or vice versa in improvement of DPA and SQ. Moreover, although it is an attractive theoretical hypothesis that a combination of AE and RE would be superior to either exercise modality alone as they act on different specific fitness and health targets, we did not find any positive additive effect of CE on DPA and SQ in the present study. There are several possible explanations for these nonsignificant results. First, our sample size was insufficient. Second, the intervention period of 12 weeks was relatively short. Third, ceiling effects could weaken the intervention effects. Because our study subjects (recruited according to inclusion and exclusion criteria) were in relatively stable condition, there would be a possibility that the levels of DPA and SQ in participants before the intervention are already relatively high levels. Finally, our IDE protocol might be of insufficient workload to stimulate positive effects.
In our secondary analysis, at 12 weeks compared with baseline, we observed significant improvements in METs (in AE and CE), bouts (in CE), sedentary bouts (in AE and CE), daily average of sedentary bouts (in AE, RE, and CE), and the percentage of time spent in MVPA (in CE) from among the DPA parameters. The results of the present study were partly supported by a recent non-randomized study, which showed that intradialytic RE training improved physical activity in 75 MHD patients [33]. A recent meta-analysis of IDE in MHD patients showed that IDE modality in the included randomized controlled trials was of various types [22]. Our data showed CE improved more DPA parameters than AE and RE. However, CE is more complex, and compliance may be poorer than the other two choices. In general, more studies, particularly comparative studies, are needed to identify the most suitable IDE modality for MHD patients.
There is currently no specified pharmaceutical treatment guideline for SQ improvement in MHD patients, and benzodiazepines, non-benzodiazepine, anxiolytics, and melatonin are all prescribed for these patients [34]. Concerns about drug tolerance, habituation, complications, excessive accumulation, and interactions with the numerous medications used in MHD population are frequently raised in pharmacological approach. Surprisingly, only a few small-sized pharmacological studies revealed improvements in SQ in MHD patients [35, 36]. As a result, extra care and caution should be exercised when prescribing drugs for sleep disorders in MHD patients. Recently, growing evidence indicates favorable effects and less adverse events using non-pharmacological interventions (including exercise training) on sleep disorders, which are promising methods to improve the SQ in a dialysis-dependent population [37]. Various pathways have been proposed to explain the relationship between exercise and sleep. Proposed mechanisms include body temperature changes, cytokine concentration changes, increased energy consumption/metabolic rate, central nervous system fatigue, changes in mood/anxiety symptoms, changes in heart rate and heart rate variability, growth hormone secretion, brain-derived neurotropic factor secretion, improved fitness level, and body composition change [38]. While most exercise and sleep studies have focused on AE, a small number of studies have examined RE on SQ [39]. Little is still known on the underlying mechanisms that can explain the effects of RE on sleep. RE could potentially improve SQ by improved symptoms of depression or anxiety, alterations in PAEE, increase in body temperature, or relief of musculoskeletal pain [40]. Several previous studies based on questionnaires have suggested that IDE had benefits on the potential improvement in sleep disturbance in MHD patients [41, 42]. In our secondary analysis, SQ data demonstrated significant improvements in average MI (in AE, RE, and CE) and SFI (in AE and RE), while other SQ parameters showed no change over the IDE duration. It has been demonstrated that use of sleep-inducing medication is a potential factor contributing to alter SQ in dialysis patients [8]. Moreover, chronic auto-administration of hypnotic medication may be more frequent in MHD patients relative to the general population. In addition, SQ may be affected by possible lifestyle factors, such as tobacco, alcohol, and caffeine use [43]. Thus, the possibility remains that these common factors could have affected our results. Several psychosocial factors also contribute to impaired SQ in MHD patients. Depression is very common and can be a cause, as well as a result, of insomnia in MHD patients [44]. Anxiety, which typically interferes with sleep onset, also may cause early morning awakening [45]. In case of depression, there are several previous trials showing that AE training significantly alleviated depression in MHD patients [42, 46, 47]. However, our exercise interventions had little effect on either depression or anxiety, which suggests that psychosocial factors may have had a very limited effect on SQ in this study population, at least during the 12-week study period.
The major strength of this study was the objective (accelerometer) assessment of changes in DPA and SQ in MHD patients as a consequence of a 12-week IDE. DPA and SQ are typically measured using self-report instruments. However, subjective methods based on questionnaires and diaries rely on individual memory and interpretation, and may be afflicted by inconsistencies and bias. Therefore, objective methods, based on specific monitoring devices, may be an alternative approach to measure DPA and SQ more accurately. Recently, small, lightweight, wearable devices capable of sampling and storing raw, triaxial acceleration data for up to several weeks are commercially available. These accelerometers are sensitive to combined gravitational and dynamic acceleration, which makes it possible to both derive inclinometric information and assess intensity of movements [48]. Accelerometry also provides objective monitoring of sleep–wake rhythms and may be considered a cost-effective substitute for polysomnography (PSG), the gold standard for sleep monitoring [49]. Therefore, accelerometry systems may be used to concurrently assess sleep and DPA behaviors in free-living settings. While most reports of DPA and SQ have relied upon self-reports, this study used more accurate measures of DPA and SQ, gathering data over 7 days of accelerometer use [37, 50].
In general, accelerometry has reasonable validity and reliability in normal individuals with relatively good sleep patterns. In comparison with PSG, however, the validity of accelerometry in special populations (e.g., elderly people, individuals with other major health problems or individuals with poor SQ) is still more questionable. The most problematic validity issue is the low specificity of accelerometry in detecting wakefulness within sleep periods reported for certain devices, algorithms, and populations. In addition, in the final analysis accelerometry only measures movements and not sleep per se, and therefore, it is affected by other neurobehavioral systems and control mechanisms that are unrelated to sleep (e.g., disorders of the motor system) [51].
Surprisingly, there is little evidence whether accelerometry is a valid alternative PSG for sleep measures in MHD patients. A recent systematic review, which utilized only studies performed using PSG, verified the high prevalence of sleep disorders (sleep apnea, RLS, and PLMD) in MHD patients [52]. Such frequent sleep-related problems have a negative impact on SQ in MHD patients [9]. As far as we know, PSG is routinely indicated for the diagnosis of sleep apnea and PLMD [53]. RLS is a diagnosis based on history with standardized clinical criteria [54]. Although PLMD can exist independent of RLS, it is estimated that approximately 80% of individuals with RLS have evidence of PLMD on PSG, so PSG may be helpful in increasing the confidence in the RLS [53]. However, PSG is a relatively expensive, time and labor-intensive test and may not be feasible for lengthy data collection. It may also difficult to convince dialysis patients, already spending a substantial amount of their time each week attached to a machine in the dialysis center, to spend two nights in a sleep laboratory [13]. Thus, because we used only accelerometry in the objective assessment of sleep–wake (activity and rest) pattern in this study, the prevalence of these sleep disorders remains unknown from our objective data, which may also limit its use as a valid alternative to PSG.
This study had several limitations. Because of the single center design and small sample size, our study might have been underpowered to detect significant changes in some variables. Another limitation was the potential selection bias in our cohort, since patients who were eligible to participate in this study were healthier than average MHD patients. They had not been hospitalized for at least 3 months before the study and could ambulate independently. Additionally, there was an inherent selection bias since not all patients who were approached agreed to participate in the study. Perhaps the patients who agreed to participate in the study were more interested in performing intradialytic training and wearing an accelerometer relative to their peers. Finally, there was a baseline imbalance in dialysis vintage between groups, which might have affected our results.
In conclusion, this study suggests that IDE may play an important role in the improvement of DPA and SQ in MHD patients, although future studies with larger sample sizes and a longer intervention duration are needed to confirm our findings and lead to improved clinical outcomes, such as QOL and mortality.
References
Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, Kent-Braun JA (2000) Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int 57(6):2564–2570. https://doi.org/10.1046/j.1523-1755.2000.00116.x
Kim JC, Shapiro BB, Zhang M, Li Y, Porszasz J, Bross R, Feroze U, Upreti R, Kalantar-Zadeh K, Kopple JD (2014) Daily physical activity and physical function in adult maintenance hemodialysis patients. J Cachexia Sarcopenia Muscle 5(3):209–220. https://doi.org/10.1007/s13539-014-0131-4
Kosmadakis GC, Bevington A, Smith AC, Clapp EL, Viana JL, Bishop NC, Feehally J (2010) Physical exercise in patients with severe kidney disease. Nephron Clin Pract 115(1):c7–c16. https://doi.org/10.1159/000286344
Tawney KW, Tawney PJ, Kovach J (2003) Disablement and rehabilitation in end-stage renal disease. Semin Dial 16(6):447–452
O’Hare AM, Tawney K, Bacchetti P, Johansen KL (2003) Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis 41(2):447–454. https://doi.org/10.1053/ajkd.2003.50055
Stack AG, Molony DA, Rives T, Tyson J, Murthy BV (2005) Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis 45(4):690–701
Lopes AA, Lantz B, Morgenstern H, Wang M, Bieber BA, Gillespie BW, Li Y, Painter P, Jacobson SH, Rayner HC, Mapes DL, Vanholder RC, Hasegawa T, Robinson BM, Pisoni RL (2014) Associations of self-reported physical activity types and levels with quality of life, depression symptoms, and mortality in hemodialysis patients: the DOPPS. Clin J Am Soc Nephrol 9(10):1702–1712. https://doi.org/10.2215/CJN.12371213
Gusbeth-Tatomir P, Boisteanu D, Seica A, Buga C, Covic A (2007) Sleep disorders: a systematic review of an emerging major clinical issue in renal patients. Int Urol Nephrol 39(4):1217–1226. https://doi.org/10.1007/s11255-007-9262-2
Merlino G, Piani A, Dolso P, Adorati M, Cancelli I, Valente M, Gigli GL (2006) Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant 21(1):184–190. https://doi.org/10.1093/ndt/gfi144
Razeghi E, Sahraian MA, Heidari R, Bagherzadeh M (2012) Association of inflammatory biomarkers with sleep disorders in hemodialysis patients. Acta Neurol Belg 112(1):45–49. https://doi.org/10.1007/s13760-012-0003-7
Shayamsunder AK, Patel SS, Jain V, Peterson RA, Kimmel PL (2005) Sleepiness, sleeplessness, and pain in end-stage renal disease: distressing symptoms for patients. Semin Dial 18(2):109–118. https://doi.org/10.1111/j.1525-139X.2005.18218.x
Koch BC, Nagtegaal JE, Kerkhof GA, ter Wee PM (2009) Circadian sleep-wake rhythm disturbances in end-stage renal disease. Nat Rev Nephrol 5(7):407–416. https://doi.org/10.1038/nrneph.2009.88
Novak M, Shapiro CM, Mendelssohn D, Mucsi I (2006) Diagnosis and management of insomnia in dialysis patients. Semin Dial 19(1):25–31. https://doi.org/10.1111/j.1525-139X.2006.00116.x
Elias RM, Chan CT, Paul N, Motwani SS, Kasai T, Gabriel JM, Spiller N, Bradley TD (2013) Relationship of pharyngeal water content and jugular volume with severity of obstructive sleep apnea in renal failure. Nephrol Dial Transplant 28(4):937–944. https://doi.org/10.1093/ndt/gfs473
Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, Kurokawa K, Rayner HC, Furniss AL, Port FK, Saran R (2008) Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 23(3):998–1004. https://doi.org/10.1093/ndt/gfm630
Johansen KL (2007) Exercise in the end-stage renal disease population. J Am Soc Nephrol 18(6):1845–1854. https://doi.org/10.1681/ASN.2007010009
Heiwe S, Jacobson SH (2014) Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis 64(3):383–393. https://doi.org/10.1053/j.ajkd.2014.03.020
Tentori F, Elder SJ, Thumma J, Pisoni RL, Bommer J, Fissell RB, Fukuhara S, Jadoul M, Keen ML, Saran R, Ramirez SP, Robinson BM (2010) Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant 25(9):3050–3062. https://doi.org/10.1093/ndt/gfq138
Parsons TL, Toffelmire EB, King-VanVlack CE (2006) Exercise training during hemodialysis improves dialysis efficacy and physical performance. Arch Phys Med Rehabil 87(5):680–687. https://doi.org/10.1016/j.apmr.2005.12.044
Konstantinidou E, Koukouvou G, Kouidi E, Deligiannis A, Tourkantonis A (2002) Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med 34(1):40–45
Barcellos FC, Santos IS, Umpierre D, Bohlke M, Hallal PC (2015) Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J 8(6):753–765. https://doi.org/10.1093/ckj/sfv099
Sheng K, Zhang P, Chen L, Cheng J, Wu C, Chen J (2014) Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am J Nephrol 40(5):478–490. https://doi.org/10.1159/000368722
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Steiber AL, Kalantar-Zadeh K, Secker D, McCarthy M, Sehgal A, McCann L (2004) Subjective global assessment in chronic kidney disease: a review. J Ren Nutr 14(4):191–200
Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 56(6):893–897
Beck AT, Steer RA, Ball R, Ranieri W (1996) Comparison of Beck depression inventories-IA and -II in psychiatric outpatients. J Pers Assess 67(3):588–597. https://doi.org/10.1207/s15327752jpa6703_13
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M (2008) Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40(1):181–188. https://doi.org/10.1249/mss.0b013e31815a51b3
Sasaki JE, John D, Freedson PS (2011) Validation and comparison of ActiGraph activity monitors. J Sci Med Sport 14(5):411–416. https://doi.org/10.1016/j.jsams.2011.04.003
Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC (1992) Automatic sleep/wake identification from wrist activity. Sleep 15(5):461–469
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191
Koh KP, Fassett RG, Sharman JE, Coombes JS, Williams AD (2010) Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: a randomized pilot study. Am J Kidney Dis 55(1):88–99. https://doi.org/10.1053/j.ajkd.2009.09.025
Saitoh M, Ogawa M, Dos Santos MR, Kondo H, Suga K, Itoh H, Tabata Y (2016) Effects of intradialytic resistance exercise on protein energy wasting, physical performance and physical activity in ambulatory patients on dialysis: a Single-Center Preliminary Study in a Japanese Dialysis Facility. Ther Apher Dial 20(6):632–638. https://doi.org/10.1111/1744-9987.12447
Molnar MZ, Novak M, Mucsi I (2006) Management of restless legs syndrome in patients on dialysis. Drugs 66(5):607–624
Koch BC, Nagtegaal JE, Hagen EC, van der Westerlaken MM, Boringa JB, Kerkhof GA, Ter Wee PM (2009) The effects of melatonin on sleep-wake rhythm of daytime haemodialysis patients: a randomized, placebo-controlled, cross-over study (EMSCAP study). Br J Clin Pharmacol 67(1):68–75. https://doi.org/10.1111/j.1365-2125.2008.03320.x
Sabbatini M, Crispo A, Pisani A, Ragosta A, Cesaro A, Mirenghi F, Cianciaruso B, Federico S (2003) Zaleplon improves sleep quality in maintenance hemodialysis patients. Nephron Clin Pract 94(4):c99–c103. https://doi.org/10.1159/000072493
Yang B, Xu J, Xue Q, Wei T, Xu J, Ye C, Mei C, Mao Z (2015) Non-pharmacological interventions for improving sleep quality in patients on dialysis: systematic review and meta-analysis. Sleep Med Rev 23:68–82. https://doi.org/10.1016/j.smrv.2014.11.005
Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW (2015) The effects of physical activity on sleep: a meta-analytic review. J Behav Med 38(3):427–449. https://doi.org/10.1007/s10865-015-9617-6
Ferris LT, Williams JS, Shen CL, O’Keefe KA, Hale KB (2005) Resistance training improves sleep quality in older adults a pilot study. J Sports Sci Med 4(3):354–360
Uchida S, Shioda K, Morita Y, Kubota C, Ganeko M, Takeda N (2012) Exercise effects on sleep physiology. Front Neurol 3:48. https://doi.org/10.3389/fneur.2012.00048
Afshar R, Emany A, Saremi A, Shavandi N, Sanavi S (2011) Effects of intradialytic aerobic training on sleep quality in hemodialysis patients. Iran J Kidney Dis 5(2):119–123
Giannaki CD, Hadjigeorgiou GM, Karatzaferi C, Maridaki MD, Koutedakis Y, Founta P, Tsianas N, Stefanidis I, Sakkas GK (2013) A single-blind randomized controlled trial to evaluate the effect of 6 months of progressive aerobic exercise training in patients with uraemic restless legs syndrome. Nephrol Dial Transplant 28(11):2834–2840. https://doi.org/10.1093/ndt/gft288
Parker KP (2003) Sleep disturbances in dialysis patients. Sleep Med Rev 7(2):131–143
Kimmel PL, Cukor D, Cohen SD, Peterson RA (2007) Depression in end-stage renal disease patients: a critical review. Adv Chronic Kidney Dis 14(4):328–334. https://doi.org/10.1053/j.ackd.2007.07.007
Theofilou P (2013) Association of insomnia symptoms with kidney disease quality of life reported by patients on maintenance dialysis. Psychol Health Med 18(1):70–78. https://doi.org/10.1080/13548506.2012.674144
Ouzouni S, Kouidi E, Sioulis A, Grekas D, Deligiannis A (2009) Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil 23(1):53–63. https://doi.org/10.1177/0269215508096760
Kouidi E, Karagiannis V, Grekas D, Iakovides A, Kaprinis G, Tourkantonis A, Deligiannis A (2010) Depression, heart rate variability, and exercise training in dialysis patients. Eur J Cardiovasc Prev Rehabil 17(2):160–167. https://doi.org/10.1097/HJR.0b013e32833188c4
Godfrey A, Conway R, Meagher D, ÓLaighin G (2008) Direct measurement of human movement by accelerometry. Med Eng Phys 30(10):1364–1386. https://doi.org/10.1016/j.medengphy.2008.09.005
Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A Jr, Coleman J, Lee-Chiong T, Pancer J, Swick TJ, Standards of Practice C, American Academy of Sleep M (2007) Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 30(4):519–529
Haskell WL (2012) Physical activity by self-report: a brief history and future issues. J Phys Act Health 9(Suppl 1):S5–S10
Sadeh A (2011) The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev 15(4):259–267. https://doi.org/10.1016/j.smrv.2010.10.001
Fonseca NT, Urbano JJ, Nacif SR, Silva AS, Peixoto RA, Urbano GJ, Oliveira EF, Santos IR, Oliveira CS, Insalaco G, Oliveira LV (2016) A systematic review of sleep disorders in patients with chronic kidney disease undergoing hemodialysis. J Phys Ther Sci 28(7):2164–2170. https://doi.org/10.1589/jpts.28.2164
Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong T, Loube DL, Owens J, Pancer JP, Wise M (2005) Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep 28(4):499–521
Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, Zucconi M, Ferri R, Trenkwalder C, Lee HB, International Restless Legs Syndrome Study G (2014) Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med 15(8):860–873. https://doi.org/10.1016/j.sleep.2014.03.025
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Cho, JH., Lee, JY., Lee, S. et al. Effect of intradialytic exercise on daily physical activity and sleep quality in maintenance hemodialysis patients. Int Urol Nephrol 50, 745–754 (2018). https://doi.org/10.1007/s11255-018-1796-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1796-y