Abstract
Anthropogenic land-use change impacts ecological communities in urban and rural landscapes, and wetlands are particularly vulnerable despite the valuable ecosystem services they provide. Urbanized non-wetland systems are often enriched in non-native plant species, and similar patterns in wetlands would have implications for ecosystem function and biodiversity. We evaluated landscape-scale patterns of plant community diversity across gradients of rural to urban land-use, testing whether diversity was related to environmental conditions indicative of surrounding land-use. We surveyed vegetation and collected soil samples from 45 wetlands throughout Ohio, USA. Sites were categorized based on surrounding land-use as intense urban, moderate urban, or rural, representing 15 replicate urban to rural gradients. Non-native richness was 56% greater and non-native relative abundance 74% greater in intense urban sites compared to rural sites. Structural equation modeling indicated that high non-native relative abundance caused reductions in native plant richness but not native Shannon diversity, which was instead related to high concentrations of urban-associated soil contaminants such as cadmium and sodium. Our results support both versions of the driver-passenger model of invasion impacts, depending on the response: native richness is directly limited by competition with non-native species (the driver model), while native diversity is limited more by urban-associated stressors that also affect non-natives (the passenger model). The few wetlands remaining in highly urban areas thus experience a range of constraints affecting multiple dimensions of wetland health. We argue it is in these sites specifically where the benefits of restoring wetland ecosystems will be maximized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Changes in land-use from human development have led to global ecosystem degradation (DeFries et al. 2004; Foley et al. 2005), with both urbanization and intensive agriculture contributing to habitat fragmentation, soil erosion, excess runoff, climate change, and biodiversity loss (DeFries et al. 2004). Wetland systems are particularly vulnerable to anthropogenic land-use change, resulting in widespread wetland loss and degradation that diminishes wetland functions and services (Brinson and Malvárez 2002). Both globally and in the United States, around 50% of wetland area has been lost, either due to drainage for intensive agriculture or due to urban development (Dahl 2000; NRC 1995; Zedler and Kercher 2005). Such losses sever hydrologic connections, hindering the movement of nutrients, sediments, and organisms among the wetlands that remain (Poff et al. 1997). Within urban regions, wetlands experience increased variability in their hydrology, largely driven by the type and extent of the hydrologic disturbances occurring in these areas (Ehrenfeld et al. 2003; Ehrenfeld 2000). Urban wetlands that are hydrologically connected to watercourses and surrounded by impervious surfaces may experience increased surface runoff with large volumes of sediment, nutrients, and other contaminants (Faulkner 2004). Alternatively, urban wetlands that are disconnected hydrologically can experience lower flooding frequency and become more groundwater-dominated (Ehrenfeld 2000). Wetlands surrounded by agriculturally intensive rural land-use are affected in different ways, in particular experiencing increased sedimentation and sediment-bound phosphorus compared to more natural wetlands due to agricultural practices in these areas (Stapanian et al. 2016). Although many impacts of urban and rural land-use on wetland systems have been documented (Ehrenfeld 2000; Zedler 2003) the distinctions between urban- versus rural-specific anthropogenic stressors are more poorly understood, as are their implications for wetland biota.
Wetland plant communities are key indicators of ecosystem health (Mack et al. 2000; Moges et al. 2016) and are critical for prioritizing management decisions (Miller et al. 2006) since plants are immobile, can be susceptible to stressors resulting from land-use change (Mahaney et al. 2004; Maurer and Zedler 2002; Miller et al. 2006; Thomas and Moloney 2013; Vilà and Ibáñez 2011), and influence food webs above and below ground (Gratton and Denno 2006; Levin et al. 2006). In particular, differential responses by the native and non-native components of wetland plant communities to anthropogenic stressors represents an important dimension of plant community structure that may yield valuable insights into the condition of wetlands and the services they provide (Ehrenfeld 2008).
Although data from wetlands are lacking, findings from other ecosystems indicate that plant species richness often varies across the gradient from rural to urban land-use and that these patterns often differ for native versus non-native species (Burton et al. 2005; Duguay et al. 2007; Loewenstein and Loewenstein 2005; Wania et al. 2006). Such studies often find more non-native plant species in urban compared to rural areas, likely due to a greater degree of habitat degradation as well as propagule pressure in highly urbanized settings (Kowarik 2011). Non-native species may be adapted to tolerate conditions such as higher temperatures and increased disturbance in urban areas (Knapp et al. 2008; Maskell et al. 2006), but the presence of more people in urban areas also leads to more potential introduction pathways compared to rural or natural areas (Cadotte et al. 2017; Lockwood et al. 2005; Pyšek et al. 2010). Rural areas with intensive agricultural activity may provide exceptions to this pattern, where large numbers of non-native species can occur in these areas due to a high degree of disturbance (Deutschewitz et al. 2003; Kuhman et al. 2011). In contrast, native plant species richness tends to decrease with increased urbanization in most cases, perhaps reflecting replacement of natives by non-natives (Aronson et al. 2015; Ranta and Viljanen 2011). Yet, exceptions to this pattern also occur, with the number of native plant species sometimes increasing with increased urbanization, either due to cities acting as biodiversity hotspots or harboring large numbers of native ruderal species that are relatively tolerant of urban conditions (Deutschewitz et al. 2003; Kühn et al. 2004; McCune and Vellend 2013).
The commonly observed pattern of declines in native plant species coinciding with increases in non-natives along a gradient of increasing urbanization could result from many different mechanisms. At one extreme, native declines may be a direct result of competitive interactions between native and non-native species, with competitive non-natives more likely to occur in highly urbanized sites. This mechanism may be especially important in wetlands, which contain high individual species abundances of both native and non-native species and are particularly prone to invasion (Doherty and Zedler 2014; Zedler and Kercher 2004). But competition by non-natives is rarely the sole cause of native species loss (Gurevitch and Padilla 2004), thus a reasonable alternative is that native species are inhibited by environmental stressors that are associated with urbanization, followed by subsequent increases in non-native species that may be more resistant to such conditions (Tuchman et al. 2009). We note that native ruderal species may illustrate an important exception to this pattern, as they could be relatively tolerant of urban-associated anthropogenic stressors and thus increase with urbanization similar to non-natives (McCune and Vellend 2013). These contrasting scenarios fit well within the framework of the driver-passenger model (MacDougall and Turkington 2005), which makes a distinction between non-native species acting as drivers or as passengers of change when associated with native species loss or decline. Such distinctions are needed because specifying the mechanisms that lead to ecosystem change is an important step towards guiding effective management of degraded sites and minimizing future declines in ecosystem health.
Although summary metrics such as richness and diversity are useful indicators of community responses to anthropogenic stress, they may mask important patterns in community composition resulting from differential stressors across the urban to rural gradient. Highly urbanized environments may be dominated by urban-exploiter species (McKinney 2002) that are better adapted to the high levels of disturbance and unique environmental conditions found in urban areas (Vakhlamova et al. 2014). Urban-exploiter species are often non-native or ruderal species (McKinney 2002; Nobis et al. 2009) such as grasses or annual plants that can tolerate such conditions. In contrast, rural and suburban areas may be occupied by a distinct complement of species that are not urban-exploiters and are more sensitive to high levels of human disturbance. Such compositional differences are likely to be particularly important for wetland plant communities because in these systems the identity of a given community dominant can have a large influence on co-occurring species abundances as well as ecosystem services (Doherty and Zedler 2014; Skultety and Matthews 2018).
Patterns of plant diversity and composition across urban to rural gradients have been assessed previously, but we address two key limitations of this work with our current study. First, compared to terrestrial systems wetlands have received relatively little attention in urban to rural gradient studies (but see Ehrenfeld 2005, Ehrenfeld 2008; Skultety and Matthews 2018), despite the fact that healthy wetlands provide valuable ecosystem services such as biodiversity support and carbon sequestration and storage (Zedler and Kercher 2005; Clarkson et al. 2013). Second, the design of many previously published urban to rural gradient studies limits the ability to make general inferences regarding landscape-scale patterns of plant diversity. For instance, many such studies focus on a single city or urban region (Malkinson et al. 2018; Porter et al. 2001; Skultety and Matthews 2018; Vakhlamova et al. 2014; Zerbe et al. 2003), where broad generalizations across the landscape may not be possible. At the other extreme, most studies that have examined plant diversity across multiple cities or urban regions do not incorporate detailed field-collected data that are important for documenting and explaining plant community variation, including data on species abundances and site-level environmental conditions (Aronson et al. 2015; Deutschewitz et al. 2003; Kühn et al. 2004; Ranta and Viljanen 2011). Few studies have addressed both of these issues, incorporating detailed field-collected data from sites located across multiple cities or urban regions (Moffatt et al. 2004; Wania et al. 2006; Vallet et al. 2010). The current study attempts to address these prior limitations and thus provide a clear link between environmental conditions that vary with land-use patterns broadly, perhaps influencing plant communities as a result.
To understand how wetland plant species richness and abundances vary in response to anthropogenic land-use and associated environmental conditions, we surveyed wetland plant communities and recorded site-level environmental conditions across 15 replicate urban to rural gradients (45 sites total). We then tested the hypothesis that surrounding land-use influences the richness and abundance of native and non-native species, at least partially due to land-use specific environmental conditions. We predicted that wetlands surrounded by intense urban land-use would contain more non-native species and fewer natives compared to wetlands surrounded by rural land-use. This could result in part from increased propagule pressure and introduction pathways in urban areas, but due to the unique environmental stressors often found in urban environments, we predicted that richness and diversity patterns would support the passenger model in which variation in the native plant community is driven primarily by environmental conditions rather than by direct interactions with non-native species.
Methods
Site selection
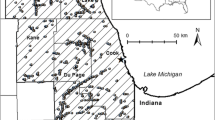
Using the National Wetlands Inventory (NWI), we considered for site selection all freshwater emergent wetlands greater than 0.04 ha in area within the state of Ohio, USA. This gave us a large pool of wetlands to select from (n = 54,519) that varied considerably in both hydrologic connectivity and wetland size (n = 31,220 for 0.04–0.28 ha, n = 16,312 for 0.28–1.2 ha, and n = 6987 for greater than 1.2 ha). We classified all such wetlands as either intense urban, moderate urban, or rural based on land-use in a 30-m buffer surrounding the wetland (National Land Cover Database 2011). We recognize this is a fairly small buffer compared to that used in other studies to evaluate the impacts of land-use on plant diversity (Charbonneau and Fahrig 2004; Duguay et al. 2007; Houlahan et al. 2006); however, a larger buffer made it impossible to compile a pool of sites to select from that had our least common land-use category (intense urban) as the majority of surrounding land-use within the buffer. National Land Cover Database (NLCD) categories of ‘Developed High Intensity’ and ‘Developed Medium Intensity’ were considered intense urban, NLCD categories of ‘Developed Low Intensity’ and ‘Developed Open Space’ were considered moderate urban, and NLCD categories of ‘Pasture/Hay’ and ‘Cultivated Crops’ were considered rural. For wetlands to be categorized as moderate urban or rural we required ≥90% of the buffer area consist of that land-use category. Only twenty intense urban wetlands across the state met this high threshold, so for this category only we lowered it so that ≥50% of the buffer area needed to be classified as intense urban. Doing so yielded 153 intense urban wetlands from which to select (before considering site access and confirming wetland status in the field).
Wetlands categorized as moderate urban based on the criteria outlined above were a heterogeneous collection of sites that were not necessarily intermediate between rural and intense urban wetlands. We therefore applied a second selection criteria to this group, requiring that at least some of the buffer area, but no more than 1% of it, be categorized as rural land-use. This ensured that wetlands classified as moderate urban contained >90% moderate urban land-use in the surrounding buffer while also having land-use characteristics that were intermediate relative to our other categories.
We randomly selected intense urban wetlands from the database first, as this was our limiting category (153 intense urban wetlands vs >1000 wetlands in each of the other two categories). We then chose moderate urban and rural wetlands within each triad based on site access (74% of sites surveyed were privately owned) and the nearest distance to the selected intense urban wetland, confirming site access and wetland status in the field prior to surveying. The average distance between wetland sites within a triad was 12.8 km. The total spatial coverage across all sites spanned an area of approximately 29,400 km2. The final forty-five selected wetlands had no overlap in their surrounding landscape buffers and were considered independent observations in analysis (Fig. 1). Nearly all of our selected wetlands (41 of 45) are hydrologically isolated, based on their being more than 10 m away from any National Hydrography Dataset (NHD) line or polygon; this distance threshold accounts for inaccuracies in the NHD and has been used previously to determine wetland isolation (Cohen et al. 2016; Lane et al. 2012).
Field surveys
We conducted vegetative surveys between June and August 2017. At each site, we sampled vegetation and environmental conditions in 1-m2 plots arranged along equally spaced transects running parallel to any elevation gradient from the edge of standing water to the edge of herbaceous wetland vegetation. To account for variable wetland sizes in our sampling scheme we increased the number of plots in larger wetlands, sampling 9 plots across three transects in wetlands of 0.04–0.28 ha (n = 30 wetlands), 12 plots across four transects in wetlands of 0.28–1.2 ha (n = 14), and 15 plots across five transects in the single wetland greater than 1.2 ha. Our largest wetlands (sampled with twelve or more plots) were fairly equally distributed across land-use categories (six intense urban, five moderate urban, and four rural). To quantify variation in nutrient availability, we collected two 2 × 15 cm soil cores from each transect at each site and stored them at 4 °C until they were processed for nutrient and elemental analysis (see below). To quantify variation in light availability, we took hemispherical photographs of the canopy from each plot using a fisheye lens situated above the tallest standing herbaceous vegetation. From these photographs, we calculated mean percent canopy openness for each site using the Gap Light Analyzer Program (Burnaby, British Columbia).
We identified herbaceous vegetation in each plot to species and estimated species abundance by eye as percent cover using the following classification: 0% = 0, <1–5% = 1, >5–25% = 2, >25–50% = 3, >50–75% = 4, >75–95% = 5, >95% = 6 (Daubenmire 1959). All cover classifications were converted to the midpoint of the range for further analysis. We averaged the midpoints for each species across plots (including instances of zero) to obtain the mean abundance for each species at each site. We used these per-species abundance estimates at the site-level to calculate Shannon diversity, Simpson evenness, and Berger-Parker dominance (Berger and Parker 1970; Shannon 1948; Simpson 1949). We determined the total richness of the site by counting the number of species present across the entire site. We categorized species as either native or non-native (USDA, NRCS 2006) so that species richness and Shannon diversity of each group could be determined separately at the site level. We also estimated the relative abundance of native and non-native species within each plot by dividing the sum of either native or non-native per-species abundances by the sum of all per-species abundances in a given plot. These values were then averaged across plots to obtain site-level relative abundances for each group. We estimated the percent of bare ground for each plot using the same cover classification as above and averaged across all plots to obtain percent bare ground at each site.
Soil analysis
To estimate plant-available nitrogen and phosphorus (NO3−, NH4+, PO43−), we used ion-exchange membranes (IEMs, 4 × 2.5 cm; SUEZ Water Technologies & Solutions: product numbers AR204R and CR67R, Trevose, PA). This method is cost-effective for measuring the relative availability of soil nutrients and correlates better with plant nutrient uptake than chemical extractions (Fernandes and Warren 1995; Qian et al. 1992). We created a slurry of 6.0 g of soil from each soil core mixed with 70 mL of deionized water (Qian and Schoenau 2002) and then submerged a cation and anion membrane in the slurry, stirring them for approximately 15 s and then leaving them to incubate at room temperature for three hours. By saturating the soil, this method provides an upper bound on plant available nutrients. After incubation, the IEMs were rinsed with deionized water and stored together at 4 °C until further processing.
We eluted nutrients bound to the membranes by submerging them in 50 mL of 2 N KCl and shaking for one hour on an orbital rotator. The resulting solution was filtered and stored at 4 °C. We estimated NO3−, NH4+, and PO43− concentrations separately using spectrophotometric procedures with standard protocols (Weatherburn 1967; Doane and Horwáth 2003; Lajtha and Jarrell 1999, respectively), quantifying them using a microplate reader (BMG LabTech: FLUOstar Omega). We processed all soil samples individually (one sample per transect, 150 total samples), averaging the final concentrations to obtain a single estimate of plant-available nitrogen and phosphorus for each site.
To estimate soil mineral content, we used nitric acid microwave digestion (EPA Method 3051) followed by ICP analysis (conducted by The Ohio State University’s Service Testing and Research Laboratory). For these analyses we combined all soil cores from a site into a single pooled sample, from which we measured a suite of elements that included macronutrients (P, K, Ca, Mg, S), micronutrients (Na, Fe, B, Mn, Zn, Cu, Co, Mo), and heavy metals (Al, As, Cd, Cr, Ni, Pb).
Statistical analysis
To determine whether wetlands surrounded by intense urban, moderate urban and rural land-use differed in plant community metrics and soil conditions, we used general linear mixed effects models. Each model included a random effect indicating the triad comprising a group of three nearest neighbor wetlands (a categorical block effect intended to account for spatial autocorrelation). We used Tukey adjustments to determine pairwise differences among our three wetland categories. Response variables were transformed as needed to meet the assumptions of linear models, including log10 transformations of mean non-native abundance, bare ground, phosphate, boron, chromium, copper, magnesium, manganese, sodium, phosphorus, lead, sulfur, and zinc; square root transformations of arsenic, iron, potassium, molybdenum, and nickel; and a logit transformation of canopy openness.
We performed Principal Component Analysis (PCA) on the soil element and nutrient data to better describe variation among sites and for subsequent use in structural equation models (SEM). All variables were transformed as above and standardized prior to running the PCA. We retained the first five axes, which were needed to account for ≥75% of explained variation. One intense urban site contained very high levels of both zinc and lead compared to the other sites. All analyses were run with and without this site, but its inclusion did not qualitatively affect our results so it remains in all analyses presented here.
We used structural equation modeling (SEM) to integrate multiple predictor and response variables and test competing hypotheses regarding the effects of land-use variation on native plant communities by way of impacts on environmental conditions and/or non-native plant communities. SEM is appropriate for these purposes as it is a statistical framework that can identify factors influencing a given response both directly as well as indirectly via effects on intermediate predictors (Grace 2006; Grace et al. 2012). It is particularly useful as a tool for comparing competing models that reflect alternative hypotheses which can be specified a priori (see step 7 in Grace et al. 2012). In our analyses, competing SEMs were formulated for both of our final responses: native species richness and native species Shannon diversity. In each case we assessed predictions from the driver-passenger model (MacDougall and Turkington 2005), testing whether environmental conditions were directly related to non-native species richness/relative abundance as well as native species richness/diversity (consistent with the passengers model) versus whether the number or relative abundance of non-native species directly drove native species richness or diversity (consistent with the driver model). To directly evaluate predictions from the driver-passenger model, we used AIC (Burnham and Anderson 2002) to compare three different SEMs for each final response: passenger-only, driver-only, and a combined driver-passenger SEM that we consider our baseline model. We included four separate linear regressions in each baseline SEM: (1) The effect of land-use on environmental conditions; (2) the effect of land-use and environmental conditions on non-native species richness; (3) the effect of land-use and environmental conditions on non-native species relative abundance; and (4) the effect of land-use, environmental conditions, non-native richness, and non-native relative abundance on the final response variable. For the passenger-only SEM, we omitted non-native richness and non-native relative abundance as predictors in regression (4). For the driver-only SEM, we omitted land-use and environmental conditions as predictors in regression (4). Environmental condition predictors included site scores from all five PC axes, percent bare ground, canopy openness, mean precipitation for the warmest quarter, and mean temperature for the warmest quarter. The use of PC axis scores as predictors for SEM is a common approach when reduced dimensionality of the dataset is required (e.g., Kavanagh et al. 2018; Romero et al. 2018). We obtained climatological data from WorldClim (Fick and Hijmans 2017). We used backwards stepwise selection in combination with AIC to simplify final regression models for each SEM. Missing paths were identified and included in each SEM using the sem.missing.paths function in the R package piecewiseSEM (v1.2.1; Lefcheck 2016). We transformed variables as above; mean temperature for the warmest quarter was square root transformed. All SEM path coefficients were standardized using the default method in piecewiseSEM. We calculated the total effect of a predictor on a response by summing the direct and indirect effects between these variables. Direct effects are equal to the path coefficient between two variables. Indirect effects are calculated by multiplying coefficients linking a predictor and a response along paths mediated by another variable (Grace 2006).
We ran Canonical Correspondence Analysis (CCA) using the R package vegan (v2.4; Oksanen et al. 2019) to analyze the effect of land-use category and environmental conditions on plant community composition. Environmental variables used in the CCA include the following: mean temperature for the warmest quarter, mean precipitation for the warmest quarter, canopy openness, bare ground, and the five PC axes from principal component analysis. Environmental variables were transformed as above. The final model was determined through backwards stepwise selection using the ordistep function in vegan (Oksanen et al. 2019), yielding a final model in which all predictor variables were statistically significant (p < 0.05).
We evaluated relationships between individual species abundances and soil nutrient/element concentrations for the three most common species across all sites. This group consisted of one non-native species, hybrid cattail (Typha x glauca – present at 29 sites), and two native species, Canada goldenrod and rice cutgrass (Solidago canadensis and Leersia oryzoides – present at 29 and 24 sites respectively). We did not conduct genetic analysis on the Typha spp. we encountered; however, morphologically we saw no indication that our wetlands contained native broadleaf cattail (Typha latifolia). As such, we considered all Typha spp. to be the hybrid cattail. We used a series of univariate mixed effects regression models, with individual species abundances as the response, single element/nutrient concentrations as the sole predictor, and triad as a random effect. Abundances and nutrient/element concentrations were transformed to satisfy the assumptions of linear models. This included a log10 transformation for Canada goldenrod abundance and a square root transformation for rice cutgrass abundance; all other variables were transformed as above.
Results
Environmental variation among land-use categories
The first five axes of the PCA on soil elements and nutrients explained 75% of the variation in those data (Table 1). Increasing PC1 values primarily reflected increases in iron, cadmium, and zinc (PC scores of 0.32, 0.31, and 0.29 respectively), whereas higher values of PC2 were associated with increases in aluminum, nickel, and chromium (PC scores of 0.40, 0.37, and 0.36). PC3 had strong positive associations with phosphorous and phosphate (PC scores of 0.46 and 0.42). PC4 was positively associated with magnesium and boron but negatively associated with lead (PC scores of 0.43, 0.42, and − 0.47). Lastly, PC5 was mostly related to nitrogen and sodium (PC scores of 0.51 and 0.48). Intense urban sites show the most separation from both moderate urban and rural sites along PC1, PC4, and PC5 axes, as seen from the 95% confidence intervals (Fig. 2).
PCA biplots for PC axes 1 and 2 (a), PC axes 1 and 3 (b) and PC axes 4 and 5 (c). Sites are shown as points, with intense urban sites as circles, moderate urban sites as squares, and rural sites as triangles. Ellipses show 95% confidence intervals around the centroid for each land-use classification. Solid lines surround intense urban sites, dashed lines surround moderate urban sites, and dotted lines surround rural sites
Single element and nutrient concentrations often varied along the rural to urban gradient, usually with intermediate values in moderate urban sites compared to the other two land-use categories. Intense urban sites had significantly higher concentrations than either moderate urban or rural sites for magnesium, sulfur, sodium, boron and arsenic (Table 2). Manganese was higher in intense urban sites only compared to rural sites, whereas aluminum was higher in rural and moderate urban sites compared to intense urban sites. Plant-available nutrients (NO3−, NH4+, and PO43−) did not differ among land-use categories (Table 2).
Two additional indicators of potentially stressful conditions were also higher in intense urban sites compared to rural sites, with moderate urban sites intermediate. Intense urban sites had a greater proportion of bare ground compared to rural sites, with intermediate values in moderate urban sites that did not differ from the other groups (p = 0.03; mean ± se for intense urban: 0.12 ± 0.02, moderate urban: 0.09 ± 0.02, rural: 0.08 ± 0.03). Mean temperature for the warmest quarter was also higher in intense urban sites compared to rural sites (p < 0.001); intense urban: 21.87 ± 0.15 °C, moderate urban: 21.76 ± 0.17 °C, rural: 21.65 ± 0.18 °C). Canopy openness and mean precipitation for the warmest quarter did not differ among land-use categories (both p > 0.55).
Plant community variation among land-use categories
Species richness for native and non-native species groups varied depending on surrounding land-use, although there were no differences in total richness or Shannon diversity (native and non-native species combined; both p > 0.37). Non-native species richness was highest in intense urban sites, intermediate in moderate urban and lowest in rural (Fig. 3a), with significant differences between intense urban and rural sites (F2,44 = 5.96, p = 0.05, Tukey p = 0.04). Native species richness trended in the opposite direction across the gradient but with no significant differences (p = 0.21; Fig. 3a). Native and non-native diversity did not differ among land-use categories (p = 0.30 and p = 0.96 respectively; Fig. 3b), but native diversity tended to be higher in rural than urban sites. As a proportion of total richness, non-native species made up 42% of species present in intense urban sites, 31% in moderate urban and 30% in rural sites. The difference in these proportions was marginally significant across land-use categories (p = 0.07).
Species abundances differed depending on surrounding land-use when evaluating all species and native/non-native species separately. The relative abundance of non-native species as a group was greater in intense urban sites than in rural sites and marginally greater than in moderate urban sites (F2,44 = 10.77, p = 0.004, Tukey p = 0.004 and p = 0.052 respectively; intense urban: 0.66 ± 0.06, moderate urban: 0.45 ± 0.06, rural: 0.38 ± 0.08; Fig. 3c). Because the relative abundance of native species is the complement of non-native relative abundance it exhibited the opposite pattern, with values in intense urban sites lower than in rural sites and marginally lower than in moderate urban sites (F2,44 = 10.42, p = 0.005, Tukey p = 0.005 and p = 0.06 respectively; intense urban: 0.34 ± 0.06, moderate urban: 0.54 ± 0.06, rural: 0.62 ± 0.08). Simpson’s evenness for all species was higher in rural sites compared to intense urban sites (F2,44 = 5.80, p = 0.05, Tukey p = 0.05; intense urban: 0.23 ± 0.02, moderate urban: 0.31 ± 0.04, rural: 0.36 ± 0.04).
Integrating environmental and community variation among land-use categories
In both baseline SEMs, environmental variation captured by PC1 and PC2 reflected land-use variation and was also correlated with non-native plant community characteristics. PC1 scores were higher in intense urban sites than they were in rural or moderate urban sites (β = −0.74, p = 0.04 and β = −0.85, p = 0.02 respectively; Fig. 4), reflecting greater concentrations of iron, cadmium, and zinc with intense urbanization. Sites with higher PC1 scores tended to have more bare ground (β = 0.26; p = 0.09) and therefore fewer non-native species, a weak indirect negative effect of PC1 on non-native richness mediated by its effect on bare ground (indirect effect = −0.09). Consistent with our univariate analyses (Fig. 3a), baseline SEMs indicated greater non-native richness in intense urban sites than in either rural or moderate urban sites (β = −1.34; p = 0.001 and β = −0.79; p = 0.03 respectively; Fig. 4). PC2 scores were greater in rural sites relative to intense urban and positively related to the relative abundance of non-native species (β = 0.59; p = 0.07 and β = 0.36; p = 0.01 respectively), indicating greater non-native relative abundance in sites with high concentrations of aluminum, nickel, and chromium. Consistent with our univariate analysis (Fig. 3), the relative abundance of non-native species was lower in rural and moderate urban wetlands compared to intense urban sites (β = −1.24; p < 0.001 and β = −0.92; p = 0.008 respectively; Fig. 4).
Structural equation models for the two final responses of native species richness (a) and native species diversity (b). Solid arrows indicate positive effects and dashed arrows indicate negative effects. P values for individual paths are indicated by superscript symbols (+: p < 0.10; *: p < 0.05; **: p < 0.01; and ***: p < 0.001). Both SEM for native richness and native diversity contained all necessary paths as indicated by the test of directed separation using Fisher’s C statistic (Fisher’s C = 58.66; p = 0.83 and Fisher’s C = 72.34; p = 0.47 respectively)
Environmental conditions and non-native relative abundance were directly correlated with native species richness (Fig. 4a). We also detected an indirect effect of PC1 on native richness, mediated by bare ground, that was similar in magnitude to the indirect effect on non-native richness (indirect effect = −0.08). This indicates that sites with high concentrations of iron, cadmium, and zinc tended to have more bare ground and therefore fewer species overall. PC4 was positively related to native richness (β = 0.37; p = 0.01), indicating a positive association between native richness and soil magnesium and boron and a negative association with lead. We also found a negative effect of non-native relative abundance on native richness (β = −0.51; p = 0.001), while detecting no relationship between non-native and native richness.
Several environmental conditions were directly correlated with native diversity, but in contrast with the baseline native richness SEM we detected no influence of the non-native plant community on native diversity (Fig. 4b). PC1 was negatively related to native diversity (β = −0.33; p = 0.03), indicating lower native diversity in sites with high concentrations of iron, cadmium, and zinc. PC5 was also negatively related to native diversity (β = −0.35; p = 0.02), indicating lower native diversity in sites with high concentrations of sodium and plant-available nitrogen (NO3 and NH4). We found no effect of non-native richness or relative abundance on native diversity.
Comparisons between SEMs indicated that the effect non-native species have on native richness was best described by the driver-only model (driver-only AIC = 139.26; passenger-only AIC = 188.09; baseline AIC = 154.66). In contrast, non-native effects on native diversity were best described by the passenger-only model (passenger-only AIC = 166.34; driver-only AIC = 202.6). We note that for native diversity, the passenger-only model and the baseline model were identical, as both non-native richness and non-native relative abundance were omitted from the baseline model during model selection.
Community composition and individual species responses to environmental conditions
Land-use categories along with their associated environmental constraints drove variation in community composition (ANOVA permutation test from CCA; p = 0.001). Based on the CCA (Fig. 5, Table 3), axis 1 is primarily associated with light availability at the top of the herbaceous layer and percent bare ground (−0.80 and − 0.56 loading values respectively), whereas axis 2 is associated with temperature (0.69 loading value). Intense urban and rural sites were also separated along axis 1, with intense urban sites associated with greater bare ground and canopy openness.
Canonical Correspondence Analysis biplot, showing how community composition varies with land-use category and environmental conditions. Sites are shown as points, with intense urban sites as circles, moderate urban sites as squares, and rural sites as triangles. Ellipses indicate 95% confidence intervals around the centroid for each land-use type. Solid lines surround intense urban sites, dashed lines surround moderate urban sites, and dotted lines surround rural sites. Species locations in ordination space are indicated with grey text for native species and black text for non-native species. Only a subset of species included in the analysis are depicted (36 of 178), including those with relatively high axis scores along CCA 1 and CCA 2 and those with high mean abundances across all sites. See Table A1 for the coded list of species abbreviations. The overall proportion of constrained inertia in explaining the community composition is 0.14
Mean abundances of the three most commonly occurring species also varied in their response to soil conditions. Sodium concentration was the only element associated with mean abundance for all three, with the abundance of non-native hybrid cattail increasing and the abundances of the natives Canada goldenrod and rice cutgrass decreasing in sites with greater sodium concentrations (all p < 0.05; Fig. 6). Mean abundance of the most common non-native, hybrid cattail, was also positively related to concentrations of aluminum, chromium, potassium, sodium, and nickel (all p < 0.05). Mean abundance of the most common native, Canada goldenrod, was negatively related to boron, cadmium, sodium, and sulfur (all p < 0.05).
Mean abundance for the most common species in response to site-level sodium concentrations. Sodium concentration is plotted in the log10 scale. Best-fit lines are plotted using model parameter estimates from regression models. Solid lines show hybrid cattail abundance, dashed lines show Canada goldenrod abundance, and dotted lines show rice cutgrass abundance. Points represent mean abundance at each site, with black points representing hybrid cattail, grey points representing Canada goldenrod, and white points representing rice cutgrass
Discussion
By sampling replicated urban to rural gradients across a large geographic region, we have documented variation in native and non-native wetland plant communities that represents a generalized response to urbanization. In our most intense urban sites, species richness and relative abundance of non-natives was the greatest and community evenness the lowest among our land-use categories. These patterns apparently result from stressors and other environmental conditions that are land-use-specific and that have differential effects on native and non-native plant species, resulting in compositional shifts across the landscape. Yet non-native species are not simply passengers of environmental change in our wetlands, based on SEM results that highlight a direct connection between high non-native abundances and low native species richness (but not diversity) that is independent of land-use category. The effects of urbanization and other dimensions of human land-use are complex, but our work identifies key relationships that may help clarify the mechanisms underlying community change in a critically important but globally imperiled ecosystem.
Land-use variation in environmental conditions
Many soil nutrient and elemental concentrations varied systematically across the gradient from urban to rural land-use, as represented by univariate analyses (Table 2) and differentiation in principal component axis scores based on the SEM (Fig. 4). For instance, PC1 scores were greater in intense urban wetlands compared to our other land-use categories, reflecting generally higher concentrations of iron, cadmium, and zinc in those sites. Yet, for cadmium and zinc, we found no clear univariate patterns across our land-use gradient, perhaps reflecting a diversity of input sources that span urban and rural settings. Common sources of cadmium include fossil fuel combustion, vehicle tire linings, and phosphate fertilizer application (EU Risk Assessment 2007), while sources of zinc include sewage sludge, animal manure, atmospheric deposition, and galvanized structures (EU Risk Assessment 2008). Our findings are consistent with other studies, some of which have found greater concentrations of both cadmium and zinc in urban compared to rural environments (Lavado et al. 1998; Li et al. 2014), with others reporting high levels of zinc across an urban to rural gradient due to multiple input sources (Callender and Rice 2000).
Axis scores for PC4 and PC5 were also greater in intense urban wetlands compared to other land-use types. High PC4 scores reflect higher magnesium and boron and lower lead concentrations, with magnesium and boron both significantly greater in intense urban wetlands based on univariate analyses. Surveys in forested systems have also found greater concentrations of magnesium in urban compared to rural areas (Pouyat et al. 1995). High PC5 scores reflect greater sodium and nitrogen ion concentrations, although only sodium was significantly higher in intense urban wetlands based on univariate analyses (Table 2). Elevated concentrations of both sodium and magnesium in our urban soils likely come from deicing salts, which are commonly applied in areas with high road densities (Cunningham et al. 2008).
Contrary to the other axes, PC2 scores were greater in rural compared to intense urban wetlands. High PC2 scores reflect more aluminum, nickel, and chromium, with aluminum concentrations also highest in rural wetlands based on univariate analysis (Table 2). High aluminum concentrations in our rural wetlands may result from nearby agricultural applications of commercial ammonium-nitrate fertilizers, which can lower soil pH and thereby increase exchangeable aluminum in the soil (Moore and Edwards 2005).
Plant communities from urban to rural environments
The native and non-native components of wetland plant communities in our region appear to have contrasting responses to urbanization, based on landscape-scale variation in richness and relative abundance. Patterns were most pronounced for non-natives, which were present in greater numbers and greater relative abundances in intense urban versus rural wetlands. Native species followed the opposite trend. Our results for non-native species are consistent with findings from previous urban-to-rural gradient surveys in wetlands and other systems (Aronson et al. 2015; Burton et al. 2005; Skultety and Matthews 2018; Vakhlamova et al. 2014). Such associations between non-native species and urbanization could reflect more introduction pathways and thus higher propagule pressure in urban compared to rural environments due to increased human population densities (Lockwood et al. 2005; Pyšek et al. 2010). Additionally, compared to native species on average non-natives may simply be more tolerant of the conditions associated with urban environments (Kowarik 2011).
Interestingly, we found that the contrasting responses by native and non-native species appeared to offset each other, yielding total species richness and diversity that did not differ across our urban to rural gradients. Results from other studies that have estimated total plant species richness across urban to rural gradients have been inconsistent, sometimes finding more species in urban environments (Deutschewitz et al. 2003; Kühn et al. 2004; Wania et al. 2006), more species in suburban or rural environments (Ranta and Viljanen 2011; Vakhlamova et al. 2014), or no change in richness across the landscape (Aronson et al. 2015). Some of this variability may result from inconsistency across studies in the way that rural sites are defined, including non-urban conditions that range from intensive agriculture (as in the current study) to sites with pristine natural vegetation. Alternatively, a lack of change in total diversity across urban to rural gradients could result from urban floras accumulating large numbers of unique non-native species that will displace natives at some point in the future, consistent with the establishment of an extinction debt (Tilman et al. 1994). Our CCA results may represent such a pattern, as may our observations of divergent variation in native versus non-native species responses to increasing urbanization. Local extinctions of native species are difficult to capture in urban to rural gradient studies that almost always focus on a single point in time (but see Zerbe et al. 2003), and inconsistent published patterns of total richness across the gradient could reflect the unique histories of different cities (Hahs et al. 2009). Longer term studies and/or those including an experimental component would further alleviate the temporal limitation of most current urban to rural gradient studies and clarify the mechanisms behind observed patterns of total richness. Without a clear sense of the mechanisms driving variation in total richness, specific characteristics of that diversity such as native/non-native status, community composition, and functional trait distributions may be more useful in understanding community change across the landscape.
Land-use and environmental effects on plant communities
Much of the variation in native and non-native species richness and abundance we observed across the urban to rural gradient apparently results from soil nutrients and putative contaminants that are associated with urbanization, based on our structural equation models. Few previous studies have united land-use and environmental conditions under a single framework to explain changes in plant communities across the urban to rural gradient (but see Ehrenfeld 2008; Godefroid and Koedam 2003). In our case, sites with the highest PC1 scores had the lowest native Shannon diversity as well as the lowest species richness for both natives and non-natives; the relationships between PC1 and richness reflected indirect effects that were mediated by a higher proportion of bare ground in high PC1 sites, which were predominantly categorized as intense urban. High concentrations of cadmium and zinc in soil are detrimental for plants, with cadmium leading to reduced root growth and chlorophyll production (Kabata-Pendias and Pendias 2001) and zinc leading to reduced root and shoot growth (Alloway 2012). Therefore, the negative relationship between PC1 and plant community structure (for both native and non-native species) could ultimately result from cadmium and/or zinc toxicity. The more strongly negative total effect of PC1 on native Shannon diversity compared to native richness may additionally indicate that urban pollutants have a greater impact on reductions in native abundance than they do on the loss of native species.
Intense urban sites were also differentiated from our other two land-use categories based on PC5, which was in turn negatively related to native Shannon diversity but not native richness. The effect on diversity alone suggests that high sodium and nitrogen concentrations in those wetlands may inhibit native abundances, a key component of that metric. More specifically, this effect is likely from sodium and nitrogen impacting rare species to a greater extent compared to highly abundant or more common species. The same analysis using Simpson’s diversity index is consistent with this interpretation because Simpson’s index is less sensitive to rare species compared to Shannon diversity and the same significant association does not occur (results not shown). Native and non-native species respond differentially to salinity in some cases, with non-native species having greater germination rates and biomass accumulation than native species under high salinity conditions (Kolb and Alpert 2003; Noe and Zedler 2000), although these results are not entirely consistent (Callaway and Zedler 1997; Kuhn and Zedler 1997). High sodium concentrations can lead to reduced productivity by interfering with the uptake of other ions necessary for plant growth (Bryson and Barker 2002; Viskari and Kärenlampi 2000). And high levels of soil nitrogen can negatively impact plant diversity via disproportionate increases in fast-growing nitrophilic species, which then suppress or competitively exclude less nitrophilic species (Bobbink et al. 2010; Carson and Barrett 1988). Because many of the nitrophilic species in our study are highly competitive non-natives (e.g., giant reed [Phragmites australis], reed canary grass [Phalaris arundinacea] and hybrid cattail), such interactions could represent a key mechanism underlying reductions in native species across the urban to rural gradient (Daehler 2003).
PC2 scores tended to be higher in rural than intense urban sites and were also positively related to an important component of plant community structure: non-native relative abundance. The mechanisms underlying a positive relationship between PC2 and non-native relative abundance are not entirely clear, but we suggest it may reflect greater sensitivity to putative stressors such as aluminum, nickel, and chromium by native species relative to non-natives in general, and perhaps relative to invasive non-natives in particular. Indeed, for the most common non-native across our sites, the invasive hybrid cattail, its relative abundance increased in sites with higher aluminum, nickel, and chromium concentrations. Other invasive species have been previously shown to reduce or exclude uptake of metals such as cadmium, lead, and zinc from the soil, leading to minimal biomass reductions under these conditions (Yang et al. 2007).
Non-native wetland species as drivers and passengers
Non-native plants can act as both drivers and passengers in reducing native species richness and per-species abundances. Based on our analyses, native wetland plant communities across the urban to rural gradient are primarily affected by environmental conditions, suggesting that non-natives are largely passengers when it comes to native species decline (MacDougall and Turkington 2005). But, the SEMs also indicate that non-native plants contribute directly to losses in native richness by reaching high relative abundances (Fig. 4), thus their role as drivers should not be ignored. Given our study design and expectations that environmental conditions would vary substantially with increasing urbanization, it is perhaps not surprising that environmental conditions were the most important factor influencing native plant communities (and the only factor influencing Shannon diversity).
Non-native species acting as drivers in reducing native species richness (Fig. 4a) implies a role for direct competitive interactions. This result may be surprising in light of studies reporting that competition from non-native invasive plants does not cause local extinctions (Gurevitch and Padilla 2004; Sax and Gaines 2008). Of course, since our data are observational this pattern could alternatively reflect strong biotic resistance, with sites high in native species richness limiting non-native relative abundances. However, because human-impacted wetlands are often dominated by large, clonal species that grow in near monocultures (Frieswyk et al. 2007; Trebitz and Taylor 2007), and because even the per-capita effects of non-native plants are more strongly suppressive than per-capita effects of similarly abundant natives (Pearse et al. 2019) we think widespread biotic resistance is highly unlikely (see also Levine et al. 2004). We were surprised to find that non-natives were drivers only in the decline of native species numbers and not Shannon diversity (thus native abundances). Declines in richness should only come after declines in abundance, suggesting these metrics should be correlated. But if highly competitive non-natives have been present in our sites for a sufficient length of time it could be that the most susceptible natives had already declined to extinction, weakening the relationship between native richness and diversity.
Whether non-native species function as drivers or passengers with respect to changes in native communities and ecosystems will often depend on the identity of the non-native species in question. Such identity effects certainly contributed to our findings, as they would in other invaded wetland plant communities where non-natives tend to occur in high abundances (Zedler and Kercher 2004). The most common and dominant non-native species across our sites was hybrid cattail, which was present in 64% of wetlands (29 of 45) at a mean abundance of 41.0 ± 4.7% cover (range = 0.2 to 79.2%). Hybrid cattail clearly has the potential to impact other species directly by producing large amounts of litter and changing nutrient cycling dynamics, resulting in the competitive exclusion of native species (Lishawa et al. 2019; Tuchman et al. 2009). Thus, the negative relationship we found between non-native relative abundance and native richness overall could reflect the common occurrence and abundance of this species specifically. Two other common wetland invaders from our study likely have similar direct competitive effects, although they occurred in fewer sites than did hybrid cattail. Both giant reed (found in 7% of our sites) and reed canary grass (42% of our sites) limit the establishment and abundance of native species because of their dense growth, high productivity and substantial rhizome and litter biomass (Healy and Zedler 2010; Minchinton et al. 2006). Yet even highly competitive species such as these may serve dual roles as drivers as well as passengers of change (Bauer 2012), as illustrated by detailed work with reed canary grass showing that native species can outcompete it at low nutrient levels (Perry et al. 2004) and that nutrient enrichment contributes to declines in native richness regardless of its presence (Green and Galatowitsch 2002). More experimental work focused on these and other common invasive species could help further clarify the circumstances under which non-natives generally are acting as drivers or passengers of change in native systems.
Compositional differences reflecting land-use and environmental conditions
Separation of wetland plant communities based on land-use classification occurs only after also accounting for site-specific environmental conditions (Fig. 5). Within our CCA, the separation of intense urban sites from our other land-use categories is correlated with a high degree of canopy openness (fewer large trees and shrubs) and bare ground along axis 1. The latter effect complements our understanding based on the SEM, showing that more bare ground (and the underlying urban-associated conditions that increase it) is associated with native species richness declines as well as compositional shifts in the community overall.
The CCA also highlights some separation of intense urban sites from the other land-use categories based on temperature. This appears to reflect the unique non-native species found in intense urban sites at higher latitudes compared to lower latitudes. For example, several intense urban wetlands from the northern region of the state were dominated by invasive giant reed, whereas similar sites in central Ohio lacked this species (see also Saltonstall 2002).
To our knowledge, only two other studies in wetlands have reported differences in community composition based on surrounding land-use and correlated indicators of anthropogenic activity. Skultety and Matthews (2018) used presence-absence data from 1999 wetlands in the Chicago, Illinois metropolitan area and found compositional differences based on both land-use type and surrounding road type, with unique plant communities in urban wetlands near highways compared to rural wetlands near two-lane roads. Ehrenfeld (2008) reported differences in the occurrence of native versus non-native herbaceous species in forested wetlands based on the proportion of a wetland’s buffer in residential land-use as well as indicators of anthropogenic activity such as human population density, road density, and presence of rubbish. Research in other ecosystems has also found compositional differences in plant communities based on land-use type. In urban forests and grasslands, increases in urban land-use and other indicators of anthropogenic disturbance are associated with greater proportions of ruderal and non-native species in the community (Godefroid and Koedam 2003; Vakhlamova et al. 2014). Shifting plant community composition in response to anthropogenic land-use is thus a common finding, but the idiosyncratic nature of species-specific responses make generalizations across studies difficult. This is an area where we expect the widespread use of plant trait data for describing the composition of communities in functional terms to yield particularly valuable insights in the near future (e.g., Lososová et al. 2006; Williams et al. 2005).
Variation in plant community composition in relation to land-use could be influenced by adaptive variation in tolerance by key individual species to certain characteristics of urban environments. This especially applies to wetlands, which are often dominated by individual species in high abundances (Frieswyk et al. 2007; Zedler and Kercher 2004). For example, sites in our survey that had high concentrations of soil sodium (associated with intense urbanization) had greater abundances of non-native hybrid cattail but lower abundances of the natives Canada goldenrod and rice cutgrass. Similar patterns have been documented for giant reed, where the non-native lineage produces more biomass and has better survival than native lineages under high salinity (Vasquez et al. 2005). The responses of these and other common and relatively abundant species should be highly influential when it comes to overall compositional change across the urban to rural gradient.
Conclusions
Our findings document variation in the number and relative abundance of native and non-native species in response to surrounding land-use, with several mechanisms potentially driving this pattern. Urban habitats contain unique environmental stressors, which may limit the overall abundance of native species. Non-native species may also spread more rapidly in these settings because of greater tolerances to such stressors, eventually displacing native species. The high number and abundance of non-native species in intense urban wetlands compared to moderate urban or rural wetlands could be an indicator of poor wetland quality, which may have consequences for the ecosystem services these wetlands provide. Given the importance of wetland ecosystem services and global trends in the loss of urban wetlands (Clare and Creed 2014; Dahl 2000; Davis and Froend 1999; Sultana et al. 2009), we believe management and research efforts should focus more attention on intense urban wetlands to clearly assess the implications of their degradation and the potential gains from their successful restoration.
Data availability
The datasets and analysis code generated for the current study are available in the Knowledge Network for Biocomplexity (KNB) repository and can be found at the following link: https://knb.ecoinformatics.org/view/doi:10.5063/F1ZS2TVZ.
References
Alloway BJ (2012) Heavy metals in soils, 3rd edn. Springer, Dordrecht
Aronson MF, Handel SN, La Puma IP, Clemants SE (2015) Urbanization promotes non-native woody species and diverse plant assemblages in the New York metropolitan region. Urban Ecosyst 18(1):31–45
Bauer JT (2012) Invasive species: “back-seat drivers” of ecosystem change? Biol Invasions 14(7):1295–1304. https://doi.org/10.1007/s10530-011-0165-x
Berger WH, Parker FL (1970) Diversity of planktonic foraminifera in deep-sea sediments. Science 168(3937):1345–1347
Bobbink, R., Hicks, K., Galloway, J., Spranger, T., Alkemade, R., Ashmore, M., ... De Vries, W. (2010). Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl, 20(1), 30–59. https://doi.org/10.1890/08-1140.1
Brinson MM, Malvárez AI (2002) Temperate freshwater wetlands: types, status, and threats. Environ Conserv 29(2):115–133
Bryson GM, Barker AV (2002) Sodium accumulation in soils and plants along Massachusetts roadsides. Commun Soil Sci Plant Anal 33(1–2):67–78. https://doi.org/10.1081/CSS-120002378
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer-Verlag, New York
Burton ML, Samuelson LJ, Pan S (2005) Riparian woody plant diversity and forest structure along an urban-rural gradient. Urban Ecosyst 8(1):93–106. https://doi.org/10.1007/s11252-005-1421-6
Cadotte MW, Yasui SLE, Livingstone S, MacIvor JS (2017) Are urban systems beneficial, detrimental, or indifferent for biological invasion? Biol Invasions 19(12):3489–3503
Callaway JC, Zedler JB (1997) Interactions between a salt marsh native perennial (Salicornia virginica) and an exotic annual (Polypogon monspeliensis) under varied salinity and hydroperiod. Wetl Ecol Manag 5(3):179–194. https://doi.org/10.1023/a:1008224204102
Callender E, Rice KC (2000) The urban environmental gradient: anthropogenic influences on the spatial and temporal distributions of Lead and zinc in sediments. Environmental Science & Technology 34(2):232–238. https://doi.org/10.1021/es990380s
Carson WP, Barrett GW (1988) Succession in old-field plant communities: effects of contrasting types of nutrient enrichment. Ecology 69(4):984–994. https://doi.org/10.2307/1941253
Charbonneau NC, Fahrig L (2004) Influence of canopy cover and amount of open habitat in the surrounding landscape on proportion of alien plant species in forest sites. Écoscience 11:278–281. https://doi.org/10.1080/11956860.2004.11682833
Clare S, Creed IF (2014) Tracking wetland loss to improve evidence-based wetland policy learning and decision making. Wetl Ecol Manag 22(3):235–245. https://doi.org/10.1007/s11273-013-9326-2
Clarkson BR, Ausseil AE, Gerbeaux P (2013) Wetland ecosystem services. In: Dymond JR (ed) Ecosystem Services in new Zealand: conditions and trends. Manaaki Whenua Press, Lincoln, pp 192–202
Cohen, M. J., Creed, I. F., Alexander, L., Basu, N. B., Calhoun, A. J. K., Craft, C., …, Walls, S. C. (2016). Do geographically isolated wetlands influence landscape functions? PNAS, 113(8), 1978–1986. https://doi.org/10.1073/pnas.1512650113
Cunningham MA, Snyder E, Yonkin D, Ross M, Elsen T (2008) Accumulation of deicing salts in soils in an urban environment. Urban Ecosyst 11(1):17–31. https://doi.org/10.1007/s11252-007-0031-x
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34(1):183–211. https://doi.org/10.1146/annurev.ecolsys.34.011802.132403
Dahl TE (2000) Status and trends of wetlands in the conterminous United States 1986 to 1997. U.S. Department of Interior, Fish and Wildlife Service, Washington, DC
Daubenmire R (1959) A canopy-coverage method of vegetational analysis. Northwest Sci 33:43–64
Davis JA, Froend R (1999) Loss and degradation of wetlands in southwestern Australia: underlying causes, consequences and solutions. Wetl Ecol Manag 7(1):13–23. https://doi.org/10.1023/a:1008400404021
DeFries R, Foley J, Asner G (2004) Land-use choices: balancing human needs and ecosystem function. Front Ecol Environ 2(5):249–257. https://doi.org/10.1890/1540-9295(2004)002[0249:LCBHNA]2.0.CO;2
Deutschewitz K, Lausch A, Kühn I, Klotz S (2003) Native and alien plant species richness in relation to spatial heterogeneity on a regional scale in Germany. Glob Ecol Biogeogr 12(4):299–311. https://doi.org/10.1046/j.1466-822X.2003.00025.x
Doane TA, Horwáth WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36(12):2713–2722
Doherty JM, Zedler JB (2014) Dominant graminoids support restoration of productivity but not diversity in urban wetlands. Ecol Eng 65:101–111. https://doi.org/10.1016/j.ecoleng.2013.07.056
Duguay S, Eigenbrod F, Fahrig L (2007) Effects of surrounding urbanization on non-native flora in small forest patches. Landsc Ecol 22:589–599. https://doi.org/10.1007/s10980-006-9050-x
Ehrenfeld JG (2000) Evaluating wetlands within an urban context. Urban Ecosyst 4(1):69–85. https://doi.org/10.1023/a:1009543920370
Ehrenfeld JG (2005) Vegetation of forested wetlands in urban and suburban landscapes in New Jersey. J Torrey Botanic Soc 132(2):262–279. https://doi.org/10.3159/1095-5674(2005)132[262:VOFWIU]2.0.CO;2
Ehrenfeld JG (2008) Exotic invasive species in urban wetlands: environmental correlates and implications for wetland management. J Appl Ecol 45(4):1160–1169
Ehrenfeld JG, Cutway HB, Hamilton R, Stander E (2003) Hydrologic description of forested wetlands in northeastern New Jersey, USA—an urban/suburban region. Wetlands 23:685–700. https://doi.org/10.1672/0277-5212(2003)023[0685:HDOFWI]2.0.CO;2
EU (2007) European Union risk assessment report. Cadmium metal. Part I environment, vol 72. Office for Official Publications of the European Communities, Luxembourg
EU (2008) European Union risk assessment report. Zinc metal. Part I environment. Office for Official Publications of the European Communities, Luxembourg
Faulkner S (2004) Urbanization impacts on the structure and function of forested wetlands. Urban Ecosyst 7:89–106. https://doi.org/10.1023/B:UECO.0000036269.56249.66
Fernandes M, Warren G (1995) Comparison of resin beads and resin membranes for extracting soil phosphate. Fertilizer Research 44(1):1–8
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37(12):4302–4315
Foley, J., DeFries, R., Asner, G., Barford, C., Bonan, G., Carpenter, S., ... Snyder, P. (2005). Global consequences of land use. Science, 309(5734), 570–574. https://doi.org/10.1126/science.1111772
Frieswyk CB, Johnston CA, Zedler JB (2007) Identifying and characterizing dominant plants as an Indicator of community condition. J Great Lakes Res 33:125–135. https://doi.org/10.3394/0380-1330(2007)33[125:IACDPA]2.0.CO;2
Godefroid S, Koedam N (2003) Distribution pattern of the flora in a peri-urban forest: an effect of the city–forest ecotone. Landsc Urban Plan 65(4):169–185. https://doi.org/10.1016/S0169-2046(03)00013-6
Grace J (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511617799
Grace J, Schoolmaster DR, Guntenspergen GR, Little AM, Mitchell BR, Miller KM, Schweiger EW (2012) Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 3:73. https://doi.org/10.1890/ES12-00048.1
Gratton C, Denno R (2006) Arthropod food web restoration following removal of an invasive wetland plant. Ecol Appl 16(2):622–631. https://doi.org/10.1890/1051-0761(2006)016[0622:AFWRFR]2.0.CO;2
Green EK, Galatowitsch SM (2002) Effects of Phalaris arundinacea and nitrate-N addition on the establishment of wetland plant communities. J Appl Ecol 39(1):134–144. https://doi.org/10.1046/j.1365-2664.2002.00702.x
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trends Ecol Evol 19(9):470–474. https://doi.org/10.1016/j.tree.2004.07.005
Hahs AK, McDonnell MJ, McCarthy MA, Vesk PA, Corlett RT, Norton BA, Clemants SE, Duncan RP, Thompson K, Schwartz MW, Williams NSG (2009) A global synthesis of plant extinction rates in urban areas. Ecol Lett 12(11):1165–1173. https://doi.org/10.1111/j.1461-0248.2009.01372.x
Healy M, Zedler J (2010) Set-backs in replacing Phalaris arundinacea monotypes with sedge meadow vegetation. Restor Ecol 18(2):155–164. https://doi.org/10.1111/j.1526-100X.2009.00645.x
Houlahan JE, Keddy PA, Makkay K, Findlay CS (2006) The effects of adjacent land use on wetland species richness and community composition. Wetlands 26:79–96. https://doi.org/10.1672/0277-5212(2006)26[79:TEOALU]2.0.CO;2
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants, 3rd edn. CRC Press LLC, Boca Raton
Kavanagh PH, Vilela B, Haynie HJ, Tuff T, Lima-Ribeiro M, Gray RD, Botero CA, Gavin MC (2018) Hindcasting global population densities reveals forces enabling the origin of agriculture. Nat Hum Behav 2:478–484. https://doi.org/10.1038/s41562-018-0358-8
Knapp S, Kühn I, Schweiger O, Klotz S (2008) Challenging urban species diversity: contrasting phylogenetic patterns across plant functional groups in Germany. Ecol Lett 11(10):1054–1064
Kolb A, Alpert P (2003) Effects of nitrogen and salinity on growth and competition between a native grass and an invasive congener. Biol Invasions 5(3):229–238. https://doi.org/10.1023/a:1026185503777
Kowarik I (2011) Novel urban ecosystems, biodiversity, and conservation. Environ Pollut 159(8–9):1974–1983. https://doi.org/10.1016/j.envpol.2011.02.022
Kuhman TR, Pearson SM, Turner MG (2011) Agricultural land-use history increases non-native plant invasion in a southern Appalachian forest a century after abandonment. Can J For Res 41:920–929. https://doi.org/10.1139/x11-026
Kuhn NL, Zedler JB (1997) Differential effects of salinity and soil saturation on native and exotic plants of a coastal salt marsh. Estuaries 20(2):391–403. https://doi.org/10.2307/1352352
Kühn I, Brandl R, Klotz S (2004) The flora of German cities is naturally species rich. Evol Ecol Res 6(5):749–764
Lajtha K, Jarrell WM (1999) Soil phosphorus. In: Robertson GP et al (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 115–142
Lane CR, D’Amico E, Autrey B (2012) Isolated wetlands of the southeastern United States: abundance and expected condition. Wetlands 32:753–767. https://doi.org/10.1007/s13157-012-0308-6
Lavado RS, Rodríguez MB, Scheiner JD, Taboada MA, Rubio G, Alvarez R, Alconada M, Zubillaga MS (1998) Heavy metals in soils of Argentina: comparison between urban and agricultural soils. Commun Soil Sci Plant Anal 29(11–14):1913–1917. https://doi.org/10.1080/00103629809370081
Lefcheck JS (2016) piecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol Evol 7(5):573–579
Levin L, Neira C, Grosholz E (2006) Invasive cordgrass modifies wetland trophic function. Ecology 87(2):419–432. https://doi.org/10.1890/04-1752
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7(10):975–989. https://doi.org/10.1111/j.1461-0248.2004.00657.x
Li L, Holm PE, Marcussen H, Bruun Hansen HC (2014) Release of cadmium, copper and lead from urban soils of Copenhagen. Environ Pollut 187:90–97. https://doi.org/10.1016/j.envpol.2013.12.016
Lishawa SC, Lawrence BA, Albert DA, Larkin DJ, Tuchman NC (2019) Invasive species removal increases species and phylogenetic diversity of wetland plant communities. Ecology and Evolution 9:6231–6244. https://doi.org/10.1002/ece3.5188
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20(5):223–228
Loewenstein NJ, Loewenstein EF (2005) Non-native plants in the understory of riparian forests across a land use gradient in the southeast. Urban Ecosyst 8(1):79–91. https://doi.org/10.1007/s11252-005-1420-7
Lososová Z, Chytrý M, Kühn I, Hájek O, Horáková V, Pyšek P, Tichý L (2006) Patterns of plant traits in annual vegetation of man-made habitats in Central Europe. Perspect Plant Ecol Evol System 8(2):69–81. https://doi.org/10.1016/j.ppees.2006.07.001
MacDougall AS, Turkington R (2005) Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86(1):42–55
Mack, J., Micacchion, M., Augusta, L., & Sablak, G. (2000). Vegetation indices of biotic integrity (VIBI) for wetlands and calibration of the Ohio rapid assessment method for wetlands v. 5.0. Ohio Environmental Protection Agency, Division of Surface Water, 401 Wetland Ecology Unit, Columbus, Ohio, USA
Mahaney WM, Wardrop DH, Brooks RP (2004) Impacts of stressors on the emergence and growth of wetland plant species in Pennsylvania, USA. Wetlands 24(3):538–549
Malkinson D, Kopel D, Wittenberg L (2018) From rural-urban gradients to patch-matrix frameworks: plant diversity patterns in urban landscapes. Landsc Urban Plan 169:260–268. https://doi.org/10.1016/j.landurbplan.2017.09.021
Maskell L, Firbank L, Thompson K, Bullock J, Smart S (2006) Interactions between non-native plant species and the floristic composition of common habitats. J Ecol 94(6):1052–1060. https://doi.org/10.1111/j.1365-2745.2006.01172.x
Maurer DA, Zedler JB (2002) Differential invasion of a wetland grass explained by tests of nutrients and light availability on establishment and clonal growth. Oecologia 131(2):279–288
McCune JL, Vellend M (2013) Gains in native species promote biotic homogenization over four decades in a human-dominated landscape. J Ecol 101:1542–1551. https://doi.org/10.1111/1365-2745.12156
McKinney ML (2002) Urbanization, biodiversity, and conservation: the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 52(10):883–890. https://doi.org/10.1641/0006-3568(2002)052[0883:ubac]2.0.co;2
Miller SJ, Wardrop DH, Mahaney WM, Brooks RP (2006) A plant-based index of biological integrity (IBI) for headwater wetlands in Central Pennsylvania. Ecol Indic 6(2):290–312
Minchinton T, Simpson J, Bertness M (2006) Mechanisms of exclusion of native coastal marsh plants by an invasive grass. J Ecol 94(2):342–354. https://doi.org/10.1111/j.1365-2745.2006.01099.x
Moffatt S, McLachlan S, Kenkel N (2004) Impacts of land use on riparian forest along an urban – rural gradient in southern Manitoba. Plant Ecol 174:119–135. https://doi.org/10.1023/B:VEGE.0000046055.27285.fd
Moges A, Beyene A, Kelbessa E, Mereta S, Ambelu A (2016) Development of a multimetric plant-based index of biotic integrity for assessing the ecological state of forested, urban and agricultural natural wetlands of Jimma highlands, Ethiopia. Ecol Indic 71:208–217
Moore PA, Edwards DR (2005) Long-term effects of poultry litter, alum-treated litter, and ammonium nitrate on aluminum availability in soils. J Environ Qual 34(6):2104–2111. https://doi.org/10.2134/jeq2004.0472
National Research Council (1995) Wetlands: characteristics and boundaries. National Academies Press, Washington, DC
Nobis MP, Jaeger JAG, Zimmermann NE (2009) Neophyte species richness at the landscape scale under urban sprawl and climate warming. Divers Distrib 15:928–939. https://doi.org/10.1111/j.1472-4642.2009.00610.x
Noe G, Zedler J (2000) Differential effects of four abiotic factors on the germination of salt marsh annuals. Am J Bot 87(11):1679–1692. https://doi.org/10.2307/2656745
Oksanen, F., Blanchet, G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., ... Wagner, H. (2019). Vegan: community ecology package. R package version 2.5–4. https://CRAN.R-project.org/package=vegan
Pearse IS, Sofaer HR, Zaya DN, Spyreas G (2019) Non-native plants have greater impacts because of differing per-capita effects and nonlinear abundance–impact curves. Ecol Lett 22:1214–1220. https://doi.org/10.1111/ele.13284
Perry LG, Galatowitsch SM, Rosen CJ (2004) Competitive control of invasive vegetation: a native wetland sedge suppresses Phalaris arundinacea in carbon-enriched soil. J Appl Ecol 41(1):151–162. https://doi.org/10.1111/j.1365-2664.2004.00871.x
Poff NL, Allan JD, Bain MB, Karr JR, Prestegaard KL, Richter BD, Sparks RE, Stromberg JC (1997) The natural flow regime. BioScience 47(11):769–784
Porter EE, Forschner BR, Blair RB (2001) Woody vegetation and canopy fragmentation along a forest-to-urban gradient. Urban Ecosyst 5(2):131–151
Pouyat RV, McDonnell MJ, Pickett STA (1995) Soil characteristics of oak stands along an urban-rural land-use gradient. J Environ Qual 24(3):516–526. https://doi.org/10.2134/jeq1995.00472425002400030019x
Pyšek, P., Jarošík, V., Hulme, P. E., Kühn, I., Wild, J., Arianoutsou, M., ... Essl, F. (2010). Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc Natl Acad Sci, 107(27), 12157–12162
Qian P, Schoenau J (2002) Practical applications of ion exchange resins in agricultural and environmental soil research. Can J Soil Sci 82(1):9–21
Qian P, Schoenau J, Huang W (1992) Use of ion exchange membranes in routine soil testing. Commun Soil Sci Plant Anal 23(15–16):1791–1804
Ranta P, Viljanen V (2011) Vascular plants along an urban-rural gradient in the city of Tampere, Finland. Urban Ecosyst 14(3):361–376
Romero GQ, Gonçalves-Souza T, Kratina P, Marino NAC, Petry WK, Sobral-Souza T, Roslin T (2018) Global predation pressure redistribution under future climate change. Nat Clim Chang 8:1087–1091. https://doi.org/10.1038/s41558-018-0347-y
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci 99(4):2445–2449. https://doi.org/10.1073/pnas.032477999
Sax DF, Gaines SD (2008) Species invasions and extinction: the future of native biodiversity on islands. Proc Natl Acad Sci 105(Supplement 1):11490–11497. https://doi.org/10.1073/pnas.0802290105
Shannon CE (1948) A mathematical theory of communication. Bell Syst Techn J 27:379–423
Simpson EH (1949) Measurement of diversity. Nature 163:688
Skultety D, Matthews JW (2018) Human land use as a driver of plant community composition in wetlands of the Chicago metropolitan region. Urban Ecosyst 21(3):447–458. https://doi.org/10.1007/s11252-018-0730-5
Stapanian M, Schumacher W, Gara B, Monteith S (2016) Negative effects of excessive soil phosphorus on floristic quality in Ohio wetlands. Sci Total Environ 551:556–562. https://doi.org/10.1016/j.scitotenv.2016.02.041
Sultana MS, Islam GMT, Islam Z (2009) Pre- and post-urban wetland area in Dhaka City, Bangladesh: a remote sensing and GIS analysis. J Water Resour Protect 01(06):414–421. https://doi.org/10.4236/jwarp.2009.16050
Thomas SM, Moloney KA (2013) Hierarchical factors impacting the distribution of an invasive species: landscape context and propagule pressure. Landsc Ecol 28(1):81–93
Tilman D, May RM, Lehman CL, Nowak MA (1994) Habitat destruction and the extinction debt. Nature 371(6492):65–66. https://doi.org/10.1038/371065a0
Trebitz AS, Taylor DL (2007) Exotic and invasive aquatic plants in Great Lakes coastal wetlands: distribution and relation to watershed land use and plant richness and cover. J Great Lakes Res 33(4):705–721. https://doi.org/10.3394/0380-1330(2007)33[705:EAIAPI]2.0.CO;2
Tuchman NC, Larkin DJ, Geddes P, Wildova R, Jankowski K, Goldberg DE (2009) Patterns of environmental change associated with Typha x glauca invasion in a Great Lakes coastal wetland. Wetlands 29(3):964–975. https://doi.org/10.1672/08-71.1
USDA, NRCS (2006) The PLANTS Database, 6 March 2006 (http://plants.usda.gov). National Plant Data Center, Baton Rouge, LA 70874-4490 USA
Vakhlamova T, Rusterholz H-P, Kanibolotskaya Y, Baur B (2014) Changes in plant diversity along an urban–rural gradient in an expanding city in Kazakhstan, Western Siberia. Landsc Urban Plan 132:111–120
Vallet J, Beaujouan V, Pithon J, Rozé F, Daniel H (2010) The effects of urban or rural landscape context and distance from the edge on native woodland plant communities. Biodivers Conserv 19:3375–3392. https://doi.org/10.1007/s10531-010-9901-2
Vasquez EA, Glenn EP, Brown JJ, Guntenspergen GR, Nelson SG (2005) Salt tolerance underlies the cryptic invasion of north American salt marshes by an introduced haplotype of the common reed Phragmites australis (Poaceae). Mar Ecol Prog Ser 298:1–8
Vilà M, Ibáñez I (2011) Plant invasions in the landscape. Landsc Ecol 26(4):461–472
Viskari E-L, Kärenlampi L (2000) Roadside scots pine as an Indicator of deicing salt use – a comparative study from two consecutive winters. Water Air Soil Pollut 122(3):405–419. https://doi.org/10.1023/a:1005235422943
Wania A, Kühn I, Klotz S (2006) Plant richness patterns in agricultural and urban landscapes in Central Germany - spatial gradients of species richness. Landsc Urban Plan 75(1–2):97–110. https://doi.org/10.1016/j.landurbplan.2004.12.006
Weatherburn M (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39(8):971–974
Williams NSG, Morgan JW, McDonnell MJ, McCarthy MA (2005) Plant traits and local extinctions in natural grasslands along an urban–rural gradient. J Ecol 93(6):1203–1213. https://doi.org/10.1111/j.1365-2745.2005.01039.x
Yang R, Tang H, Yang Y, Chen X (2007) Invasive and non-invasive plants differ in response to soil heavy metal lead contamination. Bot Stud 48(4):453–458
Zedler J (2003) Wetlands at your service: reducing impacts of agriculture at the watershed scale. Front Ecol Environ 1(2):65–72. https://doi.org/10.1890/1540-9295(2003)001[0065:WAYSRI]2.0.CO;2
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Crit Rev Plant Sci 23(5):431–452
Zedler JB, Kercher S (2005) Wetland resources: status, trends, ecosystem services, and restorability. Annu Rev Environ Resour 30:39–74
Zerbe S, Maurer U, Schmitz S, Sukopp H (2003) Biodiversity in Berlin and its potential for nature conservation. Landsc Urban Plan 62(3):139–148. https://doi.org/10.1016/S0169-2046(02)00145-7
Acknowledgements
We thank Franklin County Metro Parks, City of Columbus Parks and Recreation, City of Dublin, Allen County Farm Park, and the fourteen private landowners who granted access to conduct vegetative surveys on their property. We thank Colin Day and Nate Hofford for helping with field surveys and sample collections. We also thank Danielle Frevola and Nikhil Rivera for assisting in soil nutrient and canopy openness measurements. Funding for this research was provided by The Ohio State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 74 kb)
Rights and permissions
About this article
Cite this article
King, C.M., Hovick, S.M. Wetland plant community variation across replicate urban to rural gradients: non-native species as both drivers and passengers in systems impacted by anthropogenic land-use. Urban Ecosyst 23, 1209–1226 (2020). https://doi.org/10.1007/s11252-020-01012-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-020-01012-3